Effects of cations, temperature, and creosote on degradation of indole by Desulfobacterium indolicum (DSM 3383)
Abstract
This work describes the activity of Desulfobacterium indolicum under different conditions. Hydroxylation of indole by the sulfate-reducing marine bacterium D. indolicum was related to the concentration of Na+ and Mg2+, whereas K+ and Ca2+ had no influence. Hydroxylation of indole by D. indolicum was possible in normal medium with cation concentrations corresponding to seawater at low temperatures (10-26°C). The effect of reduced cation concentrations was most pronounced at high temperatures (29 and 36°C) where only an incomplete hydroxylation was observed in the medium with reduced cation concentrations. Desul-fobacterium indolicum was more sensitive to the presence of an artificial creosote mixture when depleted for cations. Sixty milligrams of artificial creosote per liter inhibited hydroxylation of indole by 30% in a normal growth medium compared to an 86% inhibition in a medium with low concentrations of cations. Indole and quinoline were the only compounds degraded from a creosote mixture consisting of 25 compounds. It was concluded that D. indolicum is active towards indole and quinoline under growth conditions quite distinct from its optimal laboratory conditions.
INTRODUCTION
The major contributor of heterocyclic compounds to the environment is creosote contamination at abandoned gasworks [1]. Creosote is a complex mixture consisting of approximately 3% heterocyclic compounds, 12% phenolic compounds, and 85% polycyclic aromatic hydrocarbons (PAHs) [2]. Many of the nitrogen-heterocyclic compounds are toxic [3-5], terato-genic [4], mutagenic [6, 7], and/or carcinogenic [8].
Desulfobacterium indolicum is a sulfate-reducing bacterium able to grow on the nitrogen-heterocyclic compounds indole and quinoline [9], two of the most abundant heterocyclic compounds in creosote [2]. Desulfobacterium indolicum was isolated from marine mud with indole as the sole electron donor and carbon source. The isolation was performed at 28°C and pH 7. The cultivation medium contained 360 mM NaCl and 23 mM MgCl2 as well as 1.0 mM CaCl2 and 6.7 mM KCl. Bak and Widdel [9] found that any changes in the concentrations of NaCl, MgCl2, or CaCl2 retarded the growth of D. indolicum. The cation concentrations in ground water are typically 0.1 to 2 mM of Na+, Mg2+, and Ca2+, and 0.01 to 0.1 mM of K+ [10].
In addition to indole and quinoline, D. indolicum can use the aromatic compounds anthranilic acid and 2-aminobenzoate as the sole electron donor and carbon source. However, growth with substrates other than indole is rather slow [9]. Shranker and Bollag [11] found that D. indolicum hydroxylated 95% of 0.13 mM of indole within 10 d, producing oxindole. The oxindole was subsequently transformed within 35 d.
The initial step of indole and quinoline degradation is hydroxylation [12-18]. The initial degradation step is less well known for other heterocyclic compounds. Some aromatic compounds are metabolized via a few central intermediates during anaerobic degradation, which maximizes the substrate spectrum of an organism and minimizes the number of enzymes required [19]. However, the substrate spectrum and the enzyme specificity during anaerobic degradation of heterocyclic compounds are unknown.
Examination of the characteristics of anaerobic organisms growing on heterocyclic compounds is of interest, as these compounds can be used for bioremediation. As a marine organism, D. indolicum has a need for cations. Therefore, definition of the need for cations, along with growth temperatures and utilization of other creosote compounds, is also of interest.
MATERIALS AND METHODS
Inoculum source and cultivation
Desulfobacterium indolicum (DSM 3383) was obtained from DSM, Deutche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany, and grown in a medium as described by Bak and Widdel [9] using anaerobic techniques. Desulfobacterium indolicum was cultivated on 200 to 300μM indole. Cells were transferred to a new medium at the beginning of the exponential growth phase. All experiments were done with 117-ml serum bottles containing 75 ml medium. Inoculum was approximately 5% (v/v), which corresponded to a cell density of 2 × 106 cells/ml. The incubation temperature was 28°C unless otherwise stated. The hydroxylation rates were estimated, using linear regression, from the linear part of the degradation curves including at least 12 measurements. The results are obtained from several experiments and are reproducible.
