Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on development of anuran amphibians
Abstract
We exposed anuran eggs and tadpoles to vehicle control (0.7% acetone) or waterborne [3H]2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) for 24 h (American toad, 0.003-30 μg/L; leopard frog, 3 μg/L; green frog, 0.3-100 μg/L) and subsequently raised them in clean water. Neither American toads nor green frogs exhibited TCDD-related mortality, but leopard frogs showed significantly increased (10%) mortality over controls. Eggs and tadpoles eliminated TCDD relatively quickly compared with published data for other vertebrates, with t1/2 of 1 to 5 d (American toad), 2 to 7 d (leopard frog), and 4 to 6 d (green frog). Elimination rates were slowest for tadpoles fed nothing, fastest for those fed a low-fat diet, and intermediate for those fed a high-fat diet. Although not significant, American toads exposed to ⩾0.03 μg TCDD/L appeared to metamorphose earlier, and those exposed to higher TCDD treatments appeared to metamorphose at a larger body mass than controls. Comparisons of these results with studies of fish early life stages suggest that anuran eggs and tadpoles eliminate TCDD more rapidly and are 100- to 1,000-fold less sensitive to its deleterious effects during development. These differences may be related to differences in metabolic rate, patterns of lipid storage and utilization, and aryl hydrocarbon receptor binding and signal transduction.
INTRODUCTION
Many toxicants can affect amphibian survival, development, and reproduction [1]. Amphibians may be particularly sensitive to waterborne pollutants because their skin is highly permeable to water [2], in which many species spend a large part of their life cycle.
Planar chlorinated hydrocarbons (PCHs) such as polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzo-furans, and polychlorinated biphenyls (PCBs) are a family of compounds that may pose problems for amphibians. Planar chlorinated hydrocarbons are globally distributed, persist in the environment by resisting biological and chemical degradation, and readily bioaccumulate [3]. 2,3,7,8-Tetrachlorodi-benzo-p-dioxin (TCDD), the best studied and most potent PCH, serves as a prototype for studying toxic effects of related PCHs [4]. It and other PCDDs are trace contaminants released into the atmosphere from municipal waste incineration [5] and into the aquatic environment via wet and dry aerosol deposition [6]. 2,3,7,8-Tetrachlorodibenzo-p-dioxin also enters the aquatic environment directly from paper and pulp mill discharge [7], as well as from the production of chlorophenols, chloroorganic pesticides and leaching from hazardous waste landfills [8]. Accumulation of TCDD, particularly through the food web and contact with contaminated sediments, is evident for all aquatic species that have been examined [9]. For juvenile and adult fish, TCDD is highly toxic and can cause wasting, mortality, histopathologic effects (e.g., edema, hemorrhages, epithelial lesions), and reproductive toxicity [9, 10]. Furthermore, PCHs can be transferred from adult female fishes to their offspring, causing developmental toxicity [11, 12].
Fishes are extremely sensitive to TCDD when exposed during early development [10]. Lake trout (Salvelinus namay-cush), among the most sensitive species of those studied to date, had an LD50 of 65 ppt (pg TCDD/g egg) when eggs were exposed for 48 h starting 4 to 7 h after fertilization [13]. Mortality, associated with subcutaneous yolk sac edema, occurred primarily after hatching during sac fry development.
Although recent evidence links PCHs to decreases in survival and reproduction of fishes, birds, and mammals in nature [14-16], relatively little is known about the exposure and possible effects of PCHs to amphibians [17-20]. Bullfrogs (Ranacatesbeiana) collected near a site at which the chlorophenols 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) and 2,4-dichloro-phenoxyacetic acid (2,4-D) were previously produced had substantial body burdens of TCDD, particularly in the ovaries and fat [20], but the impact of this TCDD body burden was not known.
One study showed that frogs were sensitive to TCDD when exposed during early development. South African clawed frog (Xenopus laevis) tadpoles (total length, 27-37 mm; hind leg length, 1-2 mm) exposed to 0.5 and 1 ppb waterborne TCDD in a static renewal system with water changed every 8 d exhibited decreased total length and died by 23 and 16 d of exposure, respectively [19]. Xenopus laevis tadpoles exposed to 0.1 ppb waterborne TCDD for the first 30 d and then placed in clean water exhibited accelerated metamorphosis as well as higher mortality around the time of metamorphosis. Although the actual body burdens of TCDD in the tadpoles of this study were not determined, the data suggest that these tadpoles are responsive to TCDD-induced mortality and developmental toxicity but appear less sensitive than fish early life stages. In contrast, bullfrog tadpoles injected with up to 1 μg/g TCDD and adults injected with up to 0.5 μg/g TCDD showed no increase in mortality or histopathological changes compared to controls [17].
To assess the toxicity of TCDD on amphibian development, we exposed eggs within approx. 42 h of fertilization (prior to neurula stage) using a modified FETAX exposure regimen [21] to mimic maternal deposition of TCDD. To accurately relate the effects of TCDD on development to actual body burdens in the eggs and tadpoles, we determined the accumulation and elimination of TCDD during anuran early development. We studied the influence of diet on elimination and also assessed the effects of TCDD on mortality, deformities, pigmentation, growth, swimming, and metamorphosis.
MATERIALS AND METHODS
Chemicals
We obtained [1,6-3H]TCDD from Chemsyn Science (Le-nexa, KS, USA) (specific activity, 37.7 Ci/mmol, American toad [Bufo americanus] eggs; 34.7 Ci/mmol, American toad tadpoles, leopard frog [R. pipiens], green frog [R. clamitans]). The [3H]TCDD used for the first American toad study was purified to >99% by reverse-phase high-performance liquid chromatography (HPLC) [22], whereas [3H]TCDD in the other experiments was determined to be 98% pure by Chemsyn Science. We used [3H]H20 (25 mCi/g; Bufo) and [3H]toluene (1.12 μCi × 10; Rana) to label samples used for quench curves. Acetone used as a carrier solvent was 99.9%+ pure, HPLC grade (Sigma Chemical Co., St. Louis, MO, USA). We purchased Soluene-350 tissue solubilizer and Hionic-Fluor liquid scintillation cocktail from Packard Instrument Company (Mer-iden, CT, USA). For lipid analyses, we used hexane (pesticide grade, Fisher Scientific, Fair Lawn, NJ, USA), isopropanol (99%, Fisher Scientific, Fair Lawn, NJ, USA), and Na2SO4 (Mallinckrodt, Chesterfield, MO, USA).
