Inhibition of pyrene biotransformation by piperonyl butoxide and identification of two pyrene derivatives in Lumbriculus variegatus (Oligochaeta)
Abstract
Using the freshwater annelid Lumbriculus variegatus (Oligochaeta), the presence of cytochrome P450 (CYP) isozymes was investigated by analyzing metabolites of the polycyclic aromatic hydrocarbon (PAH) pyrene in treatments with and without the CYP inhibitor piperonyl butoxide (PBO). The results show a low biotransformation capability of L. variegatus (7% of total pyrene body burden as metabolites at 168 h). Addition of PBO resulted in a significant reduction of metabolites, suggesting the presence of a CYP in L. variegatus. Besides 1-hydroxypyrene, three peaks representing unknown metabolites were detected in LC-FLD (liquid chromatography with fluorescence detection) chromatograms of L. variegatus. Deconjugations showed that sulfonation and glucosidation are involved in the formation of these unknowns. Further studies with the time of flight mass analyzer provided the identification of the glucose-sulfate conjugate of 1-hydroxypyrene. The same metabolites were detected in the solvent-nonextractable fraction by incubation of the tissue residues with proteinase K, suggesting that part of these metabolites are bound to proteins. Overall, the slow biotransformation of pyrene by L. variegatus (involving CYP) supports the use of this species in standard bioaccumulation tests; however, the tissue-bound metabolite fraction described in the current study deserves further investigation for its toxicity and availability to upper trophic levels through diet. Environ. Toxicol. Chem. 2011; 30:1069–1078. © 2011 SETAC
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs) are a group of contaminants widely spread in the aquatic environment, mainly produced by anthropogenic activities 1, 2. The most important sources of PAH in the aquatic environment are atmospheric deposition (subsequent to combustion of fossil fuels), municipal and industrial runoffs, and oil spills 2.
Exposure and accumulation of PAHs in aquatic organisms varies considerably and depends on several factors, such as physical and chemical properties of the PAHs, physiological condition, behavior and feeding rate of organisms, and exogenous factors such as temperature and dissolved organic matter 2. Benthic animals may be exposed to PAHs from both water and sediment 3, and the exposure can take place through ingested food or body membranes. The presence of PAHs in tissues of organisms is highly dependent on their biotransformation and excretion capabilities. Fish and other vertebrates are known to be more effective in terms of PAH biotransformation than invertebrates 4. Wide interspecies variation in the biotransformation of PAHs has been reported 5-7, even among closely related sibling species 8.
The biotransformation of PAHs may result in detoxification, because increasing their water solubility would theoretically make them easier to excrete. However, sometimes metabolites can be more toxic or mutagenic than the parent compounds 4.
Pyrene, included in the U.S. Environmental Protection Agency's list of priority pollutants (9; http://www.epa.gov/waterscience/methods/pollutants.htm), is a four-ringed PAH, widely used as a model PAH in a large number of studies 3, 10-16.
1-Hydroxypyrene has been described as the major cytochrome P450 (CYP)-mediated phase I metabolite of pyrene in eukaryotes 13, 14, but recent studies have also described other phase I metabolites in snails 17.
A number of different phase II metabolites have been found in invertebrates: pyrene-1-sulfate and pyrene-1-glucuronide in Nereis virens 13; pyrene-1-glucoside in Porcellio scaber 14; glucose-sulfate conjugate in aquatic crustaceans 12; pyrene diol glucuronide sulfate in marine snails 17, and pyrene-1-O-(6″-O-malonyl)-glucoside in isopods and springtails 15 are only some examples.
Lumbriculus variegatus (Oligochaeta), usually found in freshwater bodies throughout North America and Europe, is one of the most common and important species used in sediment-water bioaccumulation and toxicity tests, as recommended in guidelines provided by several organizations 18. The efficient and reliable use of this standard test species requires knowledge of its biotransformation capacity. For example, L. variegatus was reported to biotransform benzo[a]pyrene 6, 19 and phenanthrene 20. However, a study by Verrengia-Guerrero et al. 7 indicates no biotransformation of [14C] pyrene by L. variegatus after 48 h.
Recent findings offer more detailed information on the biotransformation of pyrene by L. variegatus: Lyytikäinen et al. 21 reported the presence of 1-hydroxypyrene in pyrene-exposed L. variegatus extracts, and moreover, Mäenpää et al. 22 found two unknown peaks of exogenous origin in extracts of worms previously exposed to pyrene in water.
A useful indirect method of studying the presence and activity of CYP isozymes is piperonyl butoxide (PBO), which acts as an inhibitor of CYP and thus limits the detoxification of many xenobiotics 23, determining whether their biotransformation is CYP dependent. Piperonyl butoxide has been used as a CYP inhibitor with pyrene 10 and other PAHs 24.
The aim of the present study was to obtain more detailed information on the fate of PAHs in L. variegatus, using pyrene as a model compound: first, to obtain evidence of the existence of a CYP isoform (responsible for the biotransformation of pyrene into 1-hydroxypyrene) through the use of a known CYP inhibitor (PBO); second, to identify the potential metabolites of pyrene produced by L. variegatus, and finally, to determine whether the bound, solvent-nonextractable, fraction consists of parent or metabolites.
MATERIALS AND METHODS
Test animals
Lumbriculus variegatus (Oligochaeta), originally obtained from the Great Lakes Environmental Research Laboratory (Ann Arbor, MI, USA), were reared in culture aquaria at 20 ± 1°C in a 16:8 h light:dark cycle at the University of Eastern Finland (former University of Joensuu, Joensuu, Finland). Shredded and presoaked paper towels were used as a substrate, and the organisms were fed with powdered Tetramin fish food (Tetrawerke) three times a week. The worms to be used in the present study were removed from culture and kept in clean water to empty their guts for 24 h before the experiment.
