Persistence and fate of 2,3,4,6-tetrachlorophenol and pentachlorophenol in limnocorrals
Abstract
Three separate limnocorral experiments were conducted to assess the fate and effects of a commercial 2,3,4,6-tetrachlorophenol (TeCP) formulation (DIATOX®; 19.4% TeCP, 4.8% pentachlorophenol [PCP], 75.8% “inert” buffers) in an aquatic ecosystem. Nominal treatment concentrations for experiments 1 and 2 were 0.075 and 0.75 mg active ingredient (a.i.)/L and 0.75 and 1.50 mg a.i./L DIATOX®, respectively, with three replicates of each treatment. Pesticide was applied in the morning for experiment 1 and in the evening for experiment 2. For Experiment 3, unreplicated concentrations of 0.1, 0.25, 0.5, 1.0, 2.0, 4.0, and 7.3 mg a.i./L DIATOX® were chosen; pesticide was applied in the evening. In all three experiments, the pesticide was applied as a single, uniform surface treatment. Both TeCP and PCP dissipated rapidly from the water of treated limnocorrals. Times to 50% dissipation for TeCP and PCP in the integrated water column were generally similar within experiments but ranged from 0.4 to 1.1 d following the morning application of experiment 1 to 3.4 to 7.3 d following the evening applications of experiments 2 and 3. The faster initial dissipation of TeCP and PCP from the water following the morning application implicated photolysis as the primary degradation mechanism, as did the faster dissipation of chlorophenols from the surface layer than from other water depths during experiment 2. Aqueous dissipation rates for both compounds from the integrated water column were similar in all three experiments after the initial mixing period (1–4 d); the three-experiment average first-order rate coefficient for either compound after day 4 was 0.09/d. From a mass balance perspective, the majority of the chlorophenols remained in the water column. Neither compound accumulated in the sediments to any significant extent (< 0.1% of applied mass), but sediment-associated residues did dissipate at a considerably slower rate than residues in the water column. From 97 to >99% of the applied chlorophenols had dissipated from the water of treated enclosures by the end of the various experiments (42–63 d).
INTRODUCTION
Wood constitutes one of Canada's most valuable resources [1, 2]. Freshly sawn wood generally requires some form of protection from deterioration by sapstain and other fungi during transportation and storage. This ensures the commercial and aesthetic value of the lumber and has resulted in demands for short-term wood protection [3]. Until recently, the sodium salts of 2,3,4,6-tetrachlorophenol (TeCP) and pentachlorophenol (PCP) were two of the most widely used compounds for these purposes in Canada [4]. In 1985, an estimated 2.28 million kg of these compounds was used nationally, with 41% of that used in British Columbia [5]. Because of recent legislation, TeCP and PCP are no longer used for sapstain control in Canada [2].
Because most major sawmills and lumber export terminals that used these compounds are located adjacent to large bodies of water, there was great potential for surface water contamination. Both TeCP and PCP were usually present in the various commercial antisapstain formulations and were generally encountered together as common environmental contaminants. They found their way into the environment mainly as a result of runoff from wood treatment facilities, leaching from freshly treated lumber, contaminated pulp mill effluents, and direct spills [6]. In addition, TeCP is a degradation product of PCP [7-9]. Although residue levels in most Canadian surface waters were usually in the range of 1 to 1,000 ng/L, TeCP and PCP concentrations several hundred times higher were routinely recorded near wood treatment facilities, pulp and paper mills, and some industrial discharge sites [5, 9-13].
Although TeCP had been used for decades and was a widespread contaminant in Canadian aquatic ecosystems, relatively little was known about its fate and toxicity in the natural environment. The fate of PCP, however, had been investigated in a number of laboratory [14-17] and field studies [18-23]. These studies documented the rapid dissipation or degradation of PCP in shallow waters and either showed or suggested that photolysis is the main mode of degradation. In addition, results strongly suggested that the environmental conditions under which a study is conducted, especially water depth, can drastically affect the persistence and fate of the compound. Reported half-lives in experimental aquatic ecosystems range from 3.0 h in the surface waters of artificial freshwater streams [21] to 3.0 d in 1-m-deep artificial ponds [23]. To our knowledge, similar experiments with TeCP have not been conducted. A number of reports have documented the presence of TeCP, along with other chlorinated phenolics, in industrially contaminated environments [5, 7, 9, 24-29], but the persistence and fate of this chemical has not been investigated in a replicated scientific manner.
A series of limnocorral studies was therefore initiated to address some of the unanswered questions on the persistence, dissipation pattern, and fate of TeCP and PCP in freshwater and sediment under environmental conditions that are representative of aquatic ecosystems in southern Ontario. These experiments were conducted to compare the persistence and fate of TeCP and PCP in an aquatic environment, to assess the effects of sunlight on degradation, and to evaluate the rate of dissipation of TeCP at different treatment concentrations. The effects of TeCP and PCP on the biota within these enclosures have been published elsewhere [30, 31].
| Variablea | Experiment 1 (Jun 4-Jul 30, 1987) | Experiment 2 (Aug 7-Oct 15, 1987) | Experiment 3 (Jun 16-Aug 25, 1988) |
|---|---|---|---|
| pH | 8.2 (7.9–8.4) | 8.1 (7.9–8.3) | 8.4 (8.2–8.6) |
| Ca2+ (mg/L) | 52.6 (40.8–60.5) | 52.5 (47.4–58.1) | 60.9 (47.6–67.3) |
| Temperature (°C) Surface | 25.0 (22.0–27.5) | 22.5b (17.5–25.5) | 25.0 (20.0–29.0) |
| 3.0 m depth | 22.0 (18.0–25.0) | 21.0 (17.0–23.5) | 21.5 (19.5–26.0) |
| DOc (mg/L) | 11.8 (9.8–13.8) | 8.5 (6.2–11.0) | 10.4 (8.9–11.3) |
| DIC (mg/L) | 35.5 (26.2–43.6) | 33.9 (28.1–37.6) | 36.6 (30.7–40.1) |
| DOC (mg/L) | 6.7 (5.9–7.5) | 7.3 (6.9–7.7) | 8.1 (6.6–9.4) |
| POC (mg/L) | 1.8 (0.6–2.8) | 1.3 (0.7–2.0) | 0.6 (0.4–0.7) |
| Secchi depthd (m) | 2.5 (2.0–3.4) | 3.0 (2.0–4.2) | 4.4+c |
- aDO = dissolved oxygen; DIC = dissolved inorganic carbon; DOC = dissolved organic carbon; POC = particulate organic carbon.
- bDay 63 posttreatment (Oct 15, 1987) not included.
- cMean integrated value from 0 to 3.0 m depth.
- dLow and high of range observed at start and end of all experiments, respectively.
- eBottom of enclosures (4.4 m) always visible.