Effect of cation-low medium
The influence of cations on indole degradation by D. indolicum was tested using a two-factorial test [20]. Combinations of normal cation concentrations of D. indolicum medium and concentrations reduced to 10% of normal Na+ and Mg2+ concentrations and 20% of normal concentrations of K+ and Ca2+ were tested (Table 1). These cation concentrations were chosen by the computer program Design-Ease® [20] in order to investigate the significance of each of the cations in the high concentration. The indole concentration was 150 μM, and the effect of cations was quantified as indole hydroxylation rates. A similar experiment was done with normal concentrations of K+ and Ca2+ (6.7 and 1.0 mM, respectively) and various concentrations of Na+ and Mg2+ [21]. A medium containing 360 mM Na+ and 23 mM Mg2+ as well as 6.7 mM K+ and 1.0 mM Ca2+ was chosen as “cation-low medium” for experiments with low concentrations of cations. This medium combination was chosen because it supported growth of D. indolicum and contained lower concentrations of cations than the normal growth medium. Conductivity was measured on a CDM 83 conductivity meter with a CD 81 electrode (Radiometer, Copenhagen, Denmark). The biomass was quantified as protein after 21 d.
| Sample No. | Na+ (mM) | K+ (mM) | Ca2+ (mM) | Mg2+ (mM) | Conductivity (mmho/cm) | %Indole hydroxylateda | Biomass (mg protein/L)b |
|---|---|---|---|---|---|---|---|
| 1 | 36 | 6.7 | 1.0 | 15 | 15.4 | 100 | 2.7 |
| 2 | 36 | 1.3 | 0.2 | 15 | 14.5 | 76 | 2.1 |
| 3 | 36 | 6.7 | 1.0 | 1.5 | 13.0 | 42 | 2.2 |
| 4 | 36 | 1.3 | 1.0 | 15 | 14.5 | 94 | 2.5 |
| 5 | 360 | 6.7 | 0.2 | 15 | 38.8 | 100 | 1.1 |
| 6 | 360 | 1.3 | 1.0 | 1.5 | 36.0 | 100 | 1.7 |
| 7 | 360 | 6.7 | 1.0 | 15 | 28.8 | 100 | 1.5 |
| 8 | 360 | 1.3 | 1.0 | 15 | 38.9 | 100 | 1.5 |
| 9 | 360 | 1.3 | 0.2 | 15 | 38.1 | 100 | 0.9 |
| 10 | 360 | 6.7 | 0.2 | 1.5 | 38.2 | 88 | 3.1 |
| 11 | 360 | 1.3 | 0.2 | 1.5 | 36.8 | 94 | 1.0 |
| 12 | 36 | 6.7 | 0.2 | 1.5 | 13.1 | 20 | 1.3 |
| 13 | 36 | 6.7 | 0.2 | 15 | 14.7 | 100 | 1.6 |
| 14 | 360 | 6.7 | 1.0 | 1.5 | 37.6 | 100 | 1.1 |
| 15 | 36 | 1.3 | 1.0 | 1.5 | 12.8 | 40 | 1.6 |
| 16 | 36 | 1.3 | 1.0 | 1.5 | 12.4 | 20 | 0.5 |
| Normal medium | 360 | 6.7 | 1.0 | 23 | 40.5 | 100 | 1.2 |
- a Percent of indole hydroxylated after 7 d.
- b Biomass quantified as protein after 21 d.
Effect of temperature
Indole degradation was tested at temperatures between 10 and 36°C in the normal growth medium and in the cation-low medium. Indole was added in a concentration of 150 μM. The experiment was carried out in triplicate.