Chemicals
Pyrene (98%) and radiolabeled pyrene (specific activity of 40 µCi/µmol) were purchased from Sigma Aldrich; 1-hydroxypyrene and its glucuronide conjugate were purchased from Dr. Ehrenstorfer, and piperonyl butoxide (98.2%; Pestanal®) from Riedel de Haën. The enzymes used for deconjugations were sulfatase (from Helix pomatia), alpha-glucosidase type I (from baker's yeast), and beta-glucuronidase from Sigma Aldrich, beta-glucosidase (from almonds), and lactase (from Aspergillus oryzae) from Fluka.
The solvents (hexane, methanol, acetone, ethanol, and acetonitrile) were all reagent, high-performance liquid chromatography (HPLC) or liquid chromatography-mass spectrometry grade. Ammonium acetate (puriss pro analysi for HPLC) was purchased from Fluka. Proteinase K was purchased from Sigma Aldrich. The water used in all experiments was purified MilliQ water.
Experiment 1
Three water exposure treatments were used: pyrene (nominal concentration = 10 µg/L); pyrene (10 µg/L) + PBO (35.4 µg/L); pyrene (10 µg/L) + PBO (354 µg/L). This concentration was less than 8% of the solubility of pyrene in water. The PBO concentrations used in exposures 2 and 3 were 1/100 and 1/10 the median lethal concentration, respectively, of PBO to L. variegatus reported by Ankley and Collyard 25. Radiolabeled pyrene was additionally added to all the treatments to a nominal concentration of 0.15 µg/L. The shares of nonlabeled and radiolabeled pyrene were 98.5 and 1.5%, respectively. These exposure concentrations were in the range of sublethal concentrations used in a water test performed at our laboratory 22.
The sampling time points were 8, 24, 48, 72, 96, and 168 h in all treatments, and four replicates were used for every timepoint and treatment. For each timepoint, an identical control beaker was used, with neither pyrene nor PBO added. All the experiments were carried out under yellow light (wavelength > 500 nm), to avoid photodegradation of the pyrene.
The experimental water was prepared by adding artificial freshwater with a hardness of [Ca2+] + [Mg2+] of 1 mM to 9-L flasks and adjusting the pH to 7 with 1 M HCl. Stock solutions of pyrene, radiolabeled pyrene, and PBO were prepared in ethanol, and the amounts needed for the exposure waters were added to the 9 L flasks while stirring. The measured concentrations of nonlabeled and radiolabeled pyrene were approximately 10.1 and 0.15 µg/L, respectively.
Finally, the 400-ml beakers were filled with 350 ml experimental water, and 12 worms (∼60 mg) were added to each beaker. The worms were not fed in any treatment, and every 24 h they were placed in another beaker containing new experimental water having the same pyrene and PBO nominal concentration.
Samples of 5 ml water were taken every 24 h before and after changing the exposure medium, mixed with 5 ml scintillation cocktail (InstaGel, Packard BioScience), shaken vigorously and analyzed for radioactivity after 24 h on a Wallac WinSpectral liquid scintillation counter (Wallac).
At each sampling time point, worms were picked out of the beakers, rinsed in tap water, sieved, dried on paper towels, weighed, and immediately frozen at −20°C.
Experiment 2
A second experiment with a greater wet weight of worms was performed to gain more biomass of worms for the metabolite analyses. The nominal concentration of pyrene was 20 µg/L, and neither PBO nor radiolabeled pyrene were present. Two replicate 1-L beakers with 900 ml artificial freshwater were set up in addition to a control, and approximately 400 mg worms were placed in each beaker. For this experiment, the worms were added directly to the beakers, without purging their guts, and only a time point of 168 h was used. The water was changed daily in one replicate and was not changed in the other one. The worms were not fed during the experiment, and constant aeration was provided in the beaker where the water was not changed.
Experiment 3
A more concentrated sample was used to perform mass spectrometric analyses. Forty L. variegatus (∼200 mg wet wt) were exposed to a nominal concentration of pyrene of 20 µg/L in water for approximately two months. This exposure was part of another experiment (Carrasco Navarro et al., unpublished data). The sample was sent to Åbo Akademi University (Turku, Finland) for mass spectrometric analyses.
Extraction of pyrene and its metabolites
Frozen worms from experiment 1 were thawed and homogenized in 4 ml 50:50 acetone:hexane (v/v). The homogenates were mixed and sonicated for 20 min, and then centrifuged for 10 min at approximately 1,200 g. The supernatants were collected, and the pellets were re-extracted in the same manner with 4 ml 50:50 acetone:methanol (v/v). The supernatants were then collected again. The second pellets were re-extracted with 3 ml methanol, the third supernatants were collected, and the final tissue residue was stored at −20°C. The supernatants were treated separately before being combined: 50 µl nonane was added, then evaporated to near dryness with a gentle flow of nitrogen and resuspended in 500 µl methanol. The methanol resuspensions were filtered in Spin-X® Centrifuge tube filters (Corning) and centrifuged at approximately 540 g for 1 min to facilitate the filtration process. The three 500-µl portions were then pooled in 1.8 ml HPLC amber vials and evaporated down to the final volume of 500 µl. The samples from experiments 2 and 3 were extracted by an almost identical method. The differences were that the supernatants resulting from the three extraction steps were pooled before filtering in Spin-X filters (final volume was 1.5 ml) and that larger volumes of organic solvents were used: 5 ml for experiment 2 and 8 ml for experiment 3 (in the first two extraction steps) and 4 ml in both cases (third extraction step).