MATERIALS AND METHODS
Study site
All experiments were conducted in Lake St. George, a 10.3-ha meso-eutrophic lake situated approx. 32 km north of Toronto, ON, Canada (43°57′30N, 79°25′45W). Lake St. George is a dimictic, clinograde kettle lake composed of two basins [32]. It has a maximum depth of 16 m, is ice-covered from December to April, and the bottom is overlaid with an unconsolidated organic layer that is up to several meters deep. The mean and range of various limnological parameters measured within control enclosures are presented in Table 1. Lake St. George had been used for similar enclosure studies in the past and already contained a set of 12 reusable limnocorrals. Enclosures were of two similar types, and details regarding their initial construction and installation were described previously [30, 33-35]. All enclosures were 5 × 5 m in surface area and located in approx. 4.5-m-deep water.
Before the initiation of each study, the inside of each limnocorral was lined with a new ultraviolet-protected, 6-mil clear polyethylene plastic liner. This gave each enclosure a clean and identical inside surface and circumvented problems associated with biota growing on the original polyvinyl chloride walls. A slack of about 30 cm was maintained in the walls and liner to accommodate wave action and seasonal changes in water level. The installation of the liner was aided and checked by scuba divers to ensure that a proper seal in the sediment had been obtained.
Experimental design
Three separate limnocorral experiments were conducted during the summers of 1987 and 1988. Experiment 1 was conducted from June 4 to July 30, 1987, and was designed to determine the persistence, dissipation, and mass balance of TeCP and PCP in water and sediment following a morning application with two concentrations of commercial TeCP. Experiment 2 was conducted from August 7 to October 15, 1987, and was aimed at determining the persistence and dissipation of TeCP and PCP in the entire water column and at various near-surface depths following an evening application with two concentrations of commercial TeCP. Both experiments used a randomized complete block design (RCBD) with three replicates of each treatment concentration. During the time between experiments 1 and 2, corral walls were raised for 4 d to allow for mixing of the corral and lake waters and to ensure that homogeneous, replicate enclosures would be present at the start of experiment 2. Experiment 3 was conducted from June 16 to August 25, 1988, and was designed to allow for a determination of the relationship between treatment concentration and rate of TeCP dissipation. Seven TeCP concentrations were tested in a completely randomized design (CRD) with only one enclosure per treatment.
Pesticide treatment
The pesticide applied was DIATOX® (Diachem Ltd., Richmond, BC, Canada), a commercial TeCP formulation that was commonly used in British Columbia for sapstain control. DIATOX was formulated to contain 24.2% active ingredient (a.i.) by weight with 19.4% as 2,3,4,6-TeCP and 4.8% as PCP. Analysis of the DIATOX formulation revealed only 18.1% total chlorophenol concentration by weight with 15.7% as TeCP and 2.4% as PCP. Both compounds were present as sodium phenates, and the remainder of the formulation was described as “unspecified buffers.”
Nominal treatment concentrations for experiments 1 and 2 were 0.075 and 0.75 mg a.i./L DIATOX and 0.75 and 1.50 mg a.i./L DIATOX, respectively. For experiment 3, concentrations of 0.1, 0.25, 0.5, 1.0, 2.0, 4.0, and 7.3 mg a.i./L DIATOX were chosen. The pesticide formulation was mixed with 1 L of lake water in a portable hand-pump sprayer equipped with a single jet nozzle and applied as a single uniform surface treatment. The concentrations chosen were those predicted necessary for observation of significant biological responses and were generally higher than those encountered in natural systems. The lower concentrations (0.075 to 0.5 mg/L) could be expected near lumber mills and storage yards following heavy rain events [6].
For experiment 1, DIATOX was applied on a sunny morning (June 17, 1987) between 10:25 and 11:45 AM. The experiment was terminated 43 d later, after which time the corral walls were lifted to renew the water. Mixing was aided by a portable gasoline-powered pump. Two days after mixing, the walls were once again lowered and experiment 2 initiated. For experiment 2, the pesticide was applied in the evening (after dusk) on August 13, 1987, between 7:30 and 8:45 PM. Sunrise occurred approx. 10 h later and was followed by a sunny day. Experiment 2 ran for 63 d. The walls of the enclosures were raised the following June (1988) to once again renew the water, and again a pump was used to enhance circulation. The old plastic liners were removed and new ones installed. DIATOX application for experiment 3 took place on June 30, 1988, between 8:45 and 10:15 PM. Sunrise on July 1 occurred at approx. 6:00 AM and was followed by a mostly sunny day.
Sodium chloride (254.3 g) was added to all enclosures immediately after collection of the day 1 and day 2 posttreatment samples for experiments 1 and 2, respectively. The intent was to raise the Na+ concentration by approx. 1.0 mg/L, as compared to the outside lake, to monitor for any leakage of the corrals. The corresponding increase in Cl− concentration, based on a corral volume of 100,000 L, would be approx. 1.54 mg/L.
Sampling procedure
Three types of samples were collected and analyzed for pesticide residues, water samples, sediment samples, and strips of plastic liner material (5 × 100 cm) suspended within the limnocorrals to estimate adsorption to the enclosure walls. Samples were collected immediately before treatment and at selected posttreatment times from day 1 to the end of the various experiments. All samples on any one day were collected within a 3-h period.
Five 1-L water samples were collected from each enclosure with a depth-integrating tube sampler [36] and pooled. A 500- to 1,000-ml subsample was transferred to a glass jar, approx. 1 ml of concentrated HCl was added, and the sample was stored in a dark, ice-packed cooler for transport to the laboratory. Another 1,000-ml subsample for water chemistry analysis was transferred to a Nalgene bottle and stored in another ice-packed cooler. Water chemistry samples were preserved upon return to the laboratory according to standardized methods [37]. Analysis for dissolved inorganic carbon (DIC), dissolved organic carbon (DOC), particulate organic carbon (POC), Na+, and Cl− was later performed by the Water Quality Laboratory at the Canadian Centre for Inland Waters in Burlington, Ontario.
For experiment 2, three additional 500-ml water samples were collected from each of three depths (5, 50, and 100 cm) and pooled by depth. Samples were collected on days 1 (AM and PM), 2, and 7 to investigate the relative rate of dissipation and possible significance of photodegradation, in the surface waters during this time period. Samples were collected by lowering a capped 500-ml Erlenmeyer flask attached to a marked pole to the desired depth and then pulling the stopper (with an attached string) to allow water to fill the flask.
For experiment 3, approx. 20 ml of the integrated water sample was immediately filtered through a Whatman GF/C glass microfiber filter (1.2 μm) into a separate glass jar for later determination of soluble versus adsorbed, or biologically incorporated, TeCP and PCP. All water samples were kept in ice-packed coolers and extracted within 24 h of collection.