Effect of creosote mixture
The effect of an artificial creosote-mixture on indole degradation by D. indolicum was tested in both the normal and the cation-low media. The artificial creosote mixture contained: 32% (w/v) BTEX (6.9% benzene, 6.3% toluene, 6.8% ethylbenzene, 6.1% o-xylene, and 6.1% p-xylene), 19% phenols (5.5% phenol, 4.5% o-cresol, 4.7% 2,4-dimethylphenol, and 4.4% 3,5-dimethylphenol), 5% PAHs (2.1% naphthalene, 2.0% 1-methylnaphthalene, 0.6% phenanthrene, and 0.6% flourene), and 43% heterocyclic compounds (3.3% indole, 6.9% quinoline, 1.7% 2-methylquinoline, 9.1% pyrrole, 5.8% 1-methylpyrrole, 0.5% carbazole, 0.6% acridine, 3.2% ben-zothiophene, 0.6% dibenzothiophene, 7.5% furan, 2.0% ben-zofuran, and 1.8% dibenzofuran) to simulate the water-soluble fraction of creosote [2]. The creosote mixture was added at concentrations from 5 to 100 mg per liter of medium. Controls were run without addition of the creosote mixture. One hundred micromolar indole was added separately. The indole hydroxylation was measured continuously, whereas the degradation of the individual creosote compounds was examined at the end of the experiment. The experiment was carried out in triplicate.
Analysis of indole
For quantification of indole, 1.00-mL subsamples were made alkaline to pH 12 with 25 μl 4.0 M KOH, and extracted with 500 μl diethylether in addition to 50 μl pentane, with 0.380 mM undecane added as an internal standard. The organic phase was analyzed on a Carlo Erba Mega gas chromatograph (Carlo Erba Instruments, Italy) with a flame ionization detector and a CP-sil 8 CB column (Struers, Kebolab A/S, Alberblund, Denmark), 25 m, i.d. 0.32 mm, film 0.25 mm. The initial column temperature was 60°C and was raised to 150°C at 30°C/ min, and then to 200°C at 20°C/min. Detector temperature was 300°C and injector temperature was 285°C.
Analysis of the creosote mixture
For quantification of samples containing the creosote mixture, 10-ml subsamples were extracted with 900μl diethylether and 100μl pentane, with 0.38 mM undecane added as an internal standard. The organic phase was analyzed on a Shimadzu 14A (Spectra Chrom, Brondby, Denmark) with a flame ionization detector and a CP-sil 19 CB column (Shimadzo Corp., Kyoto, Japan), 25 m, i.d. 0.32 mm, 1.2-μm coating. The column temperature was 45°C for 2 min and was raised by 20°C/min to 270°C for 5 min. Detector temperature was 280°C and injector temperature was 250°C.
Protein analysis
For quantification of protein, 10.0-ml samples were analyzed by the Folin Phenol reagent method described by Lowry et al. [22] and modified by Peterson [23].
RESULTS
Effect of cations
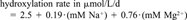
| Sample No. | Na+ (mM) | Mg2+ (mM) | Conductivity (mmho/cm) | Observed degradation ratea (μmol/L/d) | Predicted degradation rateb (μmol/L/d) | Biomass (mg protein/L)c |
|---|---|---|---|---|---|---|
| 1 | 307 | 58 | 37.5 | 85 ± 8.0 | 103 | 1.8 |
| 2 | 180 | 34 | 25.9 | 82 ± 8.0 | 63 | 2.0 |
| 3 | 180 | 68 | 29.8 | 79 ± 5.2 | 87 | 2.0 |
| 4 | 180 | 34 | 26.2 | 88 ± 6.3 | 63 | 2.0 |
| 5 | 180 | 34 | 26.3 | 84 ± 1.4 | 63 | 1.7 |
| 6 | 360 | 34 | 39.2 | 80 ± 3.1 | 97 | 2.0 |
| 7 | 52 | 58 | 19.4 | 55 ± 5.1 | 56 | 2.1 |
| 8 | 180 | 34 | 26.0 | 77 ± 1.4 | 63 | 1.8 |
| 9 | 0 | 34 | 12.2 | 12 ± 1.0 | 30 | 1.3 |
| 10 | 307 | 10 | 32.8 | 86 ± 9.1 | 70 | 2.0 |
| 11 | 180 | 0 | 22.6 | 6 ± 2.2 | 39 | 0.7 |
| 12d | 52 | 10 | 13.8 | 23 ± 1.1 | 23 | 0.8 |
| Normal medium | 360 | 23 | 41.3 | 69 ± 8.6 | 88 | 1.5 |
- a Observed rate of hydroxylation estimated by linear regression ± standard error. Initial concentration of indole was 100 μM.
