Improving ecological risk assessment in the Mediterranean area: Selection of reference soils and evaluating the influence of soil properties on avoidance and reproduction of two oligochaete species
Abstract
A current challenge in soil ecotoxicology is the use of natural soils as test substrates to increase ecological relevance of data. Despite the existence of six natural reference soils (the Euro-soils), some parallel projects showed that these soils do not accurately represent the diversity of European soils. Particularly, Mediterranean soils are not properly represented. To fill this gap, 12 natural soils from the Mediterranean regions of Alentejo, Portugal; Cataluña, Spain; and Liguria, Italy, were selected and used in reproduction and avoidance tests to evaluate the soil habitat function for earthworms (Eisenia andrei) and enchytraeids (Enchytraeus crypticus). Predictive models on the influence of soil properties on the responses of these organisms were developed using generalized linear models. Results indicate that the selected soils can impact reproduction and avoidance behavior of both Oligochaete species. Reproduction of enchytraeids was affected by different soil properties, but the test validity criteria were fulfilled. The avoidance response of enchytraeids was highly variable, but significant effects of texture and pH were found. Earthworms were more sensitive to soil properties. They did not reproduce successfully in three of the 10 soils, and a positive influence of moisture, fine sand, pH, and organic matter and a negative influence of clay were found. Moreover, they strongly avoided soils with extreme textures. Despite these limitations, most of the selected soils are suitable substrates for ecotoxicological evaluations. Environ. Toxicol. Chem. 2011; 30:1050–1058. © 2011 SETAC
INTRODUCTION
Increasing concern exists regarding protection of soil and all its ecological functions, reflected by the adoption of the Soil Thematic Strategy 1 and a proposal for a Soil Framework Directive 2 (for a recent review on the regulatory decisions for the management of contaminated land in several European Union [EU] countries see Rodrigues et al. 3). Among the several threats to soil quality identified in the Soil Thematic Strategy 1, soil contamination is one of the most relevant, affecting a large proportion of the territory within the EU and causing impairment to ecosystem services delivery.
When evaluating soil contamination, an ecological risk assessment (ERA) can be performed for two different situations: a prospective ERA, performed as part of the registration or notification of individual chemicals (e.g., pesticides) whereby compounds are spiked into artificial soil (AS) 4 and the mixture is tested, and a retrospective ERA, which addresses contaminated field soils where the contaminated soils from the site are collected and the toxicity assessed by exposing test organisms to soil samples (bulk or hole) after minimal manipulation.
In both cases, standardized ecotoxicological tests are applied in order to evaluate the risk of the contaminants 5, 6. To increase the ecological relevance of tests with individual chemicals, it has been recommended to perform them with defined representative natural field soils instead of the Organisation for Economic Co-operation and Development (OECD) artificial soil (http://ec.europa.eu/health/archive/ph_risk/committees/sct/documents/out83_en.pdf; 7-9). The need to define representative natural field soils is even more relevant in site-specific ERA, because often in tests with contaminated soils, a control or reference soil with matching soil properties is missing 10. There are several possibilities for the selection of a control soil 6. Preferably, an uncontaminated soil having properties (e.g., pH, texture) similar to those of the test soil should be used 11, because choosing another type of control soil (e.g., OECD soil) brings a high risk of obtaining biased results 12.
The use of natural soils as a control or reference soil in standard ecotoxicological tests should be based on a careful selection, focusing mainly on two criteria: first, the representativeness for a specific biogeographic region and the degree to which soil physicochemical characteristics match those of soils being assessed (e.g., texture, pH, C/N ratio, or organic matter content 9, 13, 14); second, the acceptability of the selected reference soil to support the performance of the test organisms (e.g., survival, growth, and reproduction), because some of the pedological characteristics of soils might act as stress factors for the animals 15, 16, thus confounding or influencing the test results 10. Guidance must be supplied for the use of natural soils as reference control soils in the existing guidelines, and this guidance should include criteria for the selection of acceptable reference control soils 17, 18.
The selection and characterization of natural reference soils has been undertaken in several initiatives (for a recent review see Kördel et al. 9). For example, the Euro-Soils Project aimed to obtain a set of reference soils, representing the most abundant soils within the European Union. The result of this project was the collection, pedological description, and chemical characterization of seven Euro-soils 14. However, other projects such as the Lufa-Speyer Soil (http://www.lufa-speyer.de/soil_eng.html), the RefeSols 19, and the Nordic Reference Soils 13 show that the seven Euro-soils do not represent the diversity of soils in Europe. Moreover, a reference soil taken from one specific site (as are the Euro-soils) is a finite resource. To overcome this problem, Römbke and Amorim 7 proposed that all soils having properties (texture, pH, organic matter, and C/N ratio) similar to those of the Euro-soils could be designated as SIM-soils and used as reference soils.
Another approach that can be used to address this issue is to develop predictive models that incorporate the influence of soil properties 20, 21. Thus, when analyzing site-specific toxicological data, in the absence of matching control soils, the application of these models would allow a more accurate interpretation of the test results, by separating the soil effect from the contaminant effect. In Europe, much work has been done on predicting fate of chemicals 22 and less on modeling the influence of soil properties 23-25.
