Polycyclic Aromatic Hydrocarbons Phenanthrene and Retene Modify the Action Potential via Multiple Ion Currents in Rainbow Trout Oncorhynchus mykiss Cardiac Myocytes
Abstract
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous contaminants in aqueous environments. They affect cardiovascular development and function in fishes. The 3-ring PAH phenanthrene has recently been shown to impair cardiac excitation–contraction coupling by inhibiting Ca2+ and K+ currents in marine warm-water scombrid fishes. To see if similar events take place in a boreal freshwater fish, we studied whether the PAHs phenanthrene and retene (an alkylated phenanthrene) modify the action potential (AP) via effects on Na+ (INa), Ca2+ (ICaL), or K+ (IKr, IK1) currents in the ventricular myocytes of the rainbow trout (Oncorhynchus mykiss) heart. Electrophysiological characteristics of myocytes were measured using whole-cell patch clamp. Micromolar concentrations of phenanthrene and retene modified the shape of the ventricular AP, and retene profoundly shortened the AP at low micromolar concentrations. Both PAHs increased INa and reduced ICaL and IKr, but retene was more potent. Neither of the PAHs had an effect on IK1. Our results show that phenanthrene and retene affect cardiac function in rainbow trout by a mechanism that involves multiple cardiac ion channels, and the final outcome of these changes (shortening of AP) is opposite to that observed in scombrid fishes (prolongation of AP). The results also show that retene and aryl hydrocarbon receptor (AhR) agonist have an additional mechanism of toxicity besides the previously known AhR-mediated, transcription-dependent one. Environ Toxicol Chem 2019;38:2145–2153. © 2019 SETAC.
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous contaminants that occur as complex mixtures in aquatic environments. They originate from petrogenic or pyrogenic sources, and they enter the waters via atmospheric deposition, oil accidents, municipal and industrial effluents, and urban runoff. Individual PAHs as well as PAH mixtures (such as oil) affect the development and function of the heart in several fish species (Billiard et al. 1999; Incardona et al. 2004, 2006, 2009, 2011, 2014; Dubansky et al. 2013; Brette et al. 2017; Raine et al. 2017).
Phenanthrene and retene (1-methyl-7-isopropyl phenanthrene) are 3-ring PAHs. Phenanthrene is common in both petrogenic and pyrogenic mixtures of PAHs, and it causes reversible bradycardia and atrioventricular conduction block in zebrafish (Danio rerio) and a slight increase in heart rate and reduction of circulation in marine medaka (Oryzias melastigma; Incardona et al. 2004; Mu et al. 2014; Sun et al. 2015; Cypher et al. 2017). In Pacific bluefin tuna (Thunnis orientalis), phenanthrene affects the cardiac action potential (AP) and ion currents (Brette et al. 2017). Retene is an alkylated phenanthrene, and it has been found in sediments downstream from pulp and paper mills, in landfills, and in oil sand–produced water (Leppanen and Oikari 1999a, 1999b; Legler et al. 2011; Cheng et al. 2018). Retene is an aryl hydrocarbon receptor (AhR) agonist, and it activates the AhR and causes changes in the transcription of several genes, leading to developmental defects in the cardiovascular system (Billiard et al. 1999; Scott et al. 2011; Vehniäinen et al. 2016).
Contraction of the vertebrate heart is triggered by cardiac AP, which originates from the primary pacemaker center at the border zone between the sinus venosus and the atrium (Yamauchi and Burnstock 1968; Haverinen and Vornanen 2007). From there AP spreads throughout the atrium and via the atrioventricular canal further to the ventricular wall, thereby triggering sequential contractions of atrium and ventricle (Sedmera et al. 2003). Cardiac AP is generated by the complex interaction between several voltage-gated ion currents in the sarcolemma of cardiac myocytes. In fish ventricular myocytes, there are 2 major inward currents, the fast Na+ current (INa) and L-type Ca2+ current (ICaL; long-lasting), and 2 major outward K+ currents, the fast component of the delayed rectifier K+ current (IKr) and the background inward rectifier K+ current (IK1; Vornanen 2016). Besides these major ion currents, fish ventricular myocytes may have T-type Ca2+ current (ICaT; transient) and the slow component of the delayed rectifier K+ current (IKs; Nemtsas et al. 2010; Hassinen et al. 2011; Abramochkin et al. 2018; Haverinen et al. 2018a). The shape of the cardiac AP is regulated by time- and voltage-dependent opening and closing of the Na+, Ca2+, and K+ channels. Electrical excitability of cardiac myocytes (i.e., the ease with which the cardiac AP can be triggered) is dependent on the antagonistic effects of INa and IK1 on membrane potential and an important factor in uninterrupted propagation of cardiac AP (Varghese 2016; Vornanen 2016).