The absolute recoveries for the method used in experiments 2 and 3 were calculated by extracting dead worms previously spiked with known standards and analyzing extracts by HPLC. These were 87.7 ± 4.1% for pyrene and 95.9 ± 2.5% for 1-hydroxypyrene (n = 3).
Tissue residues
The tissue residues remaining after the solvent extractions from experiment 1 were thawed and further treated with 0.5 ml Soluene®-350 (Packard BioScience) incubated overnight at 50°C, mixed with 10 ml Ultima Gold scintillation cocktail (PerkinElmer Life and Analytical life Sciences), shaken vigorously with a vortex mixer and finally analyzed for radioactivity after 24 h.
The tissue residues from experiment 2 were treated with proteinase K as follows: 400 µl Tris buffer, and 400 µl of proteinase K solution (∼200 U/ml) was added and incubated overnight at 37°C. Ethanol (600 µl) was added to denature the enzyme. The mixtures were centrifuged at approximately 1,200 g for 10 min, and the supernatants were stored in amber HPLC vials at −20°C.
HPLC analyses
The extracted samples were analyzed by the Agilent 1100 HPLC system, consisting of injector, binary pump, degasser, column thermostat (set at 30°C), diode array detector (set at 339 nm), fluorescence detector (FLD), set at excitation/emission wavelengths of 346/384 nm and automatic fraction collector. Software used for analyses was Agilent ChemStation version B.02.01-SR2. For the quantification of pyrene and 1-hydroxypyrene in the samples, standard solutions of pyrene, 1-hydroxypyrene, and glucuronide conjugate of 1-hydroxypyrene were prepared. The column used for the quantification was an Agilent Zorbax SB-C18 (3.5 µm 100× 3.0 mm: Agilent Technologies) attached to an HPLC Security Guard Cartridge System (Phenomenex). The eluents used were MilliQ water sonicated before the runs and acetonitrile (ACN). The gradient used was the same as described by Honkanen et al. 16. Briefly, it started at 5% of ACN for 4 min, then it was raised to 50% of ACN in 1 min, held in that condition for 4 min, and finally raised to 100% of ACN in 2 min and held in these conditions for 7 min. The flow rate was 0.5 ml/min.
Series of four injections of known standards on five consecutive days were performed to evaluate the repeatability of the method. The absolute standard deviation measures the percent variability of the peak area during the different runs with the formula absolute standard deviation (%) = 100 · SD/X, where SD is the standard deviation and X is the average. The peak area absolute standard deviation for this method was 9% for pyrene and 8.3% for 1-hydroxypyrene.
Detection of pyrene phase II metabolites
To study possible phase II metabolites of pyrene in L. variegatus, chromatographic separation was carried out on an Agilent Zorbax Eclipse XDB-C8 column (5 µm, 150 × 4.6 mm) (Agilent Technologies). The eluents used were 0.01 M ammonium acetate (pH 5) and ACN. The gradient started at 20% ACN and was raised to 82% in 23 min and then to 90% in 2 min, and finally held there for 2 min. The flow rate was 1 ml/min. Repeatability of this method was evaluated as described for the previous method. The peak area absolute standard deviation was 2.4 to 4.0% for pyrene, 6.0 to 12.5% for 1-hydroxypyrene, and 1.2 to 3.5% for glucuronide conjugate of 1-hydroxypyrene.
First, the retention times (tR) of available standards (pyrene, 1-hydroxypyrene and glucuronide conjugate of 1-hydroxypyrene) were compared with the tR of peaks not present in controls. However, the commercial glucuronide conjugate did not match the expected m/z value in mass spectrometric analyses (liquid chromatography-quadrupole-time-of-flight-mass spectrometry [LC-Q-ToF-MS]), as already pointed out by Beach et al. 17, who suggested that the commercial conjugate is methylated. The correct conjugate was obtained as described in literature 17. Briefly, 0.95 µg glucuronide conjugate was incubated in methanol for 21 h with an excess of potassium hydroxide (KOH). The demethylated conjugate eluted approximately 6 min earlier than the commercial conjugate.
Also, the ultraviolet (UV) absorption spectra of the peaks were surveyed. Although this does not provide conclusive structural information, it adds valuable information about the direct relation of the unknown metabolites to the parent compound 14.
Enzymatic deconjugations
The extract aliquots (17 µl of experiment 1 and 10 µl of experiment 2) were incubated separately with sulfatase, alpha- and beta-glucosidase, beta-glucuronidase (37°C), and lactase (50°C). Briefly, the aliquots were transferred to test tubes, methanol was evaporated with a stream of nitrogen, and 300 µl distilled water was added. Fifty µl enzyme solution (10 U/ml) was added, and the resulting solution was mixed and incubated for 3 h. To stop the reaction and precipitate the added proteins, 300 µl ethanol was added, and the mix was centrifuged for 3 min at approximately 1,200 g. The supernatants were collected and stored in amber HPLC vials at −20°C. The samples were concentrated by evaporating ethanol and analyzed with HPLC as described.
On the basis of the results obtained with each of the enzymes used, further deconjugations were performed to determine the total concentration of 1-hydroxypyrene produced by the worms. Beta glucosidase and sulfatase were used consecutively to deconjugate phase II metabolites. Twenty microliters extract was added to test tubes and the methanol evaporated to dryness under a stream of nitrogen. MilliQ-grade water (300 µl) was added before the addition of 100 µl beta-glucosidase (10 U/ml). The samples were incubated at 37°C for 3 h, and 350 µl ethanol was added to stop the reaction. Samples were mixed and then centrifuged at approximately 1,200 g for 3 min to precipitate proteins. The supernatants were collected, and the same procedure was followed with sulfatase after evaporation of ethanol. The final sample was analyzed with HPLC (100 µl injection volume) to quantify the total 1-hydroxypyrene.