The Mediterranean region, which is known to have extremely variable soils 26, is poorly represented in terms of the availability of representative reference soils (only one Euro-soil). To improve the accuracy and reduce uncertainty associated with ERAs, the suitability of 12 Mediterranean soils was assessed using two Oligochaete species. The objectives of the present study were to evaluate the habitat function of the selected soils, i.e., their ability to maintain viable populations of two Oligochaete species (Eisenia andrei and Enchytraeus crypticus), to evaluate the influence of soil properties on performance of these species by measuring ecologically relevant endpoints such as survival, reproduction, and avoidance behavior, and to develop predictive models using general linear model (GLM) tools that integrate the influence of soil properties with the biological responses.
Ultimately, the results of the present study will be integrated with data from more test species and tests with reference chemicals (S. Chelinho, personal communication; X. Domene, personal communication). The analyses of the larger data set should culminate in a better understanding of which soils can serve as acceptable reference soils for ecotoxicological effects assessment within the Mediterranean area.
MATERIALS AND METHODS
Test soils
The soils for the present study were initially selected using soil and land-use maps from the three countries (Portugal, Spain, and Italy; http://hidra1.icc.cat/mapasols/sols/inici.jsp; 27-29). Soils were selected to represent agricultural lands and a wide range of pedological properties. Soils were collected from sites where no pesticides or fertilizers had been applied for more than five years for the Italian and Portuguese soils and at least one year for the Spanish soils.
Twelve representative soils were collected including four (Pz, Br, Luv, and Lit) from the Alentejo region (southern Portugal), five soils (Gan, Gra, Por, Pra, and Riu) from the Cataluña region (northeastern Spain), and another three soils (It 2, It 3, and It 4) from the Liguria region (northwestern Italy). Their main pedological properties are listed in Table 1. The soils had properties that covered a wide range (e.g., texture sandy to clay loam, soil pH 4.2–7.7, organic matter content 0.6–4.8%; Table 1).
| Soil | pH (KCl) | OM (%) | C_Sand (%) | F_Sand (%) | Silt (%) | Clay (%) | Ntot (%) | C/N | MTb % | WHC (%) | CEC (cmol/kg) | Geographic coordinates (Lat; Long) | Soil use | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Portugal | Pz | 4.2 | 2.2 | 70 | 21 | 6 | 3 | 0.07 | 18.3 | 14 | 30.7 | 4.0 | 38.89307; −8.879142 | Pasture |
| Br | 6.7 | 2.5 | 10 | 18 | 23 | 49 | 0.11 | 13.2 | — | 61.1 | 26.8 | 37.986963; −7.921582 | Fallow | |
| Luv | 4.4 | 2.0 | 30 | 38 | 20 | 12 | 0.08 | 14.5 | 16 | 32.1 | 9.9 | 37.737064; −8.031483 | Pasture | |
| Lit | 4.6 | 4.2 | 42 | 25 | 21 | 12 | 0.16 | 15.3 | 17 | 42.4 | 8.6 | 37.815480; −7.671375 | Fallow | |
| Italy | It 2 | 7.4 | 4.8 | 23 | 16 | 45 | 16 | 0.25 | 11.3 | 26 | 39.8 | 18.6 | Municipality of Ventimiglia | Agriculture |
| It 3 | 7.3 | 2.8 | 23 | 28 | 36 | 13 | 0.16 | 10.1 | 22 | 43.3 | 18.4 | Fallow | ||
| It 4 | 7.3 | 2.8 | 20 | 29 | 35 | 16 | 0.13 | 12.5 | 24 | 47.4 | 18.8 | Fallow | ||
| Spain | Gan | 7.7 | 0.6 | 2 | 74 | 12 | 12 | 0.04 | 8.8 | — | 37.6 | 6.0 | 41.096235; 0.357567 | Agriculture |
| Gra | 7.6 | 1.7 | 2 | 26 | 49 | 23 | 0.11 | 9.0 | — | 49.8 | 14.2 | 41.391080; 0.619381 | Fallow | |
| Por | 6.6 | 4.3 | 46 | 22 | 20 | 12 | 0.22 | 11.3 | 17 | 38.8 | 18.6 | 41.202077; 0.868230 | Agriculture | |
| Pra | 4.5 | 2.2 | 42 | 35 | 12 | 11 | 0.12 | 10.7 | 16 | 39.4 | 11.2 | 41.343073; 1.004402 | Agriculture | |
| Riu | 6.7 | 1.9 | 24 | 35 | 14 | 27 | 0.13 | 8.5 | 18 | 45.0 | 14.9 | 41.870314; 2.796961 | Agriculture | |
| OECD-PT | 6.1 | 5.8 | 9 | 77 | 3 | 11 | 0.03 | 112.1 | — | 70.0 | 7.0 | — | — | |
| OECD-GM | 6.0 | 8.1 | 75 | 17 | 8 | 0.07 | 67.1 | — | 63.1 | 8.9 | — | — | — |
- a Codes: OM = organic matter; C_sand = coarse sand (0.2–2 mm); F_sand = fine sand (0.02–0.2 mm); C/N = organic carbon/total nitrogen; MT = moisture; WHC = water-holding capacity; CEC = cation exchange capacity; Lat = latitude; Long = longitude; for OECD-PT and OECD-GM see Materials and Methods.
- b Values from Eisenia andrei reproduction tests.