The aim of the present study was to investigate if phenanthrene and retene modulate the 4 major ion currents of the rainbow trout (Oncorhynchus mykiss) ventricle, which could reveal novel toxic effects of these PAHs on the fish heart.
MATERIAL AND METHODS
Animals
Hatchery-reared rainbow trout (Oncorhynchus mykiss; 73.43 ± 11.69 g, n = 18) were obtained from the local fish farm (Kontiolahti, Finland). In the animal facilities of the University of Eastern Finland, the trout were maintained in 500-L metal aquaria for a minimum of 3 wk before use in the experiments and fed aquarium fish food (Ewos) at least 5 times a week. Water temperature was regulated at 14 ± 0.5 °C (Computec Technologies), and oxygen saturation was maintained by aeration with compressed air. Groundwater (average pH 8.0, conductivity 13 µS/cm) was constantly flowing through the aquaria at a rate of 150 to 200 L/d (permission ESAVI/2832/04.10.07/2015).
Myocyte isolation
All experiments were conducted in vitro on enzymatically isolated ventricular myocytes. Fish were killed by a cranial concussion and pithing, and the heart was rapidly excised. Ventricular myocytes were isolated using retrograde perfusion of the heart and the standard concentrations of hydrolytic enzymes as reported for the method developed in our laboratory (Vornanen 1997). Cell isolation was conducted at room temperature (20–22 °C). Isolated myocytes were used in the experiments within 10 h from isolation.
Whole-cell patch clamp
Whole-cell current-clamp recordings were made by using an Axopatch 1D amplifier (Axon Instruments). Clampex 9.2 software was used for data acquisition, and off-line analysis of the recordings was done using the Clampfit 10.4 software package. During the experiments, myocytes were continuously superfused with external saline solution at a rate of 1.5 to 2 mL min–1. The temperature of the external solution in the recording chamber was regulated at 14 °C by using a Peltier device (CL-100 from Warner Instruments or HCC-100A from Dagan) and continuously recorded on the same file with electrophysiological data. Patch pipettes were pulled (PP-83; Narishige) from borosilicate glass (King Precision) and had a resistance of 2.7 ± 0.06 MΩ when filled with the internal saline solution. After gaining a gigaohm seal, the membrane under the pipette tip was ruptured by a short-voltage pulse (zap) to get access to the cell, transients attributable to series resistance (7.3 ± 0.26 MΩ) and pipette capacitance were canceled, and the capacitive size of ventricular myocytes was determined.
For recording of APs and K+ currents, the external saline solution contained (mmol/L–1) 150 NaCl, 5.4 KCl, 1.2 MgCl2, 1.8 CaCl2, 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and 10 glucose, with pH adjusted with NaOH to 7.6 at 20 °C (giving a pH of 7.68 at the experimental temperature). The composition of the pipette (internal) solution was as follows (mmol/L–1): 140 KCl, 4 MgATP, 1 MgCl2, 0.03 Tris-GTP, and 10 HEPES (pH adjusted with KOH to 7.2 at 20 °C). To elicit APs, ventricular myocytes were stimulated with current pulses of constant duration (4 ms) and with increasing amplitude. The initial stimulus strength was 200 pA, and it was raised with 20-pA increments until an all-or-none AP was elicited (Badr et al. 2018). The stimulation frequency was 1 Hz. The following AP parameters were analyzed off-line: resting membrane potential (Vrest, mV), threshold potential of AP (Vth, mV), threshold current (Ith, pA), critical depolarization (Vth – Vrest, mV), AP overshoot (mV), AP amplitude (AMP, mV), AP duration at 50% repolarization level (APD50, ms), maximum rate of AP upstroke (+dV/dt, mV ms–1), and the maximum rate of AP repolarization (–dV/dt, mV ms–1; Figure 1). Measures Vth, Ith, and critical depolarization are for electrical excitability of ventricular myocytes, that is, the ease with which AP can be triggered by a depolarizing current.