- b Predicted degradation rate of indole from the equation: degradation = 2.5 + 0.19·(mM Na+) + 0.76·(mM Mg2+).
- c Biomass quantified as protein after 21 d.
- d Medium composition chosen as cation-low medium.
Effect of temperature
Indole degradation was tested at temperatures between 10 and 36°C, and degradation had a maximum at about 36°C in the normal growth medium, with an initial indole hydroxylation rate at 51 μmol/L/d. In the cation-low medium, the highest indole hydroxylation rate was obtained at 29°C. However, the degradation was incomplete; not all the indole was degraded at 29°C or at 36°C (Fig. 1). The highest rate of complete hydroxylation of indole in the cation-low medium was observed at 26°C, with a hydroxylation rate of 14 μmol/L/d. A slow indole hydroxylation at 1.8 ± 0.4 μmol/L/d was observed at 10°C.
Effect of creosote mixture
Indole was hydroxylated at the same rate in all cultures with 0 to 40 mg/L of the creosote mixture added to medium with normal concentrations of cations. Indole hydroxylation was 30% inhibited in cultures with 60 mg/L creosote, and 96% inhibited in cultures with 100 mg/L (Table 3).
Indole was hydroxylated at the same rate in cultures with 0 to 20 mg/L of creosote in cation-low medium, where the salt concentrations were reduced to 52 mM Na+ and 10 mM Mg2+. However, 86% inhibition was observed in cultures with 60 mg/L of creosote added, corresponding to only 7% of the hydroxylation rate observed in the medium with normal salt content and with the same concentration of creosote added. Degradation stopped before indole was completely used in all cultures in the cation-low medium, including the medium with no creosote present. The indole hydroxylation was generally stimulated by low concentrations of creosote. Measurements showed that indole and quinoline were the only compounds from the creosote mixture that were degraded.
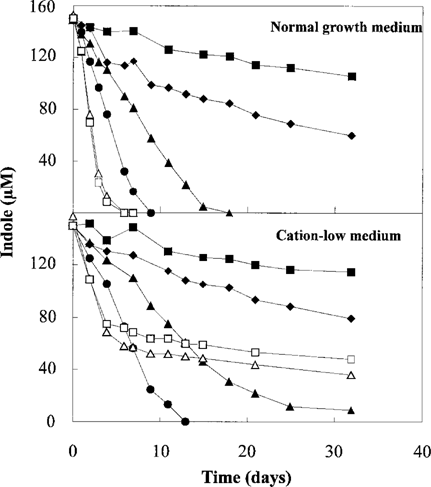
Indole hydroxylation in the normal growth medium and in the cation-low medium at temperatures between 10 and 36°C. Incubation at 10°C (▪), 17°C (○), 21°C (♦), 26°C (•), 29°C (⋄), and 36°C (□).
| Normal mediuma | Cation-low mediumb | |||
|---|---|---|---|---|
| Concentration of creosote mixture (mg/L) | Initial hydroxylation rate (μmol/L/d)c | Relative hydroxylation rate (%)d | Initial hydroxylation rate (μmol/L/d)c | Relative hydroxylation rate (%)d |
| Sterile control | 0 | 0 | 0 | 0 |
| No creosote added | 39 ± 2.9 | 100 | 13.3 ± 0.8 | 100 |
| 5 mg/L | 44 ± 3.7 | 112 | ND | ND |
| 10 mg/L | 44 ± 2.3 | 112 | 14.2 ± 0.9 | 107 |
| 20 mg/L | 45 ± 3.2 | 115 | 12.8 ± 0.9 | 96 |
| 40 mg/L | 40 ± 4.0 | 103 | 7.1 ± 0.9 | 53 |
| 60 mg/L | 27 ± 4.4 | 69 | 1.8 ± 0.9 | 14 |
| 100 mg/L | 1.7 ± 1.5 | 4 | ND | ND |
- aMedium contains 360 mM Na+ and 23 mM Mg2+.