For comparison purposes, two batches of standard Artificial OECD Soil 4 were used as control; one batch was used only in the earthworm reproduction tests performed at ECT Oekotoxikologie, Germany (GM), and hereafter designated as OECD-GM; for all other tests, which were performed in Portugal (PT), a different batch, designated as OECD-PT, was used. All soils were analyzed by Direção Regional de Agricultura de Entre-Douro e Minho (DRAEDM, Porto, Portugal), except for the OECD-GM batch. The soil properties analyzed were pH 30, organic matter 31, texture 32, total N (Kjeldhal method 33), water-holding capacity (WHC) 30, moisture (expressed by the difference between wet and dry weights after drying for at least 12 h at 105°C), and cation exchange capacity (CEC; ammonium acetate method 34). Properties from the OECD-GM batch were determined at the Free University of Berlin (Germany), where the same International Organization for Standardization (ISO) methods were used 35.
Test organisms
Both test species were obtained from laboratory cultures. The cultures of the earthworm E. andrei were maintained as described by Natal-da-Luz et al. 10 (organisms used in the avoidance tests) and Römbke et al. 8 (organisms used in the reproduction tests). The enchytraeid E. crypticus 36 has been cultured at ECT Oekotoxikologie (Germany) since September 2003 (culturing conditions were similar to those described for Enchytraeus albidus by Römbke and Moser 37), and, in March, 2005, the enchytraeids were brought to IMAR (University of Coimbra, Portugal), where they were maintained in Lufa 2.2 soil at 20°C ± 2°C with a photoperiod of 16:8 h (light:dark) and fed once a week with finely ground rolled oats.
Experimental procedure
All soils were sieved (5-mm mesh) and defaunated through freezing–thawing cycles. The moisture was adjusted to approximately 40 to 60% of the maximum WHC with deionized water, except for the soils with higher clay content (Br, Gra, and Riu). In these cases, the value was reduced to approximately 35 to 45% to provide a crumbly, moist structure, thus avoiding soil soaking.
Avoidance tests
Two test types were setup for all test organisms: the OECD soil versus natural soil, in which the standard artificial OECD soil was compared with each natural soil, and natural soil A versus natural soil B, in which all possible combinations of natural soils, within the same country, were tested. Dual-control tests (the same soil type on both sections of the test box) were also performed to check for the random distribution of the animals 38. For both test species, results revealed a homogeneous distribution of the test organisms on both sections (data not shown).
For E. andrei, test procedures were based on ISO Guideline 17512–1 38. For E. crypticus, the test procedure was based on the studies performed by Amorim et al. 39, with the exception that smaller cylindrical plastic vessels (diameter 6 cm, height 4 cm) were used and 20 g (dry wt) of soil was placed on each side of the replicates. For both organisms, the tests ran for 48 h at 20°C ± 2°C with a 16:8 h light:dark photoperiod. Five replicates per combination were set up. Both soil pH and soil moisture were measured at the beginning and at the end of the tests.
Reproduction tests
Results from the avoidance tests with E. andrei were taken into account before performing the reproduction tests. It was assumed that the most avoided soils (those that were always avoided when tested) did not fulfill the ecological requirements for the test species. For this reason, the Br (Portugal), Gan, and Gra (Spain) soils were excluded. The tests were performed with the remaining nine natural soils plus OECD-GM soil, following ISO Guideline 11268–2 40. The E. crypticus reproduction tests were based on ISO Guideline 16387 30 using the 12 natural test soils and the standard artificial OECD-PT soil as a control. Because of the smaller size and shorter reproductive cycle of this species, the test duration was reduced to 28 d, and the adults were maintained in the vessels for this period of time. At the end of the test, the number of enchytraeids was assessed after fixation with alcohol (80%), staining with Bengal rose, and wet sieving (103-µm mesh). All tests were conducted at 20°C ± 2°C with a 16:8 h light:dark photoperiod. Also, for these tests, soil pH and soil moisture were measured at the beginning and at the end of the exposure period.
Statistical analysis
Avoidance tests
The percentage of avoidance of the test organisms at each combination tested was calculated by the expression A = ([C − T]/N) · 100, where A means percentage avoidance, C means number of individuals in the control soil (soil A), T means number of individuals in the test soil (soil B), and N means total number of individuals 38. For both test species, the statistical significance of the avoidance response at each combination was evaluated by Fisher's exact test 41. With this test, comparisons between the observed distribution and a theoretical distribution (assuming a no-avoidance situation) of the organisms in the test soil compartment are performed 10. What is tested is an avoidance response toward the test soil, and the null hypothesis assumes a situation of no avoidance (i.e., the organisms were distributed randomly within the test unit). Statistical analysis was performed only taking into account surviving individuals; the mortality was low.
To develop predictive models for the influence of soil properties on avoidance behavior, GLMs were applied in Brodgar 2.5.6 software (www.brodgar.com). Previous to GLM analysis, data exploration highlighted some outliers that were removed from the data set: four for tests with earthworms (from a total of 95 observations; 19 soil combinations times five replicates) and seven for tests with enchytraeids (from a total of 92 observations; 19 soil combinations times five replicates, except for three combinations of Italian soils for which four replicates were used). Data exploration also included log transformation of soil parameters, elimination of collinear variables by removing those with high variance inflation factors, and looking for possible interactions between variables.