Solid-phase extraction
For the LC-Q-ToF-MS analysis, one sample from experiment 3 and a control sample from experiment 2 were fractioned and concentrated with solid-phase extraction (SPE) by a method modified from literature 17. The original extracts (1,500 µl of the exposed sample and 750 µl of the control) were diluted with 25 ml 10% ACN. The diluted extracts were loaded into Oasis HLB (12 cc, 500 mg; Waters) cartridges, which had been preconditioned with 2 × 4 ml methanol (MeOH) and 1 × 5 ml MilliQ-water. After the sample loading, the cartridges were washed with 2 ml MilliQ-water. The samples were eluted from the cartridges with 5 × 1-ml fractions of 20% ACN, 40% ACN, 60% ACN, 80% ACN, and 100% ACN. The fractionated extracts were evaporated to almost dryness under a stream of nitrogen and diluted to a final volume of 250 µl 0.01 M ammonium acetate in MeOH:ACN:water (38:57:5, v/v/v) for the liquid chromatography-mass spectrometry analysis.
LC-Q-ToF-MS/MS
The chromatographic separation of the pyrene metabolites was performed with a Waters XTerra RP18 analytical column (3.5 µm, 2.1 × 150 mm) equipped with a Waters XBrigde C18 guard column (3.5 µm, 2.1 × 10 mm; Waters). The mobile phase was the same as that used in the study by Beach et al. 17 and consisted of 0.01 M ammonium acetate and 0.01 M ammonium acetate in MeOH:ACN:water (38:57:5, v/v/v). The flow rate was 0.2 ml/min, and the injection volume was either 20 or 40 µl. The gradient started with 10% organic phase (0–2 min) and was then raised from 10 to 38% between 2 and 11 min, from 38 to 56% between 11 and 20 min, from 56 to 100% between 20 and 27 min, and finally was held at 100% of organic phase for 8 min.
The mass determinations were performed with a Bruker electrospray ionization quadrupole-time-of-flight mass analyzer (Q-ToF-MS) (micrOTOF-Q, Bruker Daltonics) operating in the negative ion mode. Nitrogen was used as the nebulizing gas (1.6 bar) and as the drying gas (9.0 L/min, 200°C). The capillary voltage was set to +4.5 kV. The analytes were transferred to the mass analyzer by an Agilent 1200 Series LC system consisting of a binary pump, a vacuum degasser, an autosampler, a thermostated column (30°C), and a UV detector (set at 339 nm) (Agilent Technologies). The data were collected and handled using Bruker Compass DataAnalysis 4.0 software. The scanned mass range was m/z 100 to 700. Pure 1-hydroxypyrene (0.2 µg/ml) was used to optimize the instrumental parameters by direct infusion to Q-ToF-MS.
The collision-induced dissociation experiments coupled with multiple tandem mass spectrometry employed argon as the collision gas. The collision energy, which is dependent on molecular masses, ranged from 20 to 25 eV.
Data fitting and statistical analyses
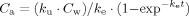 (1)
(1)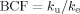 (2)
(2)The estimated parameters and BCF were compared following Bailer et al. 27, and Bonferroni correction was applied to p level (0.05) in multiple comparisons.
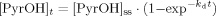 (3)
(3)The concentration data for solvent extracted 1-hydrox-pyrene and total 1-hydroxypyrene were modeled separately with (Eqn. 3). The proposed model has no theoretical basis to represent biotransformation 28, but it estimates the different steady-state concentrations of 1-hydroxypyrene among the three treatments.
The formation rate of nonextractable metabolites (present in tissue residues) was estimated from the slope of linear regression of the pyrene equivalents in tissue residues (µg/g wet wt).
Concentrations of pyrene, 1-hydroxypyrene (solvent extracted and total), and nonextractable metabolites were analyzed using univariate analyses of variance ([ANOVA]; SPSS ver 16.0, SPSS). If significant differences were found, the Games-Howell post hoc test for unequal variances was applied to determine which treatments were different. Untransformed data were used in the statistical analyses, because transformation did not reduce the variability within the treatments. The homogeneity of variances was tested by Levene's test. The p values were considered to be statistically different if ≤0.05.
Seven values of the total 1-hydroxypyrene data to complete a total of three values per timepoint were missing. Because analysis of variance (ANOVA) requires at least three replicates to be performed, a third value was calculated following the method suggested by Zar 29 for calculating missing values in quantitative analyses.
RESULTS
Pyrene uptake and data fitting
Mortality was not observed during the whole exposure. Sorption to glassware accounted for approximately one third of total pyrene during the first 24 h. Average values of pyrene concentration during the entire test in water were used because of this phenomenon. Fitting of the pyrene experimental data in (Eqn. 1) resulted in similar curves and parameters in the three treatments (Fig. 1A and Table 1). No significant differences were found among the pyrene experimental data in L. variegatus tissues belonging to the three treatments (F [2,45] = 0.007, p = 0.993). These results indicate that PBO had no measurable effects on pyrene uptake and bioaccumulation. Furthermore, no significant differences were found in conditional uptake rate coefficients between pairs of treatments.