Sediment samples were collected with a 5-cm-diameter core sampler [38] and sectioned into two subsamples (0–5 and 5–15) in the field using a specially constructed stainless-steel sectioning device attached to the top of the removable core tube of the sediment sampler. Two cores were taken from each corral at each sampling time, and the two replicate sections from each enclosure were pooled. Sediment samples were always collected after water samples to avoid contamination of the water by suspended material. All samples were brought back to the laboratory within 10 h and frozen at −17°C for later analysis. The water content was later determined by evaporation at 60°C, and the organic matter content was determined by loss on ignition after 1 h at 550°C.
One plastic strip (1,000 cm2), suspended from a wooden float within each enclosure, was removed at each selected sampling time. Strips were gently shaken to remove excess water, carefully rolled up, and wrapped in aluminum foil. All strips were stored at −17°C within 3 to 10 h of collection for later extraction and analysis.
Analytical techniques
Water samples. An aliquot of 1 to 10 ml of the water samples, depending on the sampling date, was transferred to a 250-ml Erlenmeyer flask containing 100 ml of 0.1 M K2CO3. Twenty milliliters of hexane was added, followed by 1.5 ml of double-distilled acetic anhydride. The flask was capped and the solution placed on an orbital shaker at 200 rpm for 1 h. Following this extraction, double distilled water was carefully added to raise the hexane layer to the neck of the flask. The hexane layer, containing the derivatized acetate ester residues of TeCP and PCP, was then carefully transferred with a Pasteur pipette to a funnel containing hexane-rinsed anhydrous Na2SO4 and collected in a 40-ml test tube. Additional double distilled water was added to the Erlenmeyer flask to further raise the water level, and approx. 2 to 4 ml of hexane was added. The hexane was again removed, dried through the same Na2SO4, pooled with the first hexane fraction, and the Na2SO4 rinsed with additional hexane. Approximately 3 to 4 ml of pesticidegrade isooctane (2,2,4-trimethylpentane) was added to the test tube as a “keeper,” and the volume was reduced to 2 to 3 ml under a gentle stream of nitrogen gas using an analytical evaporator operated at 40°C. The remaining isooctane-pesticide mixture was quantitatively transferred to a 5- or 10-ml volumetric flask and brought to volume with pesticide-grade isooctane. Samples were transferred to Teflon®-capped test tubes and stored in the dark at 4°C until analysis.
Sediment samples. Sediment samples were homogenized by vigorous shaking, and 10.0 g of wet sediment was transferred to a 250-ml centrifuge flask. Samples were acidified to pH <2 with concentrated HCl, 25 ml of dichloromethane (DCM): methanol (MeOH) (45:55 v/v) was added, and the samples were placed in an ultrasonic water bath at 40°C for 5 min. Flasks were then transferred to an orbital shaker operated at 200 rpm for 15 min and afterward centrifuged for 3 min at 2,500 rpm. The supernatant was carefully decanted and collected through a small glass wool plug in a 125-ml Erlenmeyer flask. The DCM MeOH extraction was repeated, and the combined supernatant was transferred to a 125-ml separatory funnel. The DCM MeOH phase was then washed twice for 2 min each time with 25 ml of distilled water acidified to pH <2 with concentrated HCl. The aqueous phase was discarded, and the DCM fraction was extracted three times with 33 ml of 0.1 M K2CO3 in the same separatory funnel. The DCM was collected in a centrifuge flask and spun for 3 min at 2,500 rpm (1,150 × g) to recover additional K2CO3 solution trapped in the DCM emulsion. The separated K2CO3 layer was then transferred with a Pasteur pipette to the separatory funnel containing the rest of the K2CO3 fraction. The K2CO3 was returned to a 125-ml separatory funnel and washed three times with 25 ml of hexane (2 min per partition). The hexane was discarded, and the K2CO3 was drained into a 250-ml Erlenmeyer flask. Twenty milliliters of hexane was added, followed by 2 ml of double-distilled acetic anhydride, and samples were derivatized and handled as described for the water samples. Final extracts were concentrated under a stream of nitrogen to a volume of 1.0 ml for analysis.
| Recovery efficiency (%)a (mean ± 1 SD) | Limit of quantificationb | Limit of detectionb | ||||
|---|---|---|---|---|---|---|
| Sample matrix | TeCPc | PCPc | TeCP | PCP | TeCP | PCP |
| Water (ng/L) | 90.4 ± 4.2 | 91.6 ± 3.9 | 200.0 | 40.0 | 50.0 | 10.0 |
| Sediment (ng/10 g) | 88.3 ± 4.2 | 76.6 ± 4.8 | 100.0 | 25.0 | 20.0 | 5.0 |
| Plastic (ng/strip) | 83.4 ± 4.0 | 84.2 ± 4.3 | 20.0 | 4.0 | 5.0 | 1.0 |
- aMean and SD are based on eight to 12 replicates.
- bMinimum attainable by chosen extraction procedures. These values could be lowered by concentrating the sample or extracting a larger sample volume.
- cTeCP = 2,3,4,6-tetrachlorophenol; PCP = pentachlorophenol.
Plastic strip samples. The previously frozen strips were thawed at 4°C overnight before extraction. The rolled-up strips were then removed from their aluminum foil wrappers and placed in 500-ml separatory funnels containing 100 ml of double-distilled water acidified to pH <2 with concentrated HCl. Twenty five milliliters of DCM was added, and the strips were unraveled in the separatory funnels by swirling and shaking. When unraveled, the strips were extracted by hand-shaking for 2 min. The extraction was repeated twice, each time with 25 ml of DCM. The combined DCM fractions were collected in another 500-ml separatory funnel and partitioned three times with 33 ml of 0.1 M K2CO3 by shaking for 2 min per partition. The DCM was discarded, and the K2CO3 fraction was returned to the same separatory funnel and cleaned by washing three times for 2 min each time with 25 ml of hexane. The K2CO3 fraction was transferred to a 250-ml Erlenmeyer flask and derivatized and stored as described for the water samples. Mass balance calculations were based on a liner surface area of 90 m2 per limnocorral.
Reference standards. All residue samples were analyzed as acetate esters of TeCP and PCP after acetylation with acetic anhydride as previously described. The 2,3,4,6-TeCP and PCP reference standards were synthesized separately according to the method of Chau and Coburn [39]. The PCP acetate ester was prepared using commercially available, 99% pure PCP (Aldrich Chemical Co., Milwaukee, WI, USA). High-purity 2,3,4,6-TeCP was not commercially available in sufficient quantities and was therefore prepared by steam distillation of DOWCIDE 6® (DOW Chemical Co., Midland, MI, USA). The purification procedure was adopted from Cserjesi [40] and Cserjesi and Johnson [41] with only minor modification. The identity and purity of the two acetate esters were confirmed by both gas chromatography and mass spectroscopy. A >99% and a 97% pure product was obtained for PCP and TeCP, respectively.