Combinations of OECD soil versus natural soil were excluded from this analysis because the first is an artificial soil, quite different from any of the natural soils tested. A binomial or quasibinomial (whenever overdispersion exceeded 1.5) model was adopted, using the logit link function and the proportion of individuals in the test soil (calculated using the formula T/N, i.e, the number of individuals in the test soil divided by the total number of individuals) as the response variable. The total number of individuals was used to weight the data (i.e., as weight variable) in the analysis. It was not possible to perform all possible combinations for the 12 soils, so a new approach involving normalization of soil parameters was adopted for the explanatory variables (the soil parameters). For each combination tested (control soil vs. test soil), a quotient for each soil parameter (test soil parameter/control soil parameter) was calculated. A value of Q < 1 indicates higher values of the soil parameter in the control soil, a Q = 1 indicates no differences, and a Q > 1 indicates higher values of the soil parameter in the test soil. A matrix containing all combinations tested and normalized values (Q quotients), expressing differences between the soils, was used as explanatory variables in the models.
Reproduction tests
Exploration of the reproduction data was the same as that for the avoidance data, and some outliers were removed from the data set: two for earthworms (from a total of 36 observations; nine tested soils times four replicates) and one for enchytraeids (from a total of 48 observations; 12 tested soils times four replicates). A one-way analysis of variance (ANOVA) followed by post hoc comparisons (Dunnett's test) was performed to compare the reproduction levels in OECD artificial soil (control) with those for the natural soils. Statistica 7.0 software (StatSoft, 2004) was used. To evaluate the role of soil properties in influencing the reproductive output of both species, GLMs were also applied to the reproduction data, using a quasi-Poisson model with the log link function. The number of juveniles was used as the response variable and the individual soil parameters as explanatory variables.
RESULTS AND DISCUSSION
Avoidance tests: Earthworms
In the present study, Eisenia andrei mortality rates were, on average, 0.1 ± 0.5%. Mediterranean soils (Med), when combined with OECD soil, were significantly avoided in seven of the 12 combinations tested (Fig. 1). This could be related to the preference of E. andrei for substrates with high organic matter content 42. A similar preference for a high OM content was also reported by Natal-da-Luz et al. 10 when comparing the avoidance response of E. andrei in several modified OECD soils.
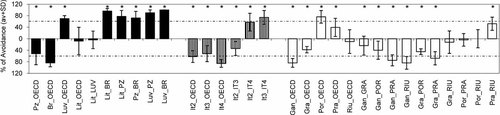
Avoidance response by Eisenia andrei within three groups of Mediterranean soils from Portugal (black bars), Italy (striped bars), and Spain (white bars). Results are expressed as average values (with standard deviation [SD]). The vertical orientation of the bars indicates which soil has been avoided in each combination: e.g., in Pz_OECD combination, Pz was avoided, whereas, in Luv_OECD, OECD was avoided. The dashed line indicates the limit value defined by International Organization for Standardization guideline (2007) for limited habitat function (more than 80% of worms in the test soil, i.e., more than 60% of avoidance). Asterisk indicates a significant avoidance response toward the test soil (Fisher's exact test, p < 0.05). OECD = Organisation for Economic Co-operation and Development.
However, an avoidance response toward the OECD soil was observed in the combinations in which Luv, Por, and Pra soils were used (Fig. 1), indicating that perhaps these natural soils were better able to fulfill the ecological requirements of this species. In Lit and Riu soils, the number of organisms found was nearly the same as in artificial soil, and no statistically significant differences were found (Fig. 1).
When comparing the different natural soils within the same country, earthworms showed a statistically significant avoidance behavior in 15 of the 19 combinations tested (Fig. 1). Within the Portuguese soils, Br was significantly avoided in all comparisons tested; Pz was always avoided except when combined with Br. In opposition, Lit and Luv were not avoided in any combination. Statistically, differences were determined for all comparisons between Italian soils, with It 4 and It 2 being the most frequently avoided. In the Spanish soils, Gan was the most avoided, followed by Gra. Soils Pra and Por were the least avoided, and Riu was significantly avoided only when combined with Pra (Fig. 1).
Referring to the definition given in the ISO guideline 38, the habitat function of Br soils is clearly limited, because more than 80% of the earthworms were found in the control soil (i.e., more than 60% avoided the test soil) in all combinations in which this soil was tested. The habitat function also seems to be diminished in Gan soil; an avoidance percentage of more than 60% was observed in three of five combinations (Fig. 1).
Influence of soil properties on avoidance behavior
With the application of GLMs to the earthworm avoidance data, a statistically significant model, explaining 70.1% of data variability, was obtained (Table 2). The model indicates that the proportion of individuals in the test soil seems to increase (the test soil is less avoided), when the ratio (Q) for sand and silt increases (the test soil has higher sand and silt percentages compared with the control soil) and decreases for pH (the test soil has lower pH than the control).