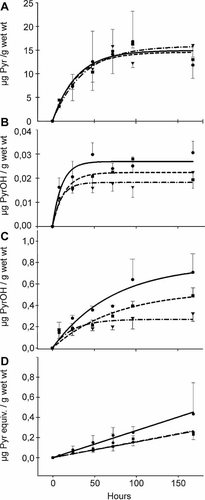
Bioaccumulation of (A) parent pyrene, (B) solvent extracted 1-hydroxypyrene, (C) total 1-hydroxypyrene, and (D) nonextractable metabolites in Lumbriculus variegatus tissues over time (0–168 h). Each panel shows the experimental data and fitted curves of the three different treatments. Continuous, dashed-dotted, and dashed lines represent the fitted curves of treatments with no piperonyl butoxide (NO PBO), PBO 354, and PBO 35.4 µg/L, respectively. The symbols circle, inverted triangle, and square indicate the experimental data (n = 3 to 4 ± standard deviation [SD]) of NO PBO, PBO 354, and PBO 35.4 µg/L treatments, respectively. Note the different scales of the y axis. Pyr = pyrene, PyrOH = 1-hydroxypyrene, Pyr equiv. = pyrene equivalents.
| Pyrene uptake | PyrOH in tissue | Total PyrOH | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | kub (SD) | kec (SD) | Adj. r2 | BCFd | 95% CI | [Pyr]sse | [POH]ss (± SD)e | Adj r2 | [POH]ss (± SD)e | Adj r2 |
| NO PBO | 59.5 (20) | 0.035 (1.5 · 10−2) | 0.52 | 1714.8 | 593.5–4954 | 14.9 | 0.027 (0.001) | 0.62 | 0.78 (0.12) | 0.75 |
| PBO 354 | 48.1 (9.7) | 0.027 (7 · 10−3) | 0.73 | 1763.3 | 919.9–3380 | 15.7 | 0.018 (9 · 10−4) | 0.55 | 0.27 (0.03) | 0.37 |
| PBO 35.4 | 55.6 (11.6) | 0.035 (9 · 10−3) | 0.71 | 1598.6 | 836.5–3055 | 14.5 | 0.022 (0.002) | 0.64 | 0.54 (0.07) | 0.81 |
- a Conditional uptake and elimination clearance coefficients (ku and ke respectively), bioconcentration factors (BCF), and estimated steady states for pyrene ([Pyr]ss), and 1-hydroxypyrene(s) ([POH]ss) in the three treatments. The adjusted coefficient of determination (Adj r2) is shown as measure of goodness of fit for every category. SD = standard deviation; CI = confidence interval.
- b ml/g · h.
- c /h.
- d (ku/ke).
- e µg/g wet wt.
Steady states were attained in all of the treatments (Fig. 1A), but no significant differences were found in the BCF between any treatment pairs (Supplemental Data, Table S1, for all z and p values described in this section).
Biotransformation of pyrene by L. variegatus
A small peak of 1-hydroxypyrene, identified based on its tR, was found in the LC-FLD chromatograms of pyrene-exposed worms in all the experiments (Fig. 2B, peak IV, tR = 16.55 min). The solvent-extracted 1-hydroxypyrene concentration was remarkably small (Fig. 1B), and it increased with time during the first 24 h, thereafter remaining at a steady state. Concentrations of pyrene (Fig. 2B, peak V, tR = 20.9 min) were more than 500 times higher than solvent-extracted 1-hydroxypyrene concentrations at steady state (Figs. 1A and 1B and Table 1).
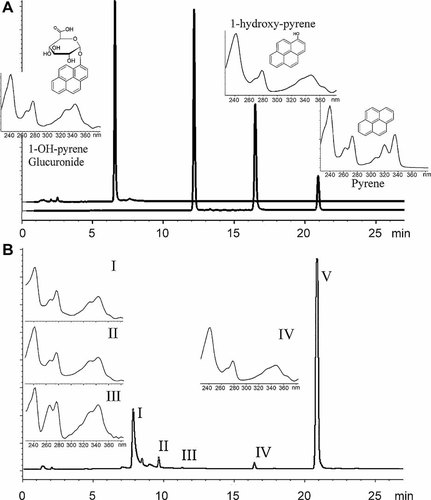
Liquid chromatography fluorescence detector (LC-FLD) chromatograms (Ex/Em = 346/384 nm) of Lumbriculus variegatus tissue extracts and standards compounds. (A) available standards (from right to left: pyrene, 1-hydroxypyrene, methylated glucuronide conjugate of 1-hydroxypyrene, and glucuronide conjugate of 1-hydroxypyrene) and (B) extracts of pyrene-exposed L. variegatus (168 h), showing peaks not present in control (peak I, tR = 8 min; peak II, tR = 9.5; peak III tR = 11.2; peak IV tR = 16.5; peak V tR = 20.9). Additionally, panel A shows ultraviolet (UV) absorption spectra (absorbance = 339 nm) and structures of standards (except methylated glucuronide conjugate) and panel B the UV absorption spectra of peaks I to IV.
Concentrations of solvent-extracted 1-hydroxypyrene (Fig. 1B) were different among treatments (ANOVA F[2,46] = 19.01, p < 0.05), specifically no PBO treatment had statistically higher concentrations than both PBO treatments (Supplemental Data, Table S1).
In addition to 1-hydroxypyrene, three unidentified peaks were found in the LC-FLD chromatograms of pyrene-exposed worms (Fig. 2B, peaks I, II, and III, with tR of 8, 9.5, and 11.2 min., respectively). None of them corresponded to the tR of the glucuronide conjugate of pyrene (Fig. 2A).
If metabolites are considered to be the sum of total 1-hydroxypyrene (solvent-extracted 1-hydroxypyrene plus deconjugated phase II metabolites) plus nonextractable metabolites, they account for only 7% of the total body burden in no PBO treatment at 168 h. Addition of PBO reduced the proportion of metabolites to 4.5 and 3% in treatments with PBO 35.4 and 354 µg/L, respectively.