Chromatography technique. Aliquots of the processed residue samples were analyzed by capillary gas chromatography on a Perkin-Elmer Sigma 2000b gas chromatograph equipped with a 63Ni electron capture detector and an AS-100b autosampler. The chromatograph was coupled to a Spectra-Physics SP-4000 integrator. Separation and quantification of chlorophenols was obtained on a DB-1-30N column (30-m-long, Durabond-packed, open-bore, fused silica capillary column with a film thickness of 0.25μm; J&W Scientific [Folsom, CA, USA]). Temperature programming started with a 2-min initial hold at 90°C, then ramped to 250°C at 3.5°C/min, with a final hold of 5 min. The carrier gas was hydrogen. Recovery efficiencies, limits of quantification, and limits of detection are listed in Table 2.
Statistical analysis
The best least-squares regression line or polynomial was determined for both compounds at all treatment concentrations, and the significance of the quadratic component was computed. Individual quadratic regressions were compared to determine significant differences in the dissipation pattern of TeCP and PCP at the various treatment concentrations tested. All contrasts were conducted using a repeated-measures polynomial analysis. Within each experiment, all dissipation patterns were compared in a CRD. A CRD was chosen only after a separate analysis had indicated that there was no block effect. That is, the rate or pattern of dissipation was not dependent on which corrals contained which treatment. Comparisons of mean point estimates were accomplished by analysis of variance (ANOVA). All analyses were performed using the software SAS®, version 6.03 [42]. A significance level of 95% was chosen in all cases. First-order rate coefficients were calculated for both TeCP and PCP for each experiment using data from day 4 onward, thus excluding the initial mixing period (days 1–2).
RESULTS
Experiment 1: Morning application
Mean TeCP concentrations of 13.6 and 320.5 μg/L were detected in integrated water samples 24 h after application with nominal concentrations of 0.065 and 0.65 mg/L, respectively. Corresponding percent reductions in mean TeCP concentrations, relative to nominal concentrations, during the first 24 h were 79.1 and 50.7%, at the low and high treatments, respectively. Similar reductions were observed in PCP concentrations (Table 3). After the first 24 h, the percent reduction in concentration of both compounds in the water column slowed progressively (Table 3). All treatments followed similar dissipation patterns with little variation among replicate enclosures (Fig. 1a). A linear relationship for log(concentration) versus time was a good approximation of all dissipation patterns in experiment 1, but the r2 value after inclusion of a quadratic term was always better, being >0.990 in all cases except one (Table 3). In two instances, however, the quadratic component was not statistically significant at the 95% level, namely at 0.065 mg/L TeCP (p = 0.096) and at 0.1 mg/L PCP (p = 0.171). The least-squares linear regression line for these two treatments were log(Y) = 1.11 - (4.24 × 10−2X) (r2 = 0.955) and log(Y) = 1.58 - (6.04 × 10−2X) (r2 = 0.953), respectively. For ease of comparison, only the quadratic equations are presented in Table 3. No significant difference was observed between the dissipation patterns of TeCP and PCP at either of the two treatment concentrations; however, the dissipation patterns for both compounds differed significantly between the low and the high treatments. The greater percent reduction in both TeCP and PCP concentrations observed during the first 24 h in the low treatment enclosures resulted in flatter dissipation curves between days 1 and 15, causing dissipation patterns to be significantly different from those observed at the high treatment. The dissipation rates were similar for all four chlorophenol concentrations from day 15 onward. First-order rate coefficients for both compounds averaged -0.11/d. The estimated time for 50% dissipation (DT50) of TeCP and PCP from the integrated water column ranged from 0.4 to 1.1 d. No significant difference was observed between TeCP and PCP DT50 estimates at either of the two treatment levels (Table 3). Overall, 99.6 to 99.9% of nominal TeCP and PCP concentrations dissipated from the water of treated enclosures over the duration of the 43-d experiment.
| Estimated % reduction | |||||||
|---|---|---|---|---|---|---|---|
| Experiment | Nominal treatment concn.a | Regression equation | r2 | DT50b (d) | First 24 h | Next 7 dc | Last 7dd |
| 1 (morning application) | 0.065 mg/L TeCP | A log(Y) = 1.20 - (5.71 × 10−2X) + (3.38 × 10−4 X2) | 0.992 | 0.44 ± 0.03 | 79.1 | 58.2 | 38.7 |
| 0.010 mg/L PCP | A log(Y) = 0.48 - (5.58 × 10−2X) + (2.64 × 10−4 X2) | 0.995 | 0.55 ± 0.10 | 71.3 | 57.7 | 43.1 | |
| 0.65 mg/L TeCP | B log(Y) = 2.56 - (8.17 × 10−2X) + (7.35 × 10−4 X2) | 0.998 | 0.99 ± 0.11 | 50.7 | 70.2 | 31.7 | |
| 0.10 mg/L PCP | B log(Y) = 1.75 - (9.24 × 10−2X) + (7.36 × 10−4X2) | 0.969 | 1.08 ± 0.12 | 47.1 | 74.9 | 42.4 | |
| 2 (evening application) | 0.65 mg/L TeCP | C log(Y) = 2.73 - (6.29 × 10−2X) + (2.62 × 10−4X2) | 0.994 | 3.75 ± 0.32 | 20.4 | 62.5 | 39.1 |
| 0.10 mg/L PCP | C log(Y) = 2.00 - (7.58 × 10−2X) + (4.51 × 10−4X2) | 0.996 | 3.44 ± 0.55 | 8.0 | 68.7 | 29.2 | |
| 1.30 mg/L TeCP | C log(Y) = 3.06 - (5.99 × 10−2X) + (3.52 × 10−4X2) | 0.997 | 5.18 ± 0.35 | 28.2 | 60.1 | 24.4 | |
| 0.20 mg/L PCP | C log(Y) = 2.28 - (5.82 × 10−2X) + (2.31 × 10−4X2) | 0.997 | 4.77 ± 0.40 | 18.8 | 59.8 | 37.9 | |
- aTreatments within the same experiment followed by different letters exhibited a significantly different (p ⩽ 0.05) dissipation pattern. Between-experiment comparisons were not made.
- bDT50 = time for 50% dissipation (mean ± 1 SD) of applied chlorophenol concentration from water column. Estimates for experiment 1 are based on nominal treatment concentrations. Estimates for experiment 2 are based on initial concentrations analytically determined before sunrise on day 1 (10 h after application).
- cPercent reduction from time of 24-h samples to day 8.
- dDays 36 to 43 and 56 to 63 for experiments 1 and 2, respectively.