| Test | Species | Regression models | V | OD | N | Q | p |
|---|---|---|---|---|---|---|---|
| Avoidance | E. andrei | Prop = (EXP(1.459 + 2.069 · logC_S + 4.040 · logF_S + 3.643 · logST − 1.951 · pH)/(1 + EXP(1.459 + 2.069 · logC_S + 4.040 · logF_S + 3.643 · logST − 1.951 · pH))) | 70.1 | 1.61 | 91 | LogC_S | < 0.001 |
| LogF_S | < 0.001 | ||||||
| LogST | < 0.001 | ||||||
| pH | 0.003 | ||||||
| E. crypticus | Prop = (EXP(1.656 + 0.136 · C_S − 1.457 · LogST − 0.979 · pH − 0.252 · (C_S · pH))/(1 + EXP(1.656 + 0.136 · C_S − 1.457 · LogST − 0.979 · pH − 0.252 · (C_S · pH))) | 45.3 | 0.21 | 85 | C_S | 0.008 | |
| LogST | < 0.001 | ||||||
| pH | 0.041 | ||||||
| C_S · pH | < 0.001 | ||||||
| Reproduction | E. andrei | No. Juv = EXP(−12.949 + 0.143 · MT + 0.328 · F_S − 1.963 · LogCY + 0.326 · pH + 1.690 · OM) | 81.8 | 5.81 | 34 | MT | < 0.001 |
| F_S | < 0.001 | ||||||
| LogCY | 0.006 | ||||||
| pH | 0.009 | ||||||
| OM | < 0.001 |
- a In avoidance models, for each soil parameter, a quotient test soil/control soil (Q) was used: for example, a positive coefficient for coarse sand (C_S) means that, when keeping all the other variables constant, an increase in this parameter ratio will increase the proportion of individuals in the test soil (Prop); i.e., the test soil will be avoided less. Codes: Prop = proportion of individuals in the test soil; No. Juv = number of juveniles; OD = overdispersion; V = percentage of variance explained; Q = quotient of soil properties; N = Nr. of observations; MT = moisture; C_S = coarse sand; F_S = fine sand; ST = silt; CY = clay; OM = organic matter; pH (KCl). Ranges: MT = 13.3–28.1; C_S = 1.5–69.8; F_S = 15.7–74.5; ST = 5.8–48.5; CY = 3.2–48.5; OM = 0.6–4.8; pH = 4.2–7.7.
The inclusion of three texture-related parameters in the model (coarse and fine sand plus silt; Table 2) with positive influence on the proportion of individuals on the test soil suggests that finely textured soils can be strongly avoided by E. andrei. This was true for Br, which had the highest clay content and was the soil that earthworms avoided most (Table 1 and Fig. 1). Furthermore, this behavior is supported by Natal-da-Luz et al. 10, who found avoidance reactions toward a finely textured (∼50% clay) modified OECD Soil by this earthworm species. The strong avoidance toward Pz may be related to its extreme texture (only 6% silt and 91% sand; Table 1 and Fig. 1).
In the present study, when combining slightly acidic soils with neutral or slightly alkaline soils, earthworms generally avoided the soil with highest pH (Table 1 and Fig. 1). Among Spanish soils, a strong avoidance response toward Gan and Gra, the most alkaline soils, occurred. The same was observed with the Portuguese Br soil (Table 1 and Fig. 1). However, the avoidance responses toward these alkaline soils by earthworms should be interpreted carefully. For the Br soil, the high amount of clay could explain the strong avoidance response by the earthworms, whereas, for Gan and Gra soils, their low organic content might have been responsible for the observed avoidance response. In a similar study, in which six natural soils plus OECD soil were tested comparatively, Hund-Rinke and Wiechering 43 did not find any particular soil property influencing the avoidance response of Eisenia fetida. However, direct comparisons with the present study are not possible because, in the paper cited 43, the data were presented in terms of percentage of worms in one of the sides of the test box, and no statistical evaluations were performed. Even so, it is possible to conclude that the most avoided soil was a forest acid soil (pH 4.1 43). Despite the wide range of pH tolerance of E. andrei and E. fetida (from pH 4 to pH 9) 15, strongly acid soils (pH lower than 4) are not suitable test substrates for these species 8.
Despite the possible influence of the organic matter content suggested by the results obtained in the combinations of natural soils with OECD soil (Table 1 and Fig. 1), this parameter did not appear as significant in the GLM models (Table 2). This was due to the noninclusion of the combinations with OECD soil in the analysis and also to the fact that, for all soils of the three countries, the range of values of OM was too narrow (Table 1). Probably, for Mediterranean soils, characterized by a low level of organic matter (in this region, ∼74% of the topsoil [30 cm] contains less than 3.4% http://eusoils.jrc.ec.europa.eu/esdb_archive/eusoils_docs/esb_rr/n15_OMsouthEurope.pdf), the influence of soil texture is more pronounced than that of organic matter.
Our results suggest that the data obtained from toxicity evaluations using avoidance tests and species that are sensitive to soil properties, such as E. andrei, should be carefully interpreted, because the response can be masked by the different properties of the soils being tested (e.g., strongly acidic pH, extreme percentages of sand, silt, or clay, and low organic matter). This is particularly relevant when assessing contaminated sites and emphasizes the importance of working with matching control soils.
Avoidance tests: Enchytraeids
The mortality rates were on average 1.4 ± 3.0%. Compared with the data for earthworms, the avoidance behavior of E. crypticus showed a greater variability (Figs. 1 and 2). For the majority of combinations with natural soils, either artificial soil was avoided (when combined with Por, Pra, Riu, It 2, and It 3 soils; Fig. 2) or no differences were detected (for the combinations OECD_Pz, OECD_Luv, OECD_Lit, OECD_It 4, and OECD_Gra; Fig. 2). Artificial soil was preferred by the enchytraeids only when the choice was between artificial soil and either Br or Gan soils. Our results are in agreement with Amorim et al. 39, who conducted a similar study with E. albidus using two standard soils (OECD and Lufa 2.2). In this case, the artificial soil was also avoided more when combined with field soils in comparison with the combination of field soils with Lufa 2.2, a natural standard soil. In the comparisons between Portuguese soils, Pz was the least avoided soil, whereas, in opposition, Lit was the most frequently avoided soil (Fig. 2). In all combinations containing Luv and Br soils, the enchytraeids avoidance pattern is similar: the soils were avoided when combined with Pz and preferred when combined with Lit (Fig. 2). Among the soils from Italy, there were no clear preference or avoidance patterns. Among Spanish soils, Por was the most frequently avoided by the enchytraeids (in three of five combinations) and Gra was the least avoided (in one of five combinations; Fig. 2); Pra was never avoided in the five combinations tested (Fig. 2).