Experimental design
We performed all experiments at the Water Science and Engineering Laboratory at the University of Wisconsin-Madison (Madison, WI, USA). We exposed American toad, leopard frog, and green frog eggs and/or tadpoles statically to 10 ml of water containing 0.7% acetone (control) or to 0.7% acetone and [3H]TCDD in 60 15-mm glass petri dishes for 24 h in a dark incubator (temperature: American toad, 20-23°C; leopard frog, 24-25.5°C; green frog, 23-23.4°C). Following a 24-h exposure, we maintained eggs and tadpoles in 9.5-L tanks supplied with flow-through (approx. three replacements per day), TCDD-free, charcoal-filtered, dechlorinated city water (dissolved oxygen, 11.5 ppm; pH, 7.7; hardness, 324 mg/L as CaCO3). We set the photoperiod on a 14:10 lightdark schedule. Water temperature (American toad, 20-23.2°C; leopard frog, 20-23.6°C; green frog, 19.7-25°C) did not differ significantly among treatments or among replicates within treatments in any of the experiments except for a replicate-within-treatment difference in the leopard frog experiment (replicate F16.428 = 2.51, p = 0.001). Effluent from tanks was pumped through cotton and charcoal filters to remove [3H]TCDD [23, 24].
We fed tadpoles boiled romaine lettuce blended into a puree and a 3:1 Rabbit Chow® (Amazon Smythe, Chilton, WI, USA): TetraMin® mix (Amazon Smythe Superior Nutrition Rabbit Food, Chilton, WI, USA; TetraMin Flake Food, TetraSales, Blacksburg, VA, USA) every 2 d. We measured food quantity based on 15% of the approximate tadpole body mass in the tanks [25] and provided by weight 90% lettuce and 10% mix. Aquaria were cleaned of feces and uneaten food prior to feeding.
We collected tadpoles to determine TCDD content over the course of development and monitored others for mortality, deformities, pigmentation (evaluated qualitatively by visual inspection to be light/white or dark/black), body size (total length or body mass), and time to metamorphosis. We also measured tadpole swimming speed [for details, see 26], which involved chasing tadpoles at apparent maximum speed back and forth with a paintbrush in a 25-cm-long static water channel. Swimming was videotaped alongside a stopwatch, and the time required to swim each 1 cm in the middle 10 cm of the channel was determined from the recording using slow-motion analysis.
American toad egg experiment
We collected a single American toad egg mass from Nevin Fish Hatchery (Madison, WI, USA) at 1000 h on May 17, 1994. The clutch was probably laid the day before. By 1600 h, we placed eggs (beginning of neurula stage; stage 13 [27]) into treatments for 24 h, until just prior to hatching (stage 16).
We exposed American toad eggs to control (carrier only) or one of five doses of [3H]TCDD (plus carrier): 0.003, 0.03, 0.3, 3, and 30 μg/L, with 25 eggs in each of seven glass petri dishes per treatment group. After exposure, we used one petri dish of eggs to determine TCDD content and divided the remaining six dishes into three replicates (two petri dishes of eggs were placed into each tank for a total of 50 eggs per tank). We sampled toad eggs and tadpoles five times after exposure to determine TCDD concentrations: day 0 (n = 25 eggs per tank), day 6 (n = 5 tadpoles per tank), day 13 (n = 4), day 19 (n = 3), and day 26 (n = 3). We raised remaining tadpoles until metamorphosis (tail ⩽ 2 mm; day 31-67), and the experiment ended when the last tadpole metamorphosed (July 22).
American toad tadpole experiment
We collected American toad tadpoles (stage 25 [27], free-swimming and -feeding, operculum covering gills; eggs laid approx. 1 week before) from a wetland near Industrial Drive, Madison, Wisconsin, USA, on May 24, 1995. We exposed the tadpoles the following day to control (three replicates) or 0.3 μg [3H]TCDD/L (four replicates) with 30 tadpoles per replicate for 24 h. During exposure, we fed controls and two TCDD-treated replicates lettuce, while the other two TCDD-treated replicates were not fed to determine whether feeding influenced TCDD absorption. After exposure, tadpoles fed lettuce during the exposure were switched to a lettuce plus Rabbit Chow/TetraMin mix, while those tadpoles that were not fed during the exposure were now fed lettuce. During exposure, three tadpoles from each [3H]TCDD-treated replicate were collected at 2, 4, 6, 8, 12, 16, 20, and 24 h. Following exposure, we weighed and sampled tadpoles at 0.5, 1, 2, 3, 4, 5, and 6 d to determine TCDD elimination, and tadpoles were measured on day 6 for total length and swimming speed. We observed remaining tadpoles until day 7.
Leopard frog experiment
Leopard frogs caught in Wisconsin were induced to breed at Nasco (Fort Atkinson, WI, USA). One clutch of eggs artificially fertilized at 0900 on April 10, 1995, was transported to Madison. By 1800, we randomly sorted eggs into groups of 60 eggs per petri dish and exposed them to control or 3 μg [3H]TCDD/L for 24 h from approximately the 16- to 32-cell stage to late gastrula. After exposure, we divided the TCDD group into three dietary regimes, no food, low fat (lettuce, 0.36% lipid), and high fat (lettuce plus the 3:1 Rabbit Chow: TetraMin mixture, 7.95% lipid), to determine whether diet influenced elimination rate. We fed controls the high-fat diet. Feeding began at 4.5 d postexposure. We replicated each treatment five times for a total of 20 tanks.
We collected embryos with jelly capsules on days 0, 0.5, 1, 1.5, 2, and 2.5 and collected tadpoles at 3, 3.5, 4, 5, 6, 6.5, 7, 7.5, 8, 9, 10, 13, 18, and 24 d postexposure (day 0-0.5, n = 15 per tank; day 1-13, 5 per tank; day 18, 2 per tank; day 24, 1 per tank) for determination of the [3H]TCDD concentration. At 2 d postexposure, we separated 15 embryos from their jelly capsules from each of 10 [3H]TCDD-treated tanks and analyzed them separately for [3H]TCDD. We collected feces at 19 and 22 d postexposure from two low-fat and two high-fat diet tanks. We monitored tadpoles until 28 d postexposure (prior to metamorphosis) for mortality, deformities, and pigmentation, and total length (n = 6 per tank) was measured at 10 and 18 d postexposure.
Green frog experiment
We collected green frog eggs (one clutch) on June 6, 1995, from the Cofrin Arboretum Prairie Pond on the University of Wisconsin-Green Bay campus (Green Bay, WI, USA). We exposed eggs to control, 0.3, 3, 10, 30, and 100μg [3H]TCDD/L (three replicates per treatment) from the neural plate stage (approx. 1.5 d after laying; stage 13) to the tail bud/muscular response stages (stages 17 and 18). Because [3H]TCDD is more expensive than unlabeled TCDD, the 0.3-,3-,10-, and 30-μg/L treatments used 10% [3H]TCDD and 90% unlabeled TCDD, and the 100-μg/L treatment used 0.15% [3H]TCDD and 99% unlabeled TCDD.