Experiment 2: Evening application
Water samples collected 10 h after application (before dawn on the morning of day 1) indicated that chlorophenol concentrations were only slightly lower than nominal values. Mean total chlorophenol concentrations of 0.71 mg/L (0.60 mg/L TeCP + 0.11 mg/L PCP) and 1.32 mg/L (1.13 mg/L TeCP + 0.19 mg/L PCP) were detected at the 0.75 mg/L (0.65 mg/L TeCP + 0.10 mg/L PCP) and 1.50 mg/L (1.30 mg/L TeCP + 0.20 mg/L PCP) DIATOX treatments, respectively. These concentrations decreased to 0.61 and 1.09 mg/L by 24 h postapplication. The rates of chlorophenol dissipation during the first 24 h after the evening application were significantly slower than those observed during the first 24 h after the morning application of experiment 1. For example, the nominal TeCP concentration of 0.65 mg/L decreased by 20.4% in experiment 2, compared to a decrease of 50.7% in enclosures that received that same nominal concentration in experiment 1 (Table 3). Similarly, the mean PCP level at the 0.10 mg/L concentration in experiment 2 decreased by only 8.0% over the first 24 h period, compared to a decrease of 47.1% at the same treatment concentration in experiment 1 (Table 3). The quadratic component of the regression equation describing TeCP or PCP dissipation was significant in all four cases, and there were no significant differences among any of the four patterns (Fig. 1b). Rate coefficients, based on first-order approximations, averaged -0.10 and -0.12/d for TeCP and PCP, respectively. The DT50s for TeCP and PCP from the water column ranged from 3.4 to 5.2 d. Observed concentrations at the end of the 63-d experiment were all <0.5% of those measured on day 1 AM. Overall, dissipation patterns were similar to those observed in experiment 1.
Experiment 3: Unreplicated evening application
Concentrations of TeCP in unfiltered water samples from experiment 3 (Fig. 1c) followed dissipation patterns similar to those observed in experiments 1 and 2. Concentrations decreased rapidly during the first 48 h at all test concentrations and then appeared to decrease at a slower rate. The dissipation rate at the 1.0-mg/L TeCP treatment from days 2 to 56 was significantly different (p < 0.05) from rates at the other six TeCP treatments. No other significant differences were observed among the rates or patterns of TeCP dissipation. Overall, the percent reduction in TeCP and PCP concentrations during the first 24 h averaged 17.9 and 19.7%, respectively; the DT50 for TeCP averaged 5.3 d. Water samples from the 0.25-mg/L enclosure were not collected after day 28 posttreatment because the corral liner detached from the bottom frame, thereby diluting the treated water with untreated water trapped between the liner and outer wall. Dissipation of PCP (data not shown) followed a trend similar to that of TeCP, with no differences in the rates of dissipation among the seven PCP concentrations. Average first-order rate coefficients for both PCP and TeCP were -0.07/d. Filtered water samples had significantly lower chlorophenol concentrations than their unfiltered counterparts. From 45 to 84% of the TeCP and 38 to 70% of the PCP measured in the day 1 AM and PM samples, respectively, were associated with particulate matter (>1.2 μm) in the water column removed by filtration. The percentage of sorbed TeCP and PCP decreased on subsequent sampling dates and maintained a mean ± 1 SD of 25 ± 14% for TeCP and 17 ± 16% for PCP for the remainder of the experiment. No correlation was apparent between percent sorbed chlorophenol and either POC or DOC concentrations. A laboratory study with spiked water samples indicated that residues did not adsorb to the filters.
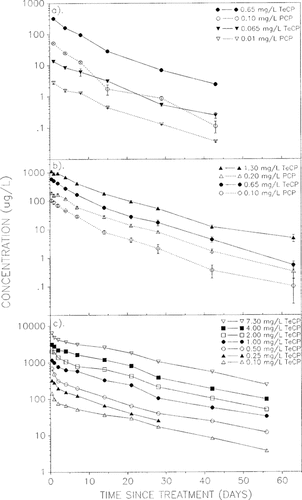
Dissipation of 2,3,4,6-tetrachlorophenol (TeCP) and pentachlorophenol (PCP) in integrated water samples from limnocorrals treated with DIATOX® (a) following a morning application (June 17, 1987), (b) following an evening application (August 13, 1987), and (c) following an evening application (June 30, 1988). Each point in a and b is the mean ± SE of three replicate enclosures. Points in c are single measurements from unreplicated enclosures.
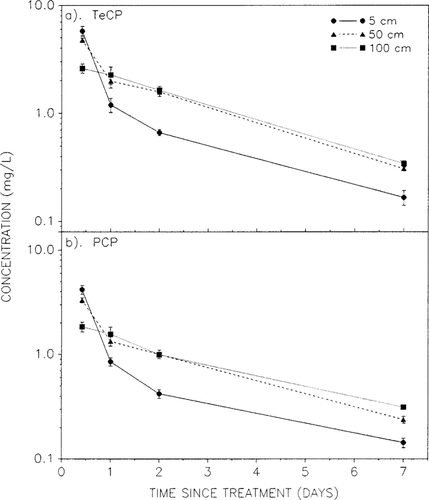
Dissipation of (a) 2,3,4,6-tetrachlorophenol (TeCP) and (b) pentachlorophenol (PCP) in water collected from 5-, 50-, and 100-cm depths of limnocorrals treated with 1.50 mg/L DIATOX® (1.30 mg/L TeCP + 0.20 mg/L PCP). Each point is the mean ± SE of three replicate enclosures.
Effect of water depth on rate of chlorophenol dissipation
Water samples collected from three depths within the enclosures treated with 1.5 mg/L DIATOX during the first week of experiment 2 showed that chlorophenol concentrations were generally lower in the surface layer (5 cm) than at depths of 50 and 100 cm. Only concentrations in the 10-h postapplication samples (day 1 AM) were highest at the 5 cm depth, suggesting that complete mixing had not yet occurred (Fig. 2a and b). Concentrations at the 5- and 50-cm depths decreased significantly during the 14-h daytime period on day 1; however, the contribution by photolysis is difficult to assess because of the confounding effect of mixing. No significant difference between residue levels in the 50- and 100-cm samples was observed after the first 24 h, suggesting a similar rate of pesticide dissipation. A comparison of dissipation rates between days 2 and 7 suggested that although concentrations in the 5-cm samples were significantly lower, the rates of dissipation were the same at all depths (Fig. 2a and b); mean rate coefficients were -0.30 and -0.25/d for TeCP and PCP, respectively. The total chlorophenol concentration in the surface layer had dropped by 96.9% on day 7 relative to the day 1 AM levels. Estimated DT50s for TeCP and PCP at the three sampled depths are presented in Table 4. Similar overall results were obtained for the enclosures treated with 0.75 mg/L DIATOX.