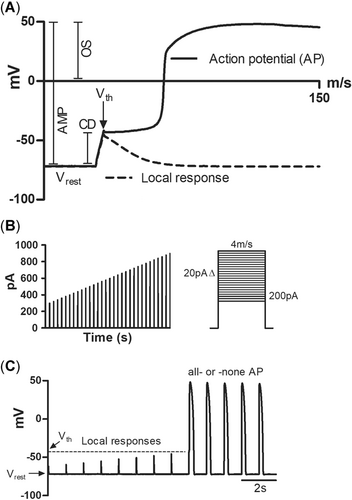
Action potential (AP) parameters measured in the current clamp experiments. (A) The first 150 ms of a rainbow trout ventricular AP showing the parameters that were determined from the recordings. The continuous line shows the all-or-none AP and the dotted line, the local passive response of the membrane. The maximum rate of depolarization (upstroke; +dV/dt) and repolarization (–dV/dt) of AP were measured from the first derivative of the AP tracing. (B) The stimulus protocol of increasing current strengths (duration 4 ms) used to search the trigger level for APs. The strength of current just able to trigger an AP is the threshold current. (C) A typical response of a ventricular cell to increasing stimulus strength. AMP = amplitude; CD = critical depolarization; Ith = threshold current; OS = overshoot; Vrest, resting membrane potential; Vth = threshold voltage.
Voltage dependency of the rapid component of the delayed rectifier K+ current (IKr) and the inward rectifier K+ current (IK1) were measured using standard stimulation protocols (Vornanen et al. 2002a) from the holding potential of –80 mV. When recording IK1, the external saline included 2 µM E-4031 (1-[2-(6-methyl-2-pyridyl)ethyl]-4-(4-methylsulfonyl-aminobenzoyl)piperidine), 0.5 µM tetrodotoxin (TTX; Tocris Cookson), and 10 µM nifedipine, to block IKr, INa, and ICaL, respectively. Also, IKr was recorded in the presence of TTX (0.5 µM), nifedipine (10 µM), and 0.2 mM BaCl2 (to block IK1).
The fast Na+ current (INa) was measured under a reduced Na+ gradient (20 mM [Na+]o, 5 mM [Na+]i) across the sarcolemma to obtain good control of the membrane voltage. The composition of the external saline was (mmol/L–1): 20 NaCl, 120 CsCl, 1 MgCl2, 0.5 CaCl2, 10 glucose, and 10 HEPES at pH 7.7 (adjusted with CsOH at 20 °C; Haverinen and Vornanen 2004). Nifedipine (10 µmol L–1) was included in the external solution to block ICaL. The pipette solution consisted of (in mmol L–1) 5 NaCl, 130 CsCl, 1 MgCl2, 5 egtazic acid (EGTA), 5 Mg2ATP, and 5 HEPES (pH adjusted to 7.2 with CsOH at 20 °C). Using established stimulus protocols, INa was elicited from a holding potential of –120 mV (Haverinen and Vornanen 2006; Haverinen et al. 2018b).
The composition of the external saline solution for recording ICaL was as follows (mmol/L–1): 150 NaCl, 5.4 CsCl, 1.8 CaCl2, 1.2 MgCl2, 10 HEPES, and10 glucose (pH adjusted to 7.6 at 20 °C with CsOH). We included TTX (0.5 μM) in this saline to block Na+ current (INa; Vornanen 1998). Because Cs+ may flow through the Erg K+ channels, 2 μM E-4031 was included in the external solution to prevent contamination by IKr. The pipette solution contained (mmol L−1) 130 CsCl, 15 tetraethylammonium chloride, 5 MgATP, 1 MgCl2, 5 oxaloacetate, 10 HEPES, and 5 EGTA (pH adjusted to 7.2 at 20 °C with CsOH; all chemicals from Sigma). We elicited ICaL from a holding potential of –80 to +10 mV at a frequency of 0.2 Hz. Recording of ICaL is complicated by time-dependent rundown (decline) of the current. To minimize the effect of rundown on results, time-dependent changes in ICaL were monitored after getting access to the whole configuration. For the same reason, the analysis of PAH effects was limited to the 2 highest concentrations. Only those cells where ICaL stabilized within approximately 5 min from the start of recording were accepted for analysis.
PAHs
The stocks of phenanthrene (Sigma-Aldrich) and retene (MP Biomedicals) were made in dimethyl sulfoxide (DMSO) at 20 mM. Test solutions at concentrations of 0.3, 1.0, 10, and 30 µM for phenanthrene and 0.1, 1.0, and 10 µM for retene were made daily in external saline solutions. Effects of the highest DMSO concentration in the experimental solutions on AP parameters and ion currents were tested in separate experiments. No statistically significant effects were noticed.