We analyzed eggs from the 0-, 0.3-, and 30-μg [3H]TCDD/L treatments for [3H]TCDD at 0.25, 0.50, 0.75, 1, 2, 9, 18, and 24 h during exposure. We collected eggs and tadpoles from all treatments at 0, 1, 2, 3, 4, 6, 8, 11, 13, 15, 18, 20, 25, and 32 d postexposure to determine elimination rates and collected feces from tanks at 35 d postexposure. We measured the total length of green frog tadpoles on days 31, 41, and 48 and their swimming speed on day 41.
Determination of [3H]TCDD concentrations
We collected samples of eggs and tadpoles over the course of development as described for each experiment to determine [3H]TCDD content per individual egg or tadpole and to calculate uptake during and elimination following exposure to TCDD. We euthanatized tadpoles with MS-222 (0.05% solution), weighed them, and placed them in covered vials with 2 ml of Soluene-350 in a 50°C drying oven overnight. After the digested samples were cooled, we added 10 ml of Hionic-Fluor and placed vials in a cold room for 2 d to reduce che-miluminescence prior to liquid scintillation counting (Packard Tri-Carb 1900 TR, Packard Instrument Co., Downers Grove, IL, USA). Using appropriate quench corrections, disintegrations per minute were calculated from counts per minute and counting efficiency.
Lipid analyses
We analyzed American toad tadpoles, leopard frog eggs and tadpoles, and green frog eggs for the percentage of total lipid (dry mass basis) using a U.S. Environmental Protection Agency method (B. Butterworth, personal communication, [28], adapted from Radin [29]) designed for lipid analyses of TCDD-treated brook trout (S. fontinalis). We homogenized tissue lipids in a 3:2 hexane:isopropanol solution, centrifuged them to separate the lipid-containing supernatant, and then washed them with 40°C 0.47 M Na2SO4 to remove isopropanol and any remaining water. Duplicate aliquots of the hexane layer were dried and weighed for lipid content. The percent lipid value was based on dry tissue mass, sample volume, and the volume of the hexane aliquot.
Statistical analyses
We analyzed egg and total cumulative (egg and tadpole) mortality, tadpole deformity, and [3H]TCDD concentrations in embryos versus jelly capsules using Kruskal-Wallis tests or t tests. When correlating mean body mass or time to metamorphosis (one mean value per tank) with treatment or number of metamorphs in a tank, we used Spearman rank correlations. We analyzed elimination rates for each treatment using analysis of variance (ANOVA) regression models, with picograms of [3H]TCDD per egg or tadpole analyzed over time. The half-life (t1/2) for whole-body elimination of [3H]TCDD was calculated assuming first-order elimination as t1/2 = In 2/k, where k is minus the slope of the least squares regression line of In [3H]TCDD content of eggs and/or tadpoles on time [19]. Wash-off effects from eggs (noted only in the green frog experiment, in which there was a 75% average drop in TCDD concentration within 24 h postexposure, considered washoff from the egg outer capsule), values below detection limits (three times background levels, <150 cpm), and TCDD doses with fewer than three time points for elimination were not used for t1/2 calculations. For the leopard frog, we had sufficient data to discriminate between eggs and tadpoles for elimination rates; all other t1/2 values are based on combined egg and tadpole data for a given experiment. Total TCDD content was used, rather than mass-specific amounts, because growth of tadpoles results in whole-body dilution of the TCDD concentration. To test the influence of diet on elimination, we calculated elimination slopes for each tank within a diet treatment and used Kruskal-Wallis tests to determine differences among treatments in elimination slopes. All other dependent variables (e.g., total length, time to metamorphosis, swimming speed) were analyzed using nested ANOVAs (individuals nested within tanks, replicate tanks nested within treatments). Replicate-within-treatment effects are reported only when significant. Covariates such as tadpole total length were incorporated where appropriate for nested analyses of covariance ANCOVAs. Data are reported as means ± SE. All statistical analyses were performed using SPSS/PC+, version 5.0 [30].
RESULTS
Mortality, deformities, and pigmentation
Neither American toads nor green frogs exhibited TCDD-related cumulative mortality compared to controls. American toads showed little mortality when exposed as tadpoles (one in the control group and one in the TCDD high-fat group died) and higher mortality when exposed as eggs (19, 10, 17, 11, 15, and 17% mortality in control, 0.003, 0.03, 0.3, 3, and 30 μg/L TCDD treatments, respectively). Green frogs also did not differ in mortality among treatments (11, 2, 2, 21, 6, and 5% mortality in control, 0.3, 3, 10, 30, and 100 μg/L TCDD treatments, respectively).
In contrast, leopard frogs exposed to 3 μg TCDD/L as eggs showed a significant increase in mortality (arcsine transformed) prior to feeding (<1.5 d posthatch) relative to controls (controls, 31 ± 4.3%; TCDD, 42 ± 2.4%; t test, t18 = −2.32,p = 0.032). Although water temperature differed among replicates in the leopard frog experiment, we found no correlation between mortality and water temperature (r18 = 0.07,p > 0.5). Leopard frog postfeeding mortality did not differ between the control and TCDD treatments (t18 = −0.15, p = 0.88).
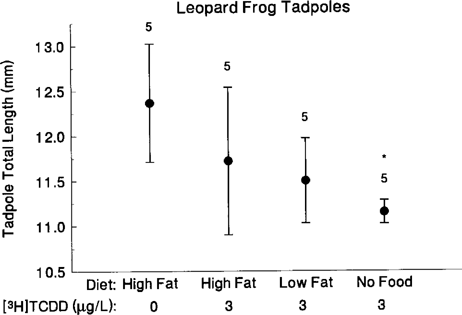
Total lengths (mm; mean ± SE) for leopard frog tadpoles 10 d after exposure to 0 (controls fed a high-fat diet) or 3 μg/L [3H]TCDD (tadpoles fed either no food, low-fat diet, or high-fat diet) (treatment: F = 3.2, d.f. = 3, 16, p = 0.051; replicate: F = 2.43, d.f. = 16,99, p = 0.004). Asterisks indicate significant differences from controls (using sequential Bonferroni corrections applied to two-tailed t tests [59]).
Cumulative deformities (e.g., bent tails, edema) did not differ significantly among treatments for any species (American toad: χ = 3.43, p = 0.63; leopard frog:t18 = −0.44, p = 0.67; green frog: χ
= 3.43, p = 0.63; leopard frog:t18 = −0.44, p = 0.67; green frog: χ = 5.58, p = 0.35). However, both leopard and green frog tadpoles were significantly lighter in pigmentation following exposure to ⩾3 μg TCDD/L (Kruskal-Wallis test; leopard frog: χ
= 5.58, p = 0.35). However, both leopard and green frog tadpoles were significantly lighter in pigmentation following exposure to ⩾3 μg TCDD/L (Kruskal-Wallis test; leopard frog: χ = 4.5, p = 0.034; green frog, χ
= 4.5, p = 0.034; green frog, χ = 11.8, p = 0.037).