Mass balance of chlorophenols in limnocorrals
Mass balance calculations for the high DIATOX treatment of experiment 1 indicated that the majority of chlorophenol residues were present in the water column (Fig. 3). Residues on the plastic strips showed that <0.01% of the total chlorophenol mass applied was associated with the plastic liner. However, these adsorbed residues dissipated at a slower rate than residues in the water (Fig. 3). Chlorophenol levels associated with the liner were higher than what could be accounted for by water adhering to the strips. Sampled strips were determined to hold approx. 0.2 to 14.6 ml of water depending on the amount of periphyton growing on the strips. If all chlorophenol residues associated with the strips were actually found in the water adhering to the plastic, then the liner/water chlorophenol ratio, on a per volume basis, should be approx. 1:1. The observed ratio for TeCP was always significantly greater, ranging from 11:1 on day 1, then increasing with time to reach a maximum of 39:1 on day 29 postapplication. Similar trends were observed for PCP. This suggests that some TeCP and PCP adsorbed to the plastic or became incorporated into the periphytic layer that developed on the strips over time. A plot (not shown) of amount of TeCP on the liner versus amount in the water displayed a linear relationship from day 4 onward, implying that an adsorption equilibrium had been reached. The amount associated with the entire corral liner was always <0.05% of the total in the water on any sample date.
| Compound | Depth (cm) | Estimated DT50 (h) |
|---|---|---|
| TeCP | 5 | 8.8 ± 0.4 |
| 50 | 12.1 ± 1.6 | |
| 100 | 55.0 ± 3.1 | |
| PCP | 5 | 8.8 ± 0.1 |
| 50 | 12.0 ± 1.9 | |
| 10 | 58.2 ± 9.3 |
Analysis of the 0- to 5-cm sediment fractions also revealed very low chlorophenol concentrations (Fig. 3). Levels on day 1 postapplication were similar to those on the plastic liner, but sediment concentrations then increased slowly over the following 14 d and maintained relatively constant levels for the duration of the 43-d experiment. Only between 0.01 and 0.001% of the applied chlorophenol was associated with the bottom sediments during the first week after application. Levels of PCP were 1.5 to 5.8 times higher than levels of TeCP, indicating a higher degree of sorption to the sediments. Values ranged from 0.08 to 0.18 mg/m2 and from 0.19 to 0.80 mg/m2 for TeCP and PCP, respectively, based on a sample depth of 5 cm. Maximum wet sediment concentrations of 2.0 and 8.8 μg/kg were observed on day 15 postapplication for TeCP and PCP, respectively. Water concentrations on day 15 were 28.6 and 1.8 μg/L for TeCP and PCP, respectively. As a result of the slight accumulation and apparent lack of degradation, PCP sediment concentrations accounted for 55.9% of the total amount of PCP in the system at the end of the experiment. Levels of TeCP in sediment accounted for only 1.2% of the total as a result of the lower level of accumulation (Fig. 3). Sediment chlorophenol concentrations were apparently never in equilibrium with water concentrations. Samples from the 5-to 15- cm sediment fraction were not analyzed because they were predicted to have even lower chlorophenol concentrations than the 0- to 5-cm fraction, if any at all.
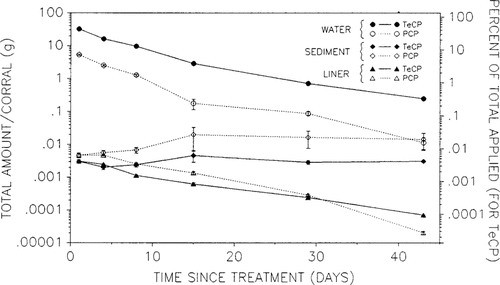
Fate of 2,3,4,6-tetrachlorophenol (TeCP) and pentachlorophenol (PCP) in water, sediment, and adsorbed to the plastic liner of limnocorrals treated with 0.75 mg/L DIATOX® (0.65 mg/L TeCP + 0.10 mg/L PCP). The left Y axis is the total amount (g) of either compound in the three phases. The right Y axis is the percent nominal amount of TeCP applied. Each point is the mean ± SE of three replicate enclosures.
Concentrations of Na+ and Cl− in limnocorrals
Concentrations of Na+ and Cl− (discussed here for experiment 2 only) decreased only slightly over the duration of the experiment and were always significantly higher within the limnocorrals than in the outside lake (Fig. 4a and b). Slightly higher Na+ and Cl− concentrations were observed in treated as opposed to control enclosures. The higher Na+ levels corresponded well with the contribution from the sodium tetrachlorophenate formulation applied (DIATOX). The decrease observed in Na+ concentration over time in control corrals suggested some Na+ loss or removal from the system. Approximately 50% of the applied Na+ disappeared from the water column of control corrals between days 4 and 63. This was significantly more than could be accounted for by diffusion into sediment pore waters or by change in limnocorral volume.
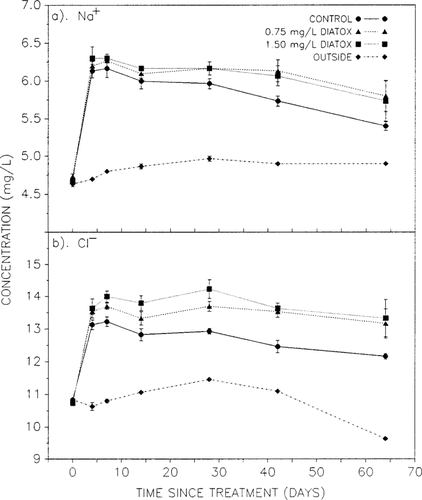
Sodium (Na+) and chloride (Cl−) ion concentrations in water from control and DIATOX®-treated limnocorrals and from outside lake sites from experiment 2. Each point is the mean ± SE of three replicate enclosures or lake sites.
Levels of Cl− showed an obvious concentration-response relationship that was highest in the 1.50-mg/L enclosures from days 4 to 28. The difference between Cl− concentrations in either of the two DIATOX treatments and the control on day 4 was greater than what could be accounted for solely by Cl− release from reductive dehalogenation of TeCP, PCP, and their various degradation intermediates. Therefore, some Cl− must have originated from unknown components of the DIATOX formulation (e.g., HCl, NaCl). The small increase in Cl− concentration in the enclosures treated with 1.50 mg/L DIATOX between days 4 and 7 should, however, be indicative of Cl− release from TeCP, PCP, and their degradation intermediates. Degradation of TeCP and PCP between days 4 and 7 was 0.112 and 0.017 mg/L, respectively, not enough to account for the observed increase in Cl− concentration, suggesting that Cl− release from degradation intermediates created between days 0 and 4 must have provided a significant contribution. To account for the observed increase, ∼40% of all Cl− introduced on day 0 in the form of TeCP and PCP must have been liberated.