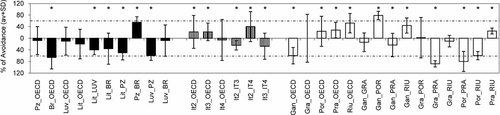
Avoidance response by Enchytraeus crypticus within three groups of Mediterranean soils from Portugal (black bars), Italy (striped bars), and Spain (white bars). Results are expressed as average values (with standard deviation [SD]). The vertical orientation of the bars indicates which soil has been avoided in each combination: e.g., in Br_OECD combination, Br was avoided, whereas, in Pra_RIU, RIU was avoided. The dashed line indicates the limit value defined by International Organization for Standardization earthworm guideline (2007) for limited habitat function (more than 80% of worms in the test soil, i.e, more than 60% of avoidance). Asterisk indicates a significant avoidance response toward the test soil (Fisher's exact test, p < 0.05). OECD = Organisation for Economic Co-operation and Development.
Influence of soil properties on avoidance behavior
The GLM developed for E. crypticus avoidance behavior explained less than 50% of the total variability (Table 2) and showed a statistically significant influence of texture and pH. In this case, and following the same line of thinking previously explained for earthworms, an increase in coarse sand Q ratios and a decrease in pH and silt Q ratios will induce a higher proportion of individuals in the test soil (lower avoidance). In the present study, the soils that were not avoided by enchytraeids (Pz and Pra) have pH slightly above 4.0 (Table 1 and Fig. 2). Most enchytraeid species prefer sand to loamy soils with pH lower than 6.5 44. Indeed, according to Jänsch et al. 15, E. crypticus prefer slightly acid soils, with an optimum pH of 6.0, avoiding only soils with a pH < 4.0. However, the ecological requirements of this species are still not entirely known, because it has so far been found only in a compost plant 36. The reasons for the strong avoidance of Por and Lit soils are not clear. These two soils are similar in terms of organic matter content and texture, but Lit has a lower pH (4.6 and 6.6, respectively, for Lit and Por; Table 1); therefore, the opposite response was expected.
In the GLM model, a significant interaction between coarse sand and pH ratios was found to have a negative impact on the behavior of enchytraeids. Test soils with higher pH tend to have a lower percentage of coarse sand, so the proportion of organisms in the test soil tends to be lower; i.e., a greater avoidance response toward the test soil tends to occur.
Recent efforts have been made to clarify the effects of soil properties on the avoidance response of these organisms. Amorim et al. 45 compared the avoidance and reproduction of E. albidus in OECD artificial soils with modified pH, OM content, and clay levels (pH 5.0, 5% organic matter, 70% clay) relative to the standard OECD artificial soil (pH of 6.0 ± 0.5, 10% organic matter, and 20% clay). They found that enchytraeids would avoid the 70% clay OECD, followed by the 5% OM OECD and the 5.0 pH OECD. Our results are partially in agreement with these findings, in that E. crypticus seemed to avoid fine textures (Table 1 and Fig. 2). However, in the present study, high pH values were also avoided (Table 1 and Fig. 2); this may be due to the different optimum pH values for the two species (E. albidus does not tolerate soils with pH below 4.8, whereas E. crypticus seems to tolerate a broader range of pH values, from 3.6 to 7.7 15).
The high variability found in the present study did not illuminate clearly the relationships between enchytraeids and the physical and chemical characteristics of the different soil types. Also, when testing the avoidance behavior of E. albidus toward several chemicals 39, 46, a highly variable response was also observed, and, at least for some chemicals, the organisms were not able to detect and avoid high concentrations. Thus, before standardizing the enchytraeid avoidance test (so it can be used as an early screening test for soil ecotoxicological evaluations), more studies including more test species, soils, and chemical classes are needed to clarify better whether the high variable response and the lack of sensitivity for some chemicals are species or family specific.
Reproduction tests: Earthworms
Eisenia andrei adults were able to survive in all tested soils. In contrast, a soil effect was observed for reproduction, in that the average number of juveniles varied by a factor of 8 (not considering Pz soil, in which only five juveniles were found in total; Fig. 3). Moreover, for only seven of the 10 soils tested, the ISO guideline validity criteria for reproduction 40 were achieved: in Pz and Riu the average number of juveniles was below 30, and in Lit the coefficient of variation was above 30%. The largest number of earthworm juveniles (215) was produced in the OECD artificial soil (Fig. 3). In all natural soils, the reproduction levels were significantly lower than in the OECD soil (p < 0.05, one-way ANOVA and Dunnett's test; Fig. 3). Nevertheless, among the tested soils, earthworms produced relatively high numbers of offspring in the Luv and It 4 soils (99 and 126 juveniles per replicate, respectively; Fig. 3).