= 11.8, p = 0.037).
Tadpole total length
American toad. American toads exposed to TCDD as tadpoles and fed a low-fat diet were significantly shorter (nested ANOVA, F2,4 = 15.3, p = 0.013) than TCDD-exposed or control tadpoles fed a high-fat diet (control, 12.2 ± 0.18 mm; TCDD low-fat diet, 10.7 ± 0.19, TCDD high-fat diet, 12.3 ± 0.18).
Leopard frog. At 10 d postexposure tadpoles exposed as eggs to 3 μg TCDD/L and fed nothing were significantly shorter than controls that were fed a high-fat diet (nested ANOVA; treatment: F3,16 = 3.21, p = 0.05; replicate: F16,99 = 2.43, p = 0.004) (Fig. 1). We found a negative correlation in tadpole length among treatments, with tadpoles becoming increasingly shorter in the following order: control, TCDD high-fat diet, TCDD low-fat diet, TCDD no food (using one mean total length value per tank, Spearman rank correlation, rs18 = −0.539, p = 0.014). By 18 d postexposure, TCDD-exposed tadpoles fed low- and high-fat diets did not differ significantly in total length (nested ANOVA, F1,5 = 0.27, p = 0.624).
Green frog. Tadpole total length differed significantly among treatments on day 31 (nested ANOVA, F5,18 = 3.35, p = 0.026) (Fig. 2) and day 48 (F5,16 = 3.68, p = 0.021) but not on day 41 (F5,12 = 2.64, p = 0.078). On day 31, tadpoles exposed to 10 μg TCDD/L were significantly shorter than control tadpoles. We found a negative correlation between tadpole length and TCDD dose, with tadpoles becoming increasingly shorter with increasing TCDD dose (using one mean total length value per tank, Spearman rank correlation, rs22 = −0.644, p = 0.001). On day 48, tadpoles in the 0.3- and 30-μg TCDD/L treatment groups were longer than control tadpoles. By day 48, treatments with longer tadpoles had fewer tadpoles in tanks, and the number of tadpoles in a tank differed significantly among treatments (Kruskal-Wallis, χ = 17.7, p = 0.003).
= 17.7, p = 0.003).
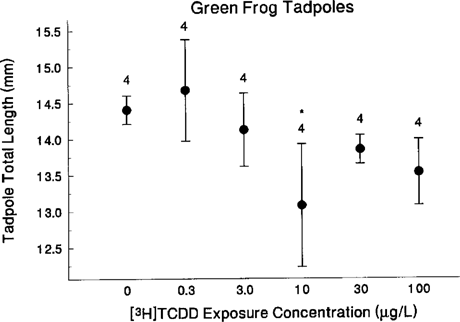
Total lengths (mm; mean ± SE) for green frog tadpoles 31 d after exposure to control or [3H]TCDD (treatment: F = 3.4, d.f. = 5,18, p = 0.026). Asterisks indicate significant differences from controls (using sequential Bonferroni corrections applied to two-tailed t tests [59]).
Swimming speed
American toad. Tadpoles exposed to 0.3 μg TCDD/L and fed a high- or low-fat diet did not differ in swimming speed from controls fed a high-fat diet. After accounting for tadpole length as a covariate (nested ANCOVA, F1,59 = 6.87, p = 0.011; r = 0.42, n = 67, p < 0.001), swimming speed again did not differ among treatments but differed significantly among replicates within treatments (F4,59 = 4.1, p = 0.005).
Green frog. Swimming speed did not differ significantly among treatments before or after accounting for tadpole total length as a significant covariate (nested ANCOVA, F1,161 = 30.3, p < 0.001).
Swimming speed for individual American toad and green frog tadpoles was highly repeatable, indicated by a high correlation between the fastest and second fastest swimming speeds (toad: r = 0.91, n = 67, p < 0.001; green frog: r = 0.72, n = 180, p < 0.001). Water temperature in the swimming channel during each individual's swim was not a significant covariate for either species. The water temperature during swimming trials ranged from 20.8 to 22°C (mean ± SE = 21.4 ± 0.38°C) for the toad and from 21.6 to 23.2°C (22.3 ± 1.67°C) for the green frog.
Metamorphosis
American toads exposed as eggs to ⩾0.03 μg TCDD/L appeared to metamorphose earlier than controls (nested ANOVA; treatment: F5,12 = 1.13, p = 0.396; replicate: F12,459 = 6.42, p < 0.001)(Fig. 3a), and those from some of the TCDD treatments appeared to metamorphose at a larger body mass than controls (treatment: F5,12 = 1.94, p = 0.16; replicate: F12,418 = 1.6, p = 0.093) (Fig. 3b). However, treatments did not differ statistically. Time to metamorphosis showed a negative but nonsignificant correlation with increasing TCDD dose (Spearman rank correlation, rs16 = −0.373, p = 0.127), and body mass at metamorphosis showed an almost significant positive correlation with increasing TCDD dose (Spearman rank correlation, rs16 = 0.417, p = 0.085). Because density could influence metamorphosis parameters, we correlated the number of metamorphs from each tank with mean values of time to and body mass at metamorphosis for each tank. We found almost significant negative correlations between the number of metamorphs from each tank and time to metamorphosis (Spearman rank correlation, rs16 = −0.440, p = 0.068) and body mass at metamorphosis (rs16 = −0.446, p = 0.063).
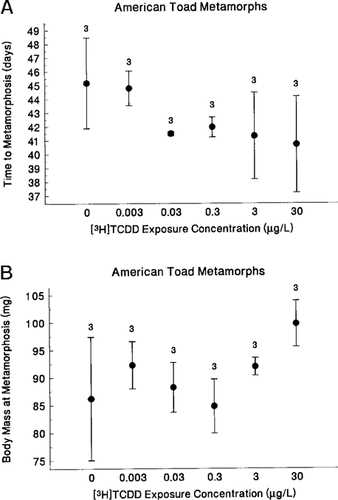
Time to metamorphosis (A) (log-transformed; treatment: F = 1.0, d.f. = 5,12, p = 0.46; replicate: F = 7.1, d.f. = 12,459, p < 0.001) and body mass at metamorphosis (B) (log-transformed; treatment: F = 2.1, d.f. = 5,12, p = 0.10; replicate: F = 2.1, d.f. = 12,418, p = 0.016) did not differ significantly among treatments but differed significantly among replicates within treatments. Data are presented as mean ± SE.