- bMedium salt reduced to 52 mM Na+ and 10 mM Mg2+.
- c Hydroxylation rate of indole estimated by linear regression of the first 4 d ± standard error.
- d Hydroxylation rate relative to the hydroxylation rate in a medium with no creosote added. ND: Not determined.
DISCUSSION
The cation content of the medium could be reduced considerably and still support growth of D. indolicum. The significant cations were Na+ and Mg2+, which could be reduced by about 15 and 60%, respectively, without reduction of the indole hydroxylation rate. The effect of reduced concentrations of cations did not reflect the lowering of the conductivity in the medium as there was no correlation between the conductivity and the hydroxylation rate. A reduction by 85 and 57% of Na+ and Mg2+, respectively, reduced the indole hydroxylation rate to one-third of normal rates. This result contradicts those of Bak and Widdel [9], who observed that any changes in the NaCl, MgCl2, or CaCl2 concentrations retarded the growth of D. indolicum. Lowering the temperature partly relieved the stress of lowering the cation concentration.
Madsen et al. [24] investigated the environmental factors affecting indole metabolism in denitrifying and methanogenic soil, sediment, and sewage sludge, and found that the indole degradation rate increased with temperatures from 15 to 35°C. Furthermore, they found nearly a threefold decrease in the maximum concentration of the intermediary product, oxindole.
Desulfobacterium indolicum was stressed by reduced salt concentrations, as seen from the result with creosote: creosote was approximately five times more toxic in the cation-low medium than in the medium with normal salt concentrations. The stimulation of indole degradation at low creosote concentrations might be due to a higher concentration of indole, 100 μM indole added, plus the indole from the creosote mixture. Indole and quinoline were the only compounds from the creosote mixture that were degraded, confirming results from Bak and Widdel [9] that D. indolicum only uses a restricted spectrum of aromatic carbon sources.
Toxicity studies of creosote compounds have shown that the heterocyclic compounds in creosote are the most toxic [25]. These studies found that addition of 38.9 mg of creosote mixture per liter of aerobic enrichment culture derived from groundwater inhibited toluene degradation by approximately 38% compared to cultures with only toluene added. The creosote mixture used by Dyreborg et al. [25] contained 55% heterocyclic compounds, which might explain the relatively high inhibition.
The experiments with addition of the creosote mixture to cultures of D. indolicum showed that D. indolicum did not transform pyrrole, carbazole, benzothiophene, dibenzothiophene, furan, benzofuran, or dibenzofuran, which are all structurally related to indole and quinoline. The inability of D. indolicum to transform these compounds indicates that they are either not hydroxylated or that they are hydroxylated by an enzyme system not active for indole and quinoline.
We have found no previous reports of the effect of cations on degradation of creosote. However, the degradation of crude oil by a mixed bacterial community isolated from a marine sediment was investigated by Mille et al. [26]. They found that the amount of oil degraded increased with increasing salt concentrations, to a maximum at 400 mM Na+. Biodegradation occurred in the 100 to 2,000 mM Na+ range. Each fraction of the crude oil was degraded after a 30-d incubation period at an NaCl concentration equivalent to seawater (approx. 400 mM).
Our results indicate that D. indolicum will have a high survival under conditions expected to be found at creosote contaminations. Furthermore, the bacterium will be active at quite low temperatures and has a high tolerance towards creosote. However, cation concentrations required for growth of D. indolicum are higher than those normally found in soils and groundwaters and could limit the use of this bacterium for bioremediation. Further, the substrate spectrum of D. indolicum was limited. However, the substrates used were removed at relatively high rates even though the initial hydroxylation is an energy-requiring step. Our general impression is that bioaugmentation of soils by addition of specific degrading bacteria will demand very high amounts of inoculum [27], making this addition less attractive. Therefore, a pump and treat method for the most water-soluble creosote compounds in a bioreactor with immobilized cells would be a more interesting approach.
Acknowledgements
Anita Hauritz provided technical assistance. This work was supported by the Danish Groundwater Research Centre.