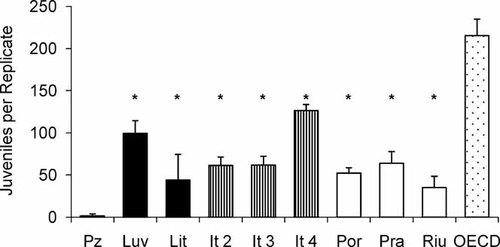
Reproduction of Eisenia andrei (average and standard deviation) in three groups of Mediterranean soils from Portugal (black bars), Italy (striped bars), and Spain (white bars) plus the Organisation for Economic Co-operation and Development (OECD) artificial soils (stippled bar). Asterisk indicates a statistically significant difference from OECD soil (p < 0.05; one-way analysis of variance followed by Dunnett's test).
The reproduction results obtained supported the avoidance behavior observed in Pz and Luv soils, insofar as the first soil was strongly avoided (and almost no juveniles were found; Figs. 1 and 3) and the second one was preferred in three of four combinations tested (and produced the third highest number of juveniles from all soils; Figs. 1 and 3).
Influence of soil properties on the reproduction of earthworms
The quasi-Poisson model developed with earthworm data (Table 2) explained 81.8% of the total variability and indicated a significant positive effect of moisture, fine sand, pH, and organic matter and a negative effect of clay on the number of juveniles produced. Thus, higher reproduction levels seem to be favored in moist soils, combining slightly alkaline pH with high organic matter and fine sand content (and lower clay content; Table 2). The positive influence of moisture on fecundity has been reported by several authors for E. andrei and E. fetida (a closely related species) 47-49. A higher number of juveniles was associated with pH values ranging from 6.6 to 7.4 (respectively for Por and It 2 soils; Tables 1 and 2), but two important exceptions were noted: Luv and Pra soils were acidic soils in which high reproduction levels were observed (Fig. 3 and Tables 1 and 2). Most lumbricid earthworms are known to be absent from soils with a pH < 3.5 and to be scarce in soils with pH < 4.5 50, although some species are able to reproduce in semiacidic soils 51. Van Gestel et al. 47 reported a significantly lower number of juveniles per worm per week at pH values of 4, 6.5, 7.5, and 8 and an optimum cocoon production of E. andrei at pH 5 to 6 in OECD artificial soil. In a study with nine natural soils (representative for temperate regions), E. andrei reproduced successfully in soils with pH values ranging from 3.8 to 7.4 8. In Luv soil, with pH and moisture levels similar to those of the Pz soil, reproduction levels were much higher. Probably, the interaction of these two factors plus the sandy texture, highest sand content of all tested soils, resulted in almost no earthworm reproduction in Pz soil. Some studies point out that abundance and biomass of earthworms decrease with increasing sand content 52, 53. However, in the present study, a positive effect of the fine sand content was found (Table 2). This probably was due to the fact that the highest reproduction levels (Fig. 3) were observed in soils with the highest fine sand content (38% for Luv and 29% for It 4; Table 1). However, extrapolations beyond the relatively small range of fine sand values tested (16% in It 2 and 38% in Luv) cannot be undertaken.
Despite the reduced range of organic matter covered by the test soils included in the regression model (1.9 for Riu and 4.8 for It 2), a positive effect was found for this parameter (Table 2). This was expected, because this species, commonly known as a compost worm, can be found in clusters of dead organic matter such as cattle dung and compost piles 15. In fact, the reproduction levels obtained in OECD-GM soil (with 8.1% OM) were clearly distinct from those for the Mediterranean soils (Fig. 3).
Reproduction tests: Enchytraeids
Enchytraeus crypticus were able to reproduce in all the test soils, and the validity criteria of the ISO guideline 30 were fulfilled. However, adult survival could not be accurately assessed in several soils (especially those with higher pH), because it was not possible to distinguish adults from juveniles at the end of the test. The number of enchytraeids found varied between 382 (in Por soil) and 1,200 (in Br soil; Fig. 4). In all natural soils except for Br, the reproduction was lower than in the OECD artificial soil (Fig. 4). With the exception of Luv, Lit, and Gan, this difference was significant (p < 0.05, one-way ANOVA and Dunnett's test; Fig. 4).
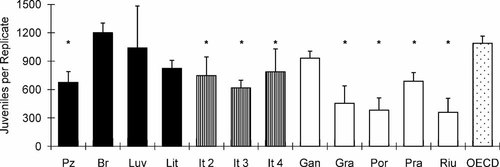
Reproduction of Enchytraeus crypticus (average and standard deviation) in three groups of Mediterranean soils from Portugal (black bars), Italy (striped bars), and Spain (white bars) plus the Organisation for Economic Co-operation and Development (OECD) artificial soil (stippled bar). Asterisk indicates a statistically significant difference from OECD soil (p < 0.05; one-way analysis of variance followed by Dunnett's test).
Influence of soil properties on the reproduction of enchytraeids
No statistically significant model could be developed for enchytraeid reproduction, because the variability explained was very low (20%; data not shown). Despite this, the differences in the reproductive output of these organisms in the tested soils indicate a possible effect of soil properties (Fig. 4). Kuperman et al. 17 investigated the reproduction of E. crypticus in eight North American soils (five natural and three composite soils) and arrived at the same conclusions. In a similar study performed by Amorim et al. 16, the reproductive performance of E. albidus and Enchytraeus luxuriosus was assessed in 17 natural soils. Results obtained by these authors varied between 0 to 90 and 0 to 60 juveniles per test vessel, respectively, for E. albidus and E. luxuriosus. Significant effects of pH on survival and reproduction of both species were found. The results obtained in the present study indicate the higher reproduction rate and broader tolerance of E. crypticus for different soil properties (compared with E. albidus) referred to by several authors 15, 36, 54.