Absorption of [3H]TCDD
Following exposure to waterborne TCDD, only 3.7% (leopard frog) and 1.2 to 1.8% (green frog) of the TCDD was found in the jelly capsule relative to the embryo (Table 1). Thus, most of the TCDD passed through the jelly capsule and was found in the embryo (t tests; leopard frog: t18 = −9.5, p < 0.001; green frog: 0.3 μg/L TCDD: t4 = −5, p = 0.007; green frog, 30 μg/L TCDD: t4 = −3.4, p = 0.027).
| Species | Exposure water (μg [3H]TCDD/ L) | Jelly capsule (pgTCDD/g) | Embryo (pgTCDD/g) | % Jelly capsule/ embryo |
|---|---|---|---|---|
| Leopard frog | 3.0 | 171 ± 13.7 | 4,665 ± 471.9 | 3.7 |
| Green frog | 0.3 | 7 ± 0.2 | 378 ± 74.1 | 1.8 |
| 30.0 | 303 ± 101.9 | 25,298 ± 7,295.5 | 1.2 |
Bioconcentration factors (BCFs), the concentration in the organism divided by the concentration in water, for eggs and tadpoles after a 24-h exposure to TCDD are shown in Table 2. American toad, leopard frog, and green frog eggs accumulated a range of 1 to 4, 4 to 7, and 1 to 3 times the nominal TCDD water concentration, respectively. American toad tadpoles showed a BCF of 17 to 20 when exposed without lettuce and a BCF of 8 to 10 when exposed with lettuce.
Absorption rate was calculated during exposure for American toad tadpoles and green frog eggs. For the toad tadpoles, TCDD absorption was maximal at the first sampling time point (2 h) after initiation of exposure (Fig. 4). The tadpoles exposed without food showed a plateau in TCDD concentration, while tadpoles exposed with food exhibited a significant decline in TCDD content during the 24-h exposure (t1/2 = 3.9 d; r = 0.36, n = 32, p = 0.041). In addition, tadpoles exposed without food absorbed approximately two times more TCDD than those exposed with food.
The TCDD content in green frog eggs exposed to 30 μg TCDD/L revealed biphasic absorption curves, with linear accumulation up to 2 h during exposure and a plateau between 9 and 24 h (Fig. 5). Eggs exposed to 0.3 μg TCDD/L also exhibited a biphasic absorption curve (data not shown).
Elimination of [3H]TCDD: Effect of diet and lipid levels
Elimination of TCDD from eggs and tadpoles was relatively rapid and varied with diet but was not related to percent egg or tadpole lipid. The elimination half-life (t1/2 of American toads, leopard frogs, and green frogs exposed as eggs ranged from 3 to 5, 2 to 7, and 4 to 6 d, respectively (Table 2). American toads eliminated TCDD more rapidly when exposed as tadpoles (Fig. 6a) than when exposed as eggs (Fig. 6b), with a mean t1/2 of 1.4 and 4 d, respectively (Table 2). Within species t1/2 did not vary in a dose-dependent manner. Excretion via feces was one route of elimination, because TCDD in the range of 2 to 6 ppt was detected in feces collected from leopard and green frog tadpoles.
American toad tadpoles fed low- and high-fat diets did not differ significantly in TCDD elimination slopes (Kruskal-Wal-lis test, χ = 2.4, p = 0.121), although tadpoles on the low-fat diet appeared to eliminate TCDD faster than those on the high-fat diet (Fig. 6a).
= 2.4, p = 0.121), although tadpoles on the low-fat diet appeared to eliminate TCDD faster than those on the high-fat diet (Fig. 6a).
Leopard frog tadpoles fed three diets between 5 and 10 d postexposure differed significantly in TCDD elimination slopes (Kruskal-Wallis, χ = 9.06, p = 0.011). The TCDD elimination rates were slowest for tadpoles fed nothing, intermediate for those fed a high-fat diet, and fastest for those fed a low-fat diet (Fig. 7). The t1/2 values for the diet treatments between 5 and 10 d postexposure were as follows: no food, 7.3 d (95% confidence limit, 5.84-8.81); high-fat diet, 5.7 d (4.06-7.30); and low-fat diet, 3.5 d (3.16-3.90). Tadpoles from the no food treatment were collected at 10 d postexposure for lipid analysis. From 5 to 18 d postexposure, the low-fat (t1/2 = 2.3 d [95% confidence limit, 1.93-2.69 ± 0.38]) and high-fat (t1/2 = 3.3 d [1.87-4.70 ± 1.42]) diet tadpoles did not differ in elimination (Kruskal-Wallis, χ
= 9.06, p = 0.011). The TCDD elimination rates were slowest for tadpoles fed nothing, intermediate for those fed a high-fat diet, and fastest for those fed a low-fat diet (Fig. 7). The t1/2 values for the diet treatments between 5 and 10 d postexposure were as follows: no food, 7.3 d (95% confidence limit, 5.84-8.81); high-fat diet, 5.7 d (4.06-7.30); and low-fat diet, 3.5 d (3.16-3.90). Tadpoles from the no food treatment were collected at 10 d postexposure for lipid analysis. From 5 to 18 d postexposure, the low-fat (t1/2 = 2.3 d [95% confidence limit, 1.93-2.69 ± 0.38]) and high-fat (t1/2 = 3.3 d [1.87-4.70 ± 1.42]) diet tadpoles did not differ in elimination (Kruskal-Wallis, χ = 1.13, p = 0.29).
= 1.13, p = 0.29).
| [3H]TCDD concn. | ||||
|---|---|---|---|---|
| Species, stage exposed | Exposure watera (μg [3H]TCDD/L) | Egg or tadpoleb (pg [3H]TCDD/g) | BCF | t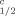 (d) (d) |
| American toad | ||||
| Egg | 0.003 | 10 | 3.33 | — |
| 0.03 | 123 | 4.10 | — | |
| 0.3 | 1,078 | 3.59 | 3.4 | |
| 3 | 7,125 | 2.38 | 4.5 | |
| 30 | 19,331 | 0.64 | 4.0 | |
| Tadpole | 0.3, No lettuce | 5,661 ± 377.4 | 18.9 ± 1.26 | 1.0 |
| 0.3, Lettuce | 2,663 ± 209.7 | 8.9 ± 0.70 | 1.7 | |
| Leopard frog | ||||
| Egg | 3 | 17,486 ± 2,611.5 | 5.8 ± 0.87 | 2.7 |
| Tadpoled | 3, No food | 7.3 | ||
| 3, High-fat diet | 3.3 | |||
| 3, Low-fat diet | 2.3 | |||
| Green frog | ||||
| Egg | 0.3 | 891 | 2.97 | 5.0 |
| 3 | 3,682 | 1.23 | 4.0 | |
| 10 | 11,482 | 1.15 | 3.9 | |
| 30 | 28,227 | 0.94 | 4.3 | |
| 100 | 73,717 | 0.74 | 5.8 | |
- a Nominal concn. of [3H]TCDD in water.