Observations of chlorophenol degradation in limnocorrals
An obvious change in water color was observed within 2 h of application at the high DIATOX concentration in experiment 1. The water color changed from green to brown, whereas the color of control and low treatment corrals remained unchanged. Similar observations were made within 3 to 4 h after exposure to direct sunlight for both DIATOX concentrations in experiment 2 and for all concentrations in experiment 3 except 0.1 mg/L. The brown color remained obvious in all three experiments at concentrations ⩾0.75 mg/L DIATOX until the end of the respective studies. The intensity and persistence of the brown color corresponded to the magnitude of the applied DIATOX concentration.
DISCUSSION
Persistence in water
Results from all three experiments suggested that surface-applied TeCP and PCP dissipated rapidly from the water column of limnocorrals via a pseudo-first-order process best described by quadratic regressions. Both compounds behaved in a similar manner, with only minor differences in rates of dissipation. Reduction in residue concentration during the first 24 h after treatment with 0.75 mg/L DIATOX was greater following a morning than an evening application, implicating photolysis as a significant factor. Because the pesticide formulation was applied to the surface in both experiments, the ∼10 h of darkness before sunrise provided by the evening application resulted in an extended mixing period and therefore a smaller percentage of the applied chlorophenols in the surface waters. This should have reduced the amount of chlorophenols exposed to rapid photodegradation. Chlorophenol residues in water samples collected from 5-, 50-, and 100-cm depths during the first week of experiment 2 supported this hypothesis. Unpublished data obtained from tests using glass tubes containing 1 mg/L TeCP in distilled water and suspended at various depths within the limnocorrals also indicated that the rate of degradation was inversely proportional to depth.
Pignatello et al. [21] studied the degradation of PCP in artificial freshwater streams in Minnesota and similarly reported that photolysis of PCP was fastest at the water surface. Observed half-lives averaged over a 24-h period were 3.0, 4.2, 16.9, 27.5, and 43.1 h at depths of 0.5, 3.8, 7.2, 10.5, and 13.8 cm, respectively. Attenuation with depth was assumed to be due to the extinction of light by suspended particles and dissolved materials in the channel water. Our DT50 estimates of 8.8 h for both PCP and TeCP at a 5-cm depth correspond well with these findings.
Results obtained by Crossland and Wolff [23], who investigated the fate of PCP in 1-m-deep outdoor experimental ponds (pH 7.3–10.3), were similar to the results of aquatic PCP persistence reported here. Crossland and Wolff [23] reported that PCP dissipated rapidly from the water column with a mean half-life of 2.9 d and a range of 2.0 to 4.7 d. Our DT50 estimates for PCP in integrated water samples ranged from 0.6 to 4.8 d. Robinson-Wilson et al. [19] also found that PCP disappeared rapidly from experimental ponds; only slightly more than half the PCP remained in the water column 1 d after treatment with 1 mg/L. This corresponded well with our estimated PCP reductions of 38.7 and 52.9% for the low and high treatments during the first 24 h of experiment 1. A rapid dissipation pattern was also observed by Sugiura et al. [20], who investigated the fate of PCP in a 70-cm-deep outdoor experimental pond. Concentrations in the water dropped by more than two orders of magnitude within 30 d of treatment at an application rate of ∼46 μg/L. This was faster than was observed from our data, but their pond was only 70 cm deep, so photodegradation may have been more rapid.
A number of possible explanations exist for the degradation patterns of TeCP and PCP observed in this study. Degradation appeared to result from a pseudo-first-order process, presumably photochemical, with deviations most likely arising from changes in the amount of light absorbed by the chlorophenols. Initially, incomplete mixing resulted in an accelerated rate of loss from the surface waters. However, as day length and the angle of the sun above the horizon decreased during the experimental period (late summer-early fall), incident solar radiation in the corrals decreased. This could theoretically explain the progressive reduction in photolysis rates observed over the period of measurement.
The rates of photolysis also could have been affected by biological phenomena. The chlorophenol-induced decreases in zooplankton and phytoplankton that occurred shortly after application [31] could have resulted in an increase in penetration of sunlight and thus increased photolysis. As the plankton populations recovered from the chlorophenol application (days 14–28), light penetration would again decrease and thus photolysis rates would also decrease slightly. However, results obtained by Hwang et al. [43] suggested that, for surface waters, changes in summer irradiance affect photolysis rates more than attenuation by particulates. Day-to-day changes in the amount of incident solar radiation caused by cloud cover and haze, as well as short-term mixing kinetics and adsorption to particulate matter, could also have contributed to the observed degradation patterns.
Mass balance distribution
Results from our study indicated that the water was the most significant compartment of the enclosures with respect to both TeCP and PCP mass balance distribution. As predicted, partitioning to the sediment and plastic liner contributed very little to the mass balance of both compounds. Liner residues, however, decreased at a slightly slower rate than water residues, perhaps as a result of protection from photolysis or incorporation into the periphyton layer that developed on the plastic. Also, for the chlorophenols to have been either adsorbed or incorporated, it must be assumed that they were in the molecular form, not the ionic phenate form. This could explain the slower rate of disappearance of plastic strip residues because the rate of photolysis is higher for the phenate ion than for the nonionized molecule [43]. Yunker (as in Jones [44]), investigating the fate of PCP in experimental marine enclosures, similarly reported that PCP did not adsorb in significant amounts onto the enclosure walls or onto particulates that could have been removed from the system as settling material.
Whole-sediment TeCP concentrations in the limnocorrals could easily be accounted for by assuming that all residues were present in the water fraction of the 0- to 5-cm deep samples. This is not surprising considering that these sediments were 95.7% water (by weight) and because TeCP and PCP should primarily be present in the water-soluble phenate form at the observed pH of 8.2. Sediment PCP concentrations were, however, greater than those for TeCP, despite the water column ratio of TeCP:PCP being approx. 6.5:1. This suggests a preferential, but low, level of PCP adsorption to the solid sediment phase (2.6% organic matter). For example, on day 4 postapplication, PCP levels in sediments were 17.6 times higher than predicted from aqueous concentrations. This observation may also be partly explained by competition between sorbates. Jafver et al. [45] reported that, in a laboratory study, PCP affected the distribution ratio of TeCP on a lake sediment at pH 8.3. Electrostatic repulsion appeared to reduce the partitioning of TeCP in the presence of PCP. A similar phenomenon may have occurred in our limnocorrals. This explanation seems reasonable because the predicted sediment/water partition coefficient for PCP is only 1.4 times greater than that for TeCP.