Enzymatic deconjugations
Single enzymatic deconjugations were performed to elucidate the structure of the unknown metabolites I to III. Incubations of worm extracts with sulfatase from Helix pomatia yielded three peaks: a peak with the same tR as that of 1-hydroxypyrene (an increase of 9 times in peak area) and two peaks with the same tR as peaks II and III (increase of 7.6 and 27 times, respectively; Fig. 3A). Area of peak I decreased approximately 81% after sulfatase treatment alone. However, beta glucosidase treatment resulted in complete conversion of peak I to 1-hydroxypyrene (its peak area increased more than 17 times; Fig. 3B), confirming that peak I is a pyrene derivative molecule. Additionally, a very small peak with a tR of 13.8 min (marked with an asterisk in Fig. 3B) also appeared after beta-glucosidase treatment. Peak II was reduced to 25% of its original area. Because of the low intensity of peak III (Fig. 3B), whether it has decreased could not be confirmed. Similar results to those of the beta-glucosidase deconjugations were found with lactase.
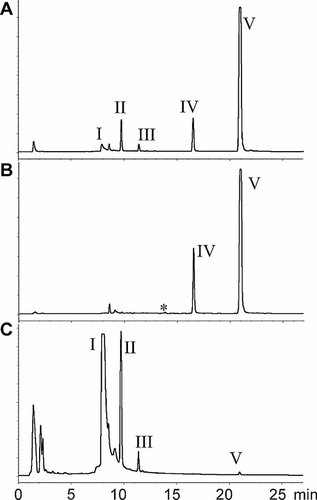
Enzymatic treatments performed with Lumbriculus variegatus tissue extracts. Liquid chromatography fluorescence detector (HPLC-FLD) chromatograms (Ex/Em = 346/384 nm) of pyrene-exposed L. variegatus (experiment 2) extracts after enzymatic incubations. Enzymes used were (A) sulfatase and (B) beta glucosidase. Panel (C) shows the chromatogram of an extract after incubation of tissue residue (experiment 2) with proteinase K. Peaks not present in controls are indicated by same nomenclature as in Figure 2 (I–V).
However, samples treated with alpha-glucosidase and beta-glucuronidase showed no differences as compared with untreated samples (chromatograms not shown), indicating that neither alpha-glucose nor glucuronide conjugates are present.
Effect of deconjugations on 1-hydroxypyrene concentrations
After the action of the single deconjugating enzymes had been tested, beta-glucosidase and sulfatase were used consecutively to liberate and quantify the total 1-hydroxypyrene and to determine the differences among the treatments.
Concentrations of solvent-extracted 1-hydroxypyrene were considerably lower compared with total 1-hydroxypyrene concentrations (Fig. 1B and 1C, respectively).
Total 1-hydroxypyrene concentrations were significantly different among the treatments (ANOVA F [2,36] = 30.59, p < 0.05), and Games-Howell post hoc test revealed that the concentrations in no PBO treatment were significantly higher than those of PBO 354 µg/L treatment (p = 0.003; Supplemental Data, Table S1).
As seen in Figure 1C, addition of PBO caused a reduction in the production of total 1-hydroxypyrene at the end of the treatment. The reduction is inversely proportional to PBO concentration: the more PBO present, the less 1-hydroxypyrene was produced.
Tissue residues
The concentrations of nonextractable pyrene equivalents present in the tissue residues increased with time (Fig. 1D). No statistical differences were found among the three treatments (ANOVA F[2,51] = 1.38, p > 0.05; Supplemental Data, Table S1 for p values). Linear regressions of the experimental data resulted in similar slopes (Supplemental Data, Table S2).
Incubation of the tissue residues from experiment 2 with proteinase K resulted in three peaks (peaks I, II, and III in Fig. 3C) in the LC-FLD chromatograms that had the same tR as the peaks I, II, and III found in the solvent-extracted samples (Fig. 2B). Also, a small peak of pyrene was found (peak V in Fig. 3C).The peaks were absent in the control tissue residues.
UV absorption spectra
Only the pyrene UV spectra was obtained in experiment 1, because of the low intensities of peaks II to V. Samples from experiment 2 were used to acquire the UV spectra from peaks I to III, and spectra from 1-hydroxypyrene (peak IV) was only obtained from samples (experiment 2) after deconjugations with lactase or beta-glucosidase (Fig. 2).
LC-Q-ToF-MS
Several peaks of low intensity were observed in the UV chromatograms of the SPE fractions (experiment 3), but only one of the peaks gave a signal of sufficient intensity for mass spectrometric analyses. The deprotonated molecular ion of the peak was observed at m/z 459.08 (tR = 20.8 min) in both the 20% ACN and 40% ACN fractions, which matched the [M-H]− ion of the glucose-sulfate conjugate of 1-hydroxypyrene (Figs. 4A and B). On fragmentation of the ion at m/z 459.08, a fragment ion with m/z 217.06 was formed, which corresponds to 1-hydroxypyrene and was consequently obtained by the loss of the glucose-sulfate moiety, [M-H-glucose-sulfate]− (Fig. 5). The loss of the glucose-sugar moiety from glucose-sugar conjugates of 1-hydroxypyrene has been also reported in literature 12.