- b Maximal egg or tadpole [3H]TCDD concn. (mean ± SE).
- c Half-life (t1/2) for whole-body elimination of [3H]TCDD was calculated assuming first-order elimination as t1/2 = ln 2/k, where -k is the slope of the least-squares regression line of ln [3H]TCDD content of eggs and/or tadpoles versus time.
- d Tadpoles were exposed as eggs.
Lipid levels in eggs and/or tadpoles of the species tested are presented in Table 3. We did not find significant Spearman rank correlations between percent lipid and TCDD concentrations in American toads (rs2 = 0.4, p = 0.6) or in leopard frogs (rs3 = 0.3, p = 0.62) during development.
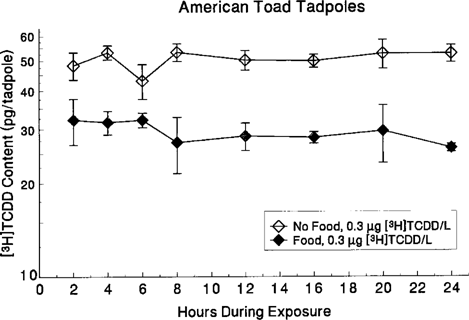
Content of [3H]TCDD in American toad tadpoles during a 24-h exposure. Data are presented as mean ± SE of four replicates.
DISCUSSION
TCDD in adult and eggs of amphibians in the wild
Little data are available on TCDD residue levels in amphibians in the wild. Bullfrogs from Arkansas, USA, near a trichlorophenoxyacetic acid production site showed TCDD levels as high as 10,400 and 68,000 ppt in female ovaries and fat, respectively [20]. One toad (unidentified species) collected from Seveso, Italy, 2 years after TCDD contamination from a chemical plant explosion had a whole-body burden of 200 ppt [18]. Southern toads (B. terrestris) from an air base in Florida, USA, where 2.8 kg TCDD had been aerially sprayed as 2,4,5-T showed a whole-body concentration of 1,360 ppt [31]. Although the percentage of TCDD transferred from mother to eggs is unknown for amphibians, the percentage transferred from adult female fishes to eggs can be as high as 50% [12]. If amphibians transfer a similar percentage, amphibian egg TCDD concentrations could reach as high as 34,000 ppt based on the body burdens in the adult frogs cited above. Concentrations of TCDD approached or exceeded this level in the egg experiments described in this article but did not appear to significantly increase mortality or deformities for early life stages of these species.
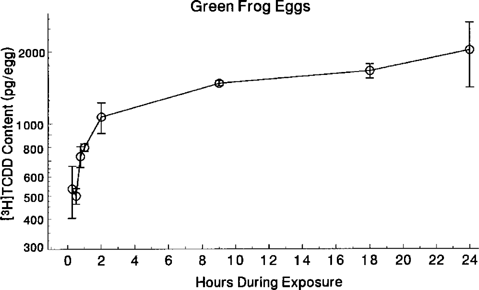
Content of [3H]TCDD in green frog tadpoles during a 24-h exposure to 30 μg [3H]TCDD/L. The linear absorption rate could be described by the following linear regression equation: TCDD content (pg/individual) = 430.4 + 327.6(time) (F = 40.2, d.f. = 1,13, p < 0.0001; r2 = 0.76, n = 23). Data are presented as mean ± SE of three replicates. Eggs exposed to 0.3 μg [3H]TCDD/L exhibited similar absorption kinetics (data not shown).
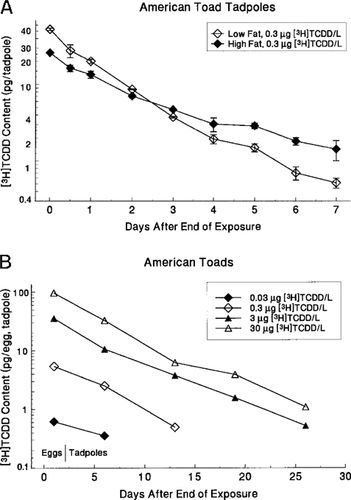
Elimination of [3H]TCDD for American toads exposed as tadpoles (A) (mean of two replicates) and exposed as eggs (B) (mean of three replicates). In B, coefficients of variation averaged 42% (range, 11-83%) of the mean values shown.
| Species, stage | % Total lipid | [3H]TCDD concn. (pg/g) |
|---|---|---|
| American toad | ||
| Eggs | 24.1 ± 1.48a | 10-19,332 (0.003-30) μg/L |
| Tadpoles, from field | 41.1 ± 2.27 | 2,663 (food), 5,661 (no food) |
| Tadpoles, high-fat diet (1 d postexposure) | 54.9 ± 0.58 | 1,395 |
| Tadpoles, high-fat diet (3 d postexposure) | 48.6 ± 1.76 | 292 |
| Tadpoles, high- and low-fat diet (7 d postexposure) | 22.8 | 32 |
| Leopard frog | ||
| Eggs | 18.8 ± 0.65 | 11,555 |
| Posthatch tadpoles (5 d postexposure) | 27.5 ± 0.04 | 4,222 |
| Tadpoles, no food (10 d postexposure) | 59.3 ± 4.04 | 1,324 |
| Tadpoles, high-fat diet (10 d postexposure) | 25.0 ± 0.86 | 940 |
| Tadpoles, high-fat diet (13 d postexposure) | 10.0 | 369 |
| Green frog | ||
| Eggs | 18.1 ± 1.74 | 890-73,717 (0.3-100) μg/L |
- a Data from Mark J. Komoroski (Savannah River Ecology Laboratory, personal communication).
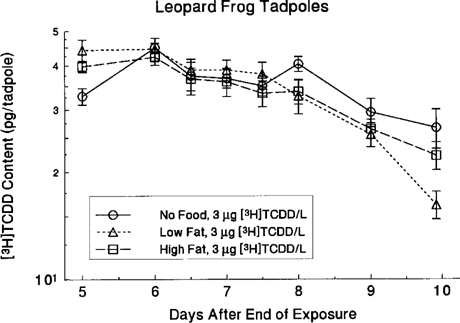
Influence of diet on elimination of [3H]TCDD in leopard frogs. Each point is the mean of five replicates.