Other research has likewise suggested that neither TeCP nor PCP accumulate to any significant extent in sediments of experimental systems. Lee et al. [46] investigated the fate of radioactive labeled PCP in enclosed marine ecosystems (MERL tanks, 1.8 m diameter × 5.5 m deep). A mass balance of PCP carried out at various times showed that >99% of the radioactivity remained in the water. Pignatello et al. [21] reported that the combined adsorption of PCP by sediment and uptake by biota in unacclimated water in artificial streams accounted for < 15%, in fact, probably <5%. Robinson-Wilson et al. [19] reported that approx. 90% of the PCP applied to experimental ponds was found in the water as compared to sediment and fish. Sugiura et al. [20] found a peak PCP sediment concentration of approx. 400 ng/cm2 (4 mg/m2) 2 d after application with 46 μg/L PCP to an experimental pond. This value was approx. five times higher than the highest concentration observed in our study; however, the pH of their water (not reported) may have been lower than the pH of ours, thereby increasing the potential for partitioning to the sediment. Residue levels in their sediment also decreased at a slower rate than in the water.
Residue levels in filtered and unfiltered water samples from experiment 3 revealed that a large percentage of the chlorophenol residues were associated with suspended particulate matter greater than 1.2 μm in size. This contradicts other published findings [42, 44]. The fact that no correlation was observed with POC suggested either that POC levels were high enough to prevent saturation by the chlorophenols or that the residues were associated with nonorganic matter. The close approximation of the observed chlorophenol degradation pattern in the unfiltered water samples to first-order kinetics would imply that residues removed by filtering were in equilibrium with residues in the water. This was not the case for the bottom sediments.
Although higher chlorinated phenols are reported to bioconcentrate [5, 28, 29, 47-49], all residues removed by filtering were not likely associated with living biological material such as phytoplankton and zooplankton. The high chlorophenol percentage removed by filtering would imply a bioconcentration factor (BCF) of approx. 10,000, whereas the above-mentioned studies have reported much lower BCF estimates for TeCP and PCP, mostly in the range of 50 to 1,000, for various aquatic species. Also, the highest percentages were observed 24 h after treatment. At a BCF of approx. 10,000, this would not be expected if bioconcentration or bioaccumulation was accountable for the entire removal. Whatever the reason, most residues removed by filtering were apparently still bioavailable because the impacts on zooplankton communities [30, 31] were in reasonable agreement with those predicted using results from laboratory experiments [50].
Transformation and degradation
The observed change in water color from green to dark brown within hours of treatment was indicative of phototrans-formation products formed in the surface waters. A number of researchers have shown that the absorption spectrum of an aqueous solution of PCP (as phenate ion) has a λmax at 318 to 320 nm [43,51, this study]. We also found the λmax for TeCP (as phenate ion) to be in the same region, which is within the spectrum of solar radiation. Enclosure experiments similar to the ones reported here were conducted concurrently with pure PCP [52]. Visual comparison of corrals treated with 1 and 2 mg/L PCP indicated that brown compounds formed in the pure PCP corrals as well, but not to the same intensity as in the DIATOX-treated corrals. This suggests similar phototransformation products for both TeCP and PCP, but with those of TeCP origin being either more abundant or absorbing light more intensely.
Although no other field studies have reported similar findings, a number of laboratory experiments suggest possible explanations. Wong and Crosby [53] reported that a colorless aqueous 100 mg/L PCP solution (pH 9) placed outdoors in sunlight soon turned violet and then faded through brown, red, and yellow to colorless again. Identification of the photolysis products suggested that the first step was a photonucleophilic substitution of hydroxide for chloride to provide three possible tetrachlorodiols. The tetrachlorodiols were then assumed to be air-oxidized to their corresponding quinones (red), followed by further displacement of chloride to form the purple hydroxytrichloroquinones and orange dichlorohydroxyquinones (chloranilic acids). Tetrachlorophenols and trichlorophenols were also thought to be formed early by photoreduction but would be expected to undergo similar photonucleophilic and photooxidation reactions.
Munakata and Kuwahara [54] also investigated photochemical degradation products of PCP but used an even more concentrated solution of 1 kg Na-PCP in 50 L of water. Analysis at the time of 50% reduction in PCP concentration revealed tetrachlororesorcinol as one of the transformation products. Other compounds included various dimeric benzoquinones, most likely the result of the extremely high concentration of the irradiated solution. Whether these compounds would form in detectable quantities at the chlorophenol concentrations used in our studies is questionable.
The color change observed in our limnocorrals was likely the result of phototransformation of PCP to tetrachlorodiols and corresponding quinones and of TeCP to trichlorodiols and their corresponding quinones. Tetrachlorophenol is also a known photodegradation product of PCP [8, 9, 53]. However, because we did not observe a slower rate of TeCP loss, the PCP to 2,3,4,6-TeCP yield is assumed to be either very small or counteracted by a more rapid rate of TeCP degradation. Because the applied TeCP:PCP ratio was approx. 6.5:1 and because the color change in corrals treated with pure PCP [52] was less intense, the majority of the color likely resulted from phototransformation of TeCP.
CONCLUSIONS
The results reported here suggest a number of common trends. Higher chlorinated phenols dissipate rapidly from the water column of treated systems at environmental pH levels of 7.0 to 10.0, with photolysis being the major driving factor. Adsorption to suspended particulates and sediments is dependent on the limnological characteristics of the system studied (e.g., pH), but the majority of the residues reside in the water column. Once associated with sediments, dissipation occurs at a slower rate than in the water. Even though microbial degradation is possible, it usually requires an adaptation period and is not significant in the early stages of degradation in a newly contaminated environment [21]. Its significance could potentially increase in systems undergoing chronic low-level exposure to chlorophenol contamination because a microbial population capable of degrading chlorophenols could develop. Also, microbial degradation appears to be confined to the sediments or sediment/water interface and is not significant within the water column, where the majority of the chlorophenol residues are found [55]. Other removal processes, such as evaporation and hydrolysis, do not appear to be significant under most natural conditions [11, 23, 44].
All available data suggest that the fate of both TeCP and PCP can be predicted with a reasonable level of accuracy as long as a number of important chemical and environmental parameters are known [11, 23]. Those of greatest importance appear to be solar irradiation (photon flux), quantum yield of TeCP and PCP, octanol/water partition coefficient, level of light attenuation by the water, pH, and depth of water or surface area/volume ratio of the system in question. The variation in estimates of persistence, half-lives, and fate by different researchers is indicative of the different experimental conditions used. Estimates would probably be more similar if degradation was measured against some function of solar irradiation (i.e., photon flux at various depths) rather than time. Unfortunately, very few experiments, including ours, have measured the photon flux on a daily or continuous basis. In any case, this research provided new information on the persistence and fate of TeCP in freshwater environments. The results indicate that TeCP and PCP have very similar fates, that both TeCP and PCP dissipate rapidly from the water column of treated systems, that photolysis is a significant removal process for both compounds, and that there is minimal difference in the rate and pattern of dissipation of either compound in the range of concentrations tested.
Acknowledgements
We thank J. Warner, J. Virtue, and F. Grangés for their assistance with the collection of field samples and D.R.S. Lean and K. Burnison for providing the analysis of water chemistry samples. Environment Canada and the World Wildlife Toxicology Fund provided financial support of this project.