Highlights for risk assessment schemes
Overall, our results show that, in risk assessment schemes (particularly in retrospective ERA), the tolerance of the test species to the local (control and contaminated) soils must be taken into account when defining a test battery, in order to decrease the possibility of biased results. Alternatives may include the use of native species, adapted to the local soil properties, but with presumably reduced possibilities of being successfully cultured and tested in a laboratory context. Information from the literature suggests that earthworm species other than E. fetida and andrei might be more suitable as test organisms whenever the performance of these standard species is impaired by soil type. However, none of the so-called alternative species (e.g., Dendrodrilus rubidus, Lumbricus terrestris, Aporrectodea caliginosa) has been standardized 15. According to the present study, the use of E. andrei as test species might indeed be impaired in some types of soils, but the results obtained with E. crypticus (particularly in reproduction tests) hint that this organism is a good alternative oligochaete species to E. andrei. Not only does it have a lower sensitivity to soil properties but the test period is shorter (in the case of reproduction tests) and the amount of soil required is less. However, for chemical testing, the sensitivity of E. crypticus must be further evaluated because of the lack of data for this species 37.
CONCLUSIONS
The different soil properties of the Mediterranean soils tested clearly affected the behavior and reproduction of both test species, and the ecological requirements of the two can limit their use as test organisms for some soils. E. andrei was much more sensitive to the physicochemical properties of different soils than E. crypticus. In fact, in avoidance tests, earthworms presented the most robust response; they avoided soils with high pH and with fine textures. This strong influence of soil parameters was confirmed for the reproduction tests, in which they reproduced successfully in seven of 10 soils. Although avoidance patterns for enchytraeids were much more variable than those for E. andrei, and their reproduction showed some influence of soil properties, E. crypticus always reproduced above the validity criteria. Thus, the use of this enchytraeids species for site soil assessments might be advantageous when soil properties are suspected to impair E. andrei reproduction or whenever the control soil is different from the test soil. Based on the results of the present study, the following recommendations can be made (although restricting them to the range of pedological properties presented). For avoidance tests, E. andrei should be used to assess the toxic potential of Mediterranean soils with pedological properties that fall within the tolerance range for the species and should not be used for fine textured soils with a soil pH above 8. For natural soils that are extremely sandy or silty (e.g., more than 75% sand and more than 50% silt), the results of tests with E. andrei must be interpreted with caution, because these pedological characteristics can influence the results of the tests. The test design and procedures for the avoidance test with E. crypticus require modification in order to reduce the variability currently associated with the responses. Also, E. andrei should not be used to assess reproduction in substrates that have extreme textures (e.g., >80% sand), are strongly acidic (e.g., pH ≤ 4.2), and/or have low organic matter (e.g., < 2%); E. crypticus seems to be a good alternative species insofar as it showed a broader tolerance to different properties (e.g., ranges of 4.2–7.7 for pH, 0.6–4.8% for organic matter, 3–49% for clay, 6–49% for silt, 2–70% for coarse sand, and 18–74% for fine sand) compared with E. andrei.
Based on these criteria, the selected 12 Mediterranean soils can be used as reference soils for tests with E. crypticus. Tests with E. andrei should be restricted to soils with properties similar to those of Luv, It 2, It 3, It 4, Por, and Pra soils.
The range of soils considered in the present study does not cover the entire Mediterranean region, and testing more soils from the other Mediterranean countries or other areas within the countries considered (e.g., Spain and Italy) would be desirable to determine their suitability as reference control soil. However, the results obtained to date provide a good basis for the selection of reference soils for ecotoxicological effects assessment within this biogeographical region. They could be either used for prospective ERA (in which several of them can be used to investigate the influence of soil properties on the toxicity of a certain chemical) or retrospective ERA (whenever, in a contaminated area, no clean field soil is available, one of the studied soils with matching soil properties to those of the test soils can be selected and used as a control). Also, a similar approach should be considered for other regions.
SUPPLEMENTAL DATA
Figure S1. Avoidance response by Eisenia andrei within three groups of Mediterranean soils: comparison of the average observed values with those predicted by the respective regression model.
Figure S2. Avoidance response by Enchytraeus crypticus within three groups of Mediterranean soils: comparison of the average observed values with those predicted by the respective regression model.
Figure S3. Reproduction of Eisenia andrei incubated in three groups of Mediterranean soils: comparison of the average observed values with those predicted by the respective regression model. (76 KB DOC)
Acknowledgements
The present study was sponsored by Fundação para a Ciência e Tecnologia Portugal grants to S. Chelinho (SFRH/BM/18844/2004) and Conselho de Reitores das Universidades Portuguesas (CRUP), by Acções Integradas Luso-Espanholas (Acção E-5/05). We also thank the following people and institutions for facilitating the soil collection: Manuel Patanita (Escola Superior Agrária de Beja), Rita Alcazar (Liga para a Protecção da Natureza), Castro Antunes (Parque Nacional da Serra de São, Mamede), José Luís (Companhia das Lezírias), Teresa Pinto Correia (University of Évora), and Associação de Defesa do Património de Mértola. We especially thank Silvana Siehoff (University of Aachen) for helping with the laboratory work.