General effects
We found relatively few impacts of acute TCDD exposure on eggs and tadpoles. Because tadpole body burdens were eliminated rapidly and few dose-response effects were found, TCDD-related effects were expressed based on nominal TCDD exposure water concentrations. The only significant TCDD-related mortality was a 10% increase in leopard frog egg and early tadpole mortality (exposed to 3 μg TCDD/L) prior to feeding as compared to controls. The leopard frog eggs were exposed to TCDD at an earlier stage of development compared to the American toad and green frog, which may explain the increased mortality. A significantly higher incidence of lighter pigmentation was found in leopard and green frog tadpoles following exposure to ⩾3 μg TCDD/L. Lighter pigmentation was also observed in pike (Esox lucius) and rainbow trout (Oncorhynchus mykiss) fry exposed to TCDD [32, 33]. We found only a short-term reduction in tadpole total length soon after exposure in leopard and green frogs exposed to 3 or > 3 μg TCDD/L, respectively. Other studies have reported decreased total length of amphibian and fish early life stages due to TCDD exposure [19, 32, 33]. Eggs and tadpoles exposed to TCDD in our study did not show a significant increase in deformities, although deformities are commonly observed in fishes exposed to TCDD as embryos [13, 34-36].
We found no statistically significant effects of TCDD exposure on American toad swimming speed or metamorphosis. American toads exposed as eggs to higher TCDD concentrations appeared to show accelerated metamorphosis and larger body mass at metamorphosis as compared to controls, but these did not differ significantly among treatments. Because we found almost significant relationships between number of tadpoles or metamorphs in a tank and body size or time to metamorphosis, treatment effects may have been overshadowed by density-dependent effects on these parameters. Another study found a tendency for accelerated metamorphosis in South African clawed frog tadpoles exposed to 0.1 ppb TCDD for 30 d and then placed in clean water, but instead of larger-sized metamorphs they found higher mortality around metamorphosis [19]. We did not find a difference in metamorph mortality among treatments. Accelerated metamorphosis would seem to be related to earlier production of thyroxine (T4). Studies have found both reduced and increased serum T4 levels in PCH-exposed animals [37]; however, the mechanism by which TCDD causes accelerated metamorphosis is unknown. Shorter time to metamorphosis and larger body size are often associated with enhanced survival and fitness in amphibians [38].
Sensitivity to TCDD
Our findings support other studies that have found that amphibians are less sensitive than fishes to the deleterious effects of TCDD. The TCDD LD50 for adult bullfrogs is >500 μg/kg, whereas the LD50s for taxonomically varied adult fish species are <20 μg/kg [39]. Also, in our study, TCDD concentrations as high as 5.66 and 19.33 μg/kg in toad eggs and tadpoles, respectively, and 73.72 μg/kg in green frog eggs did not significantly increase mortality, whereas LD50s of <0.488, 2.5, and 25.71 μg/kg are reported for trout [13, 24, 40], zebra fish (Danio rerio) [41], and fathead minnows (Pimephales promelas) [42], respectively, exposed to TCDD as eggs. Thus, amphibians appear to be 100- to 1,000-fold less sensitive to TCDD than early life stages of fishes. We did not observe TCDD dose-related edema or hemorrhages in amphibians resembling blue-sac disease, as has been observed in fishes [13].
Rapid elimination of TCDD early in amphibian development (t1/2 = 1-7 d) means that amphibians are not exposed to high TCDD levels for prolonged periods during development, which could explain why TCDD is less toxic to amphibians. For comparison, differences in developmental timing in sal-monid fishes and therefore in the length of time they are exposed to TCDD prior to the fry stage, when TCDD is eliminated, could be used to explain differences in salmonid sensitivity to TCDD. Lake trout (120 d with maternal lipids until fry stage [13]) are most sensitive to TCDD, followed by brook trout (70 d until fry stage [43]) and rainbow trout (54 d until fry stage [44]).
Sensitivity of early life stages of fishes to TCDD is not limited to salmonids, which develop at cold water temperatures and have a long egg developmental period (>15 d). Fishes that develop at warmer temperatures and have rapid egg development (<15 d), as observed in anuran amphibians, are also sensitive to TCDD, as illustrated by northern pike [32], Japanese medaka (Oryzias latipes) [34, 35], zebra fish [36], and fathead minnow [45]. Pike exposed as eggs to 0.1 ng TCDD/L in 14°C water showed delayed growth and development [32], while Japanese medaka exposed as eggs to 13 ng TCDD/L in 23°C water exhibited a significant increase in mortality (60% above control) [34, 35]. The highest TCDD concentrations we used exceeded those used in fish early development studies, and body burdens in the anuran amphibians we studied also exceeded body burdens achieved in fish early life stage studies; however, we observed only a 10% increase in mortality in one experiment (leopard frogs exposed to 3 μg TCDD/L). Thus, it seems unlikely that the reduced sensitivity of anuran amphibians to TCDD compared to fish early life stages can be explained solely by their rapid embryonic development or higher incubation water temperatures.
South African clawed frog tadpoles exposed to 0.5 and 1 μg TCDD/L showed greater effects (mortality, decreased total length) [19] than those we found for anuran eggs or tadpoles exposed to TCDD, although exposure was longer (up to 30 d) in their study. Indeed, South African clawed frog eggs exposed for 24 h to waterborne TCDD concentrations as high as 30 μg/L did not show TCDD-related mortality (M.K. Walker, personal communication).
2,3,7,8-Tetrachlorodibenzo-p-dioxin toxicity is thought to be mediated through binding to a cytosolic aryl hydrocarbon (Ah) receptor [4]. Limited evidence suggests that amphibians possess this receptor [46]. Teleost and elasmobranch fish possess an Ah receptor [47], so it seems likely that amphibians would as well, having arisen from bony fish in the class Osteichthyes [2]. Because cytochrome P4501A1 transcription, which is regulated by the Ah receptor, is inducible in amphibians [48], this further suggests that amphibians possess an Ah receptor. If, however, the binding affinity of TCDD for the amphibian Ah receptor is substantially lower or the Ah receptor is present in low concentrations, this could explain reduced sensitivity of amphibians to TCDD. Adult green frogs require TCDD doses ⩾0.1 mg/kg to induce ethoxyresorufin-O-deethylase, further suggesting that amphibians are relatively insensitive to TCDD-induced effects (Y.W. Huang, personal communication).
Absorption
Absorption of TCDD into both eggs and tadpoles occurred rapidly (within approx. 2 h) during exposure. When eggs were exposed, TCDD was found primarily in the embryo proper. The jelly capsule, which protects the embryo from injury, fungal infestation, and predation, contained <4% TCDD relative to the embryo. The jelly capsule is composed of proteins, mucoproteins, and mucopolysaccharides [2], which might not readily store lipophilic compounds and thus would not be expected to retain significant amounts of TCDD. Similarly, wood frog (R. sylvatica) embryos exposed to waterborne DDT showed little DDT in the jelly capsule (8.8%) relative to the embryo [49]. Licht [49] also showed that embryos exposed to DDT without protective jelly capsules accumulated 10 times more DDT than eggs with jelly capsules.