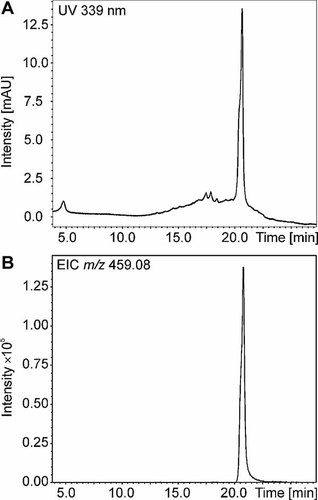
(A) Liquid chromatography-quadrupole-time of flight-mass spectrometry (LC-Q-ToF-MS) ultraviolet (UV); (B) and extracted ion chromatogram (EIC) of m/z 459.08 of the solid-phase extraction (SPE) fraction (20% acetonitrile) from a Lumbriculus variegatus sample exposed to pyrene (20 µg/L) in water for two months (experiment 3).
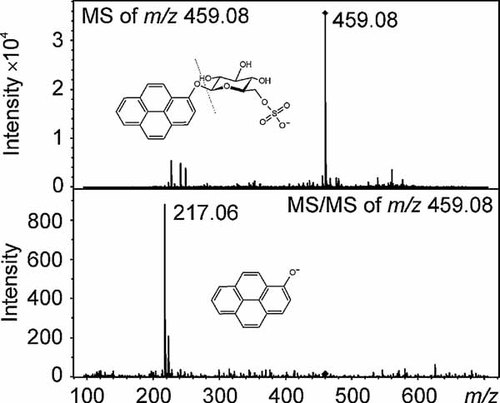
Mass spectrometry (MS) and tandem mass spectrometry of the ion m/z 459.08 recorded by LC-Q-ToF-MS of the solid-phase extraction (SPE) fraction (20% acetonitrile) from a Lumbriculus variegatus sample exposed to 20 µg/L of pyrene in water for two months (experiment 3) and the proposed chemical structure of glucose-sulfate conjugate of 1-hydroxypyrene.
DISCUSSION
The present study supports previous findings that reported biotransformation of PAHs by L. variegatus 21, 22, although its capability is low, because 7% of total body burden accounts for metabolites after 168 h. Co-exposure to PBO lowered biotransformation capability of pyrene by L. variegatus, and only 3% was found as metabolites after 168 h. Because PBO is a CYP inhibitor, a reduction of biotransformation capacity with its co-addition is evidence of the presence of a CYP isoform.
1-Hydroxypyrene and its glucose sulfate conjugate were identified in the true sense (by the use of reference standard compounds or liquid chromatography-mass spectrometry), and the presence of other metabolites was indicated by the deconjugations to release 1-hydroxypyrene. Furthermore, the content of the tissue residue is probably related to phase II metabolites of pyrene bound to proteins.
Uptake of pyrene
The addition of PBO to the exposure media had no effect on the uptake of pyrene (Fig. 1A and Table 1). This is consistent with Lee and Landrum 30, who found no differences in parent pyrene in Hyalella azteca after 8 d in the presence or absence of PBO, although the total body burdens were higher without PBO than with PBO. By contrast, accumulation of parent PAHs was shown to increase with the addition of PBO in Daphnia magna 10 and Palaemonetes pugio 24, which indicates a clear suppression of biotransformation via the CYP system. Without PBO, metabolites form between 75 and 87.5% of total body burden in D. magna after 24 h 10. A possible explanation of the similar body burdens of pyrene found in our three treatments with L. variegatus could be that, under the present conditions, the organism does not have a significant biotransformation rate compared with its capacity for uptake of pyrene.
Biotransformation of pyrene in L. variegatus
The phase I metabolite 1-hydroxypyrene has been found to be a minor (and intermediate) pyrene metabolite of invertebrates in some studies 13, 22; however, it has not been found at all in a short-term study by Verrengia Guerrero et al. 7. Such contradictory findings may be explained by the existence of at least two different species within the L. variegatus complex 31, which may have different biotransformation capabilities, or simply by the different exposure conditions.
Despite clear indications of the ability of L. variegatus to biotransform PAHs, possibly the low concentration of metabolites found in the current study could have been produced by gut or external (water phase) microbes of L. variegatus.
Presence of bacteria in the gut of some Oligochaetes has been reported 32, and it would be logical to find them also in L. variegatus. However, that the microbial gut content of L. variegatus would produce 1-hydroxypyrene is unlikely, because the worms were not fed in the whole experiment (they were fed in experiment 3, but the same peaks were found in the LC-FLD chromatograms), and because bacteria commonly biotransform pyrene through different pathways, yielding other phase I metabolites than 1-hydroxypyrene 33, even resulting in mineralization of pyrene to CO2 11, 33. Furthermore, these microbes possibly could use functional groups from phase II metabolites as a source of energy rather than producing them. For example, glucuronic acid could be used as a carbon source by microbes 11, and the microbial gut content of some fish has glucuronidase activity 34.
External, water-phase microbes also could be responsible for the biotransformation of pyrene. Because the Millipore treatment-based water was changed daily in our experiments, we do not expect microbes to play a significant role in the fate of pyrene in test beakers.
Effects of PBO
Based on the low 1-hydroxypyrene concentrations in invertebrates 13, 22 and assumption of very rapid phase II biotransformation reactions 35, we can postulate that hydroxylation of pyrene to 1-hydroxypyrene may be the limiting reaction in biotransformation of pyrene in invertebrates.
Thus, the sum of the phase II metabolites plus the solvent-extracted 1-hydroxypyrene could be used to estimate the total quantity of 1-hydroxypyrene produced by CYP.
Deconjugations of phase II metabolites yielded concentrations of total 1-hydroxypyrene that make differences among treatments more pronounced (Fig. 1C). Finding more 1-hydroxypyrene in the no-PBO treatment indicates the presence of a CYP in L. variegatus. This statement is also verified by the detection of a glucose-sulfate conjugate of 1-hydroxypyrene in the experiment 3 samples. In agreement with our findings, decreasing levels of metabolites with increasing concentrations of PBO in D. magna have been described in Akkanen and Kukkonen 10, although at PBO concentrations of 500 and 1,000 µg/L, the CYP system was near saturation. Saturation does not mean that biotransformation is completely inhibited, because metabolites were found in the current study and also by Akkanen and Kukkonen 10.