Salmonid fish eggs, which lack jelly capsules yet possess a chorionic membrane, showed TCDD BCF values of 3 to 4 (lake trout) [13], 2 to 3 (rainbow trout) [50], and 20 (brook trout) [40] after 48-h exposures. The brook trout egg accumulation exceeds the BCFs observed for amphibian eggs in this study, but the lake and rainbow trout egg accumulations are similar. Fish data show that longer exposures to TCDD in the laboratory can lead to extremely high BCFs (e.g., approx. 300,000 [9]).
Tadpoles exposed in the present study showed higher BCFs than eggs, which may be partly explained by the lack of a jelly capsule around tadpoles. In another study, wood frog tadpoles exposed to waterborne [14C]DDT also showed rapid uptake, with approximately maximal whole-body levels achieved after 7 h of exposure [51]. Tadpoles have more exposure routes than eggs because they possess gills and a thin, highly vascularized epidermis (only two to three layers thick and lacking a keratinized layer) and may ingest TCDD during exposure if the TCDD becomes attached to bacteria or protozoa in the water that they eat [2]. The tadpoles exposed to TCDD with food (lettuce) showed a lower BCF than those without food, probably because TCDD was sorbed to uneaten lettuce in the exposure dish (726 pg TCDD/g lettuce) such that less TCDD could be absorbed by the tadpoles.
However, BCFs in laboratory exposures can be quite artificial and may not accurately reflect BCFs in the wild. 2,3,7,8-Tetrachlorodibenzo-p-dioxin can stick to glassware or volatilize, and the use of organic solvents (e.g., acetone) may significantly alter TCDD absorption.
Elimination
The pattern of TCDD elimination in amphibians during both egg and tadpole development differs from that of fishes. Previous studies of cold-water fish found very slow or no elimination of PCHs during nonfeeding stages (egg and sac fry) but increased elimination in the feeding stage (fry). For example, rainbow trout exposed as eggs eliminated 2,2′,5,5′-tetrachlorobiphenyl (PCB 52) slowly in the egg and sac fry stages (t1/2 = 231 d) but more rapidly during the fry stage (t1/2 = 15 d) [44]. Lake trout eliminated TCDD only during the fry stage (2 months posthatch, t1/2 = 35–37 d) [13].
In contrast, we found relatively rapid elimination in both the amphibian egg and tadpole stages. For leopard frogs, the half-life of TCDD in eggs was 2.7 d, and the half-life in feeding tadpoles ranged from 2 to 7 d. Overall, t1/2 for American toad, leopard, and green frogs ranged from 1 to 7 d, which is consistent with t1/2 values calculated for the South African clawed frog (1.4−1.9 d; M.K. Walker, personal communication) and similar to elimination of [14C]DDT from the liver of wood frog tadpoles [51] (calculated from raw data in article: t1/2 = 3–7 d). This difference in elimination between amphibians and fish could be caused by a host of factors, including incubation temperature, metabolic rate, rate of development, and lipid turnover. Given the physiological principle of the Q10 effect, with a 13°C difference in incubation temperature between a fish (7°C, lake trout) and an amphibian (20°C), one might expect a three- to fourfold increase in metabolic rate [52]. However, we found a 10-fold difference in elimination rate. Thus, a putative three- to fourfold change in metabolic rate is likely not responsible for a 10-fold difference in elimination.
Anuran amphibian development occurs rapidly and could be considered an accelerated version of fish development. Amphibian eggs hatch within 3 to 6 d of laying, utilize maternally deposited lipids quickly, and begin feeding within a few days of hatching. This contrasts sharply with salmonid fish such as lake trout, which are eggs for 60 d and are utilizing maternal lipids for 120 d prior to feeding. The TCDD concentration is often directly correlated with lipid levels [11], and it is possible that the rapid use of maternally deposited lipids in amphibians corresponds with rapid TCDD elimination during early development. Frogs are reported to have a relatively high rate of turnover or renewal of fatty acids [53], which could contribute to more rapid elimination of lipophilic compounds. Our percent total lipid levels on a dry mass basis agree with other reports for anuran eggs (mean range, 18.8–25.2% [54]) and early developing tadpoles (25–30% [55]). Whole-body elimination of TCDD in this study could not be explained solely by changes in the percentage of lipid. Elimination differences are likely influenced by biotransformation and differences in metabolic rate [56].
Amphibian tadpole elimination corresponds more with elimination rates observed in exogenously feeding fish at the fry stage of development rather than elimination rates by eggs or sac fry. Fry may eliminate TCDD, rather than eggs and sac fry, because (1) fry have a lower fat content than other life stages, (2) fry have a higher rate of biotransformation, and/or (3) lipids or lipoproteins absorbed from the yolk sac or synthesized in the liver during the fry stage may bind the TCDD, resulting in a TCDD–lipoprotein complex that may be more rapidly eliminated in feces than unbound TCDD [57]. These conditions may also apply to exogenously feeding amphibian tadpoles.
Influence of diet on TCDD elimination
Diet significantly influenced TCDD elimination. Leopard frog tadpoles that were not fed showed slower elimination of TCDD relative to tadpoles that were fed. This result agrees with findings of previous studies [cited in 58], in which starved fish eliminated PCBs more slowly than fed fish. In fact, PCB concentrations in starved fish increased as fat levels decreased. During starvation, reduced lipid turnover or the use of alternate energy reserves (e.g., glycogen) may retard TCDD elimination. In addition, American toad and leopard frog tadpoles fed lowfat diets appeared to eliminate TCDD faster than those on highfat diets. The high-fat diet might lead to greater retention of lipophilic compounds such as TCDD.
Acknowledgements
We are indebted to R.E. Peterson for use of his laboratory facilities, and we especially thank him and William H. Karasov for ideas, support, and advice. We thank the Center for Limnology and Water Chemistry Program for use of aquatic facilities; R. Lindroth for use of his lab; P.D. Hoff for statistical help; B. Darken, T.M. Le, M. Saur, and others for assistance in the lab; and T. Garland, Jr., for comments on the manuscript. This research was sponsored by the U.S. Department of Energy, Office of Energy Research, Environmental Sciences Division, Office of Health and Environmental Research, under appointment to the Graduate Fellowships for Global Change (R.E. Jung) administered by Oak Ridge Institute for Science and Education, and was funded by the University of Wisconsin Sea Grant Institute under grants from the National Sea Grant College Program, National Oceanic and Atmospheric Administration, U.S. Department of Commerce, and from the State of Wisconsin (federal grant NA46RG0481, project R/MW-54, to W.H. Karasov). M.K. Walker was supported in part by National Institutes of Health National Research Service Award (1-F32-ES-05673–01).