Although no information on CYP at molecular level has been published for L. variegatus, the available genetic data support its existence. An expressed sequence tag library of L. variegatus (D.A. Price, University of Florida, St. Augustine, FL, USA, unpublished data) shows that it possesses several genes belonging to the CYP family. Based on a Blast search (J. Lemmetyinen, University of Eastern Finland, Joensuu, Finland, personal communication) carried out with Blast2GO software 36, the homology to some characterized CYP genes ranges from 45 to 64%. In addition, CYP genes have been isolated and purified in organisms belonging to the same class as L. variegatus: in Lumbricus terrestris (Oligochaeta) 37 and Lumbricus rubellus (Oligochaeta) 38.
Identification of metabolites
1-Hydroxypyrene was identified on the basis of tR and supported by its UV absorption spectra. Commercial standards were not available for the unknown peaks I to III and, although their UV spectra do not give any direct information about their structure, their similarity to pyrene absorption spectra (Fig. 2B) indicates that these are pyrene-derivative molecules.
Because the glucose-sulfate conjugate of 1-hydroxypyrene with m/z of 459 was detected with LC-Q-ToF-MS as the major metabolite of pyrene (Figs. 4 and 5), the identity of peak I in LC-FLD chromatograms as a glucose-sulfate conjugate is proposed.
However, peak I has a pronounced tailing that could be an indication of the co-elution of an interfering peak, possibly formed by a phase II metabolite. Enzymatic deconjugations also sustain this suggestion. Sulfatase breaks sulfate bonds, and pyrene-1-glucoside should be the product of the glucose-sulfate conjugate after incubation with sulfatase. Likely, peaks II or III are candidates for pyrene-1-glucoside, because they both grow after incubation with sulfatase. Although the glucoside conjugate has not been identified by LC-Q-ToF-MS analyses, we propose it as an intermediary step between 1-hydroxypyrene and its glucose-sulfate conjugate (Supplemental Data, Fig. S1). Invertebrates commonly biotransform pyrene using the glucosylation pathway 13-15. Both glucose and glucose sulfate conjugates of 1-hydroxypyrene have been found co-existing in tissues of some invertebrates 12.
Because the 1-hydroxypyrene peak also grew after sulfatase incubation, most likely other metabolites are present in L. variegatus tissue extracts as well, these are assumed to be sulfate conjugates of 1-hydroxypyrene. This is in agreement with the several peaks observed in the LC-UV chromatograms of SPE fractions from experiment 3 (section LC-Q-ToF-MS in Results) and with the co-elution of other molecules in peak I.
Results obtained with beta-glucosidase incubation also support the mass spectrometric identification of glucose-sulfate conjugate, because the main peak yielded is 1-hydroxypyrene.
Tissue residue
A fraction of metabolites in tissue residues that are nonextractable are commonly reported in the literature 19, 39, and the phenomenon could indicate binding of metabolites to macromolecules. By incubation of the remaining pellets with the proteolytic enzyme proteinase K, the nonextractable pyrene-related fraction in L. variegatus is seen to be composed of the same metabolites as were observed after the extractions of tissues with organic solvents (Fig. 3C). Thus, protein binding is a plausible reason for the existence of a bound fraction, which has been found previously in other organisms after exposure to PAHs 39. Interestingly, PAHs are not the only compounds that can produce a nonextractable fraction. Belden et al. 40 reported the formation of bound fraction after exposure of L. variegatus to TNT and suggested the presence of products covalently bound to proteins 40. This fraction accounted for as much as 64% of the total body burden related to TNT.
Although the share of bound fraction in our experiment with pyrene accounts for only 2.7% of the total, it is nevertheless a relevant part of the total body burden of pyrene in L. variegatus, representing approximately 40% of the total metabolites in the treatment with no PBO at 168 h (Fig. 1D). It therefore must be considered when the biotransformation capability of an organism is assessed.
Taking into account that L. variegatus biotransforms PAHs slowly, ignoring the nonextractable fraction in short bioaccumulation tests would not underestimate the whole body burden to a very great extent. However, in standard, 28-d accumulation tests, bound, nonextractable fraction can be significant. Because the biotransformation rate is probably slow, it may not affect the source–organism equilibrium of the parent pyrene. However, the role of the bound fraction should be explored in toxicity tests. The bound fraction may offer another source of PAH metabolites for predators. This transfer and the possible consequences should be investigated with PAHs.
Acknowledgements
This study was funded by a grant from the Maj and Tor Nessling Foundation (M. T. Leppänen). Additional funding was provided by the Academy of Finland (projects 123587 and 214545) and the Finnish graduate school in Environmental Science and Technology (EnSTe; V. Carrasco Navarro). The authors wish to thank Marja Noponen and Julia Keronen for their excellent laboratory assistance. We are also grateful to Anna-Maija Ruotsalainen, Merja Lyytikäinen, Juha Lemmetyinen, and Kimmo Mäenpää. Valery E. Forbes is acknowledged for her valuable comments on the manuscript.
SUPPLEMENTAL DATA
Table S1. Complete statistical pairwise comparisons (p values) of pyrene, solvent extracted, and total 1-hydroxypyrene concentrations during the 168-h experiment.
Table S2. Nonextractable metabolites present in the bound fraction.
Figure S1. Suggested main biotransformation pathway of pyrene in L. variegatus (106KB PDF).