Statistical analyses
After checking the normality of distribution and equality of variances, one-way analysis of variance (with Tukey's or Dunnett's T3 post hoc test) or nonparametric test (with Friedman's test) were used for evaluating the effect of different PAH concentrations on AP parameters and maximum ion currents. All statistical tests were performed using SPSS (IBM; Ver 21.0) software. Data are presented as mean ± standard error of the mean (SEM), and p < 0.05 was considered statistically different.
RESULTS
Phenanthrene and retene differentially modify the AP in rainbow trout ventricular myocytes
Phenanthrene had no effect on the duration of AP at the level of 50% repolarization (APD50; Figure 2A and E) but shortened it at the zero voltage level (APD0) at 30 µM (Figure 2A). Phenanthrene increased the maximum upstroke velocity (+dV/dt) at 1 and 10 µM and accelerated the maximum rate of AP repolarization (–dV/dt) at 10 and 30 µM (Figure 2C). Retene had more pronounced effects on APs than phenanthrene. The duration of the AP (APD50 and APD0) was strongly shortened at 1 and 10 µM concentrations (Figure 3A and E). Retene augmented the AP amplitude at 10 µM and increased the overshoot at 1 and 10 µM (Figure 3B). The maximum rate of AP upstroke became faster at all concentrations of retene, but the effect on –dV/dt was significant only at 10 µM (Figure 3C). Excitability of ventricular myocytes was decreased at the highest (10 µM) retene concentration as the critical depolarization needed to elicit AP was approximately 18% higher than in the control (Figure 3B).
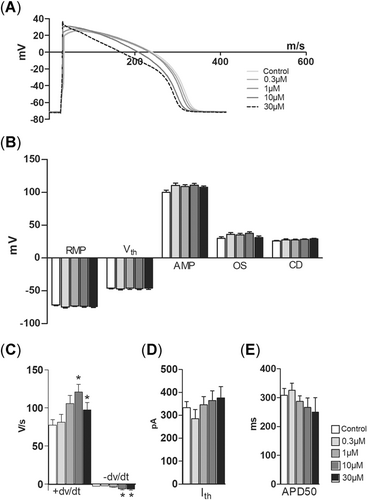
Phenanthrene modifies the shape of the action potential (AP) but has no effect on the AP duration in rainbow trout cardiac ventricle cells. (A) A representative experiment showing the effect of cumulatively increasing concentrations of phenanthrene on AP. (B, D, E) Phenanthrene has no effect on the resting membrane potential, threshold voltage, AP amplitude, overshoot, critical depolarization, threshold current, and AP duration at 50% repolarization level. (C) Phenanthrene steepens both the maximum rate of AP depolarization (+dV/dt) and repolarization (–dV/dt). An asterisk indicates statistically significant difference from control. AMP = amplitude; APD50 = AP duration at 50% repolarization; CD = critical depolarization; Ith = threshold current; OS = overshoot; RMP = resting membrane potential; Vrest, resting membrane potential; Vth = threshold voltage.
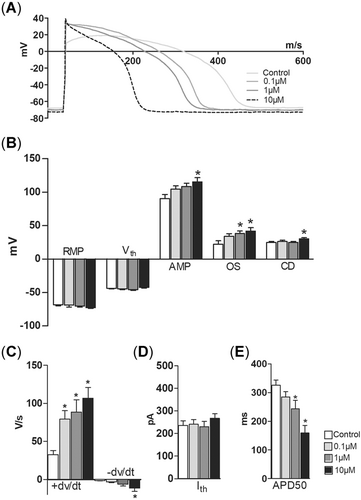
Retene shortens the action potential (AP) duration and modulates the shape of the AP in ventricular cardiomyocytes of rainbow trout. (A) A representative experiment showing the effect of cumulatively increasing concentrations of retene on AP. (B, D) Retene has no effect on the resting membrane potential, threshold voltage, and threshold current. (B) AP amplitude, overshoot, and critical depolarization are increased by retene. (C, E) Retene shortens the AP duration and steepens both the maximum rate of depolarization (+dV/dt) and the maximum rate of repolarization (–dV/dt) of the AP. An asterisk indicates statistically significant difference from control. AMP = amplitude; APD50 = AP duration at 50% repolarization; CD = critical depolarization; Ith = threshold current; OS = overshoot; RMP = resting membrane potential; Vrest, resting membrane potential; Vth = threshold voltage.
Phenanthrene and retene modulate cardiac INa, ICaL, and IKr currents but have no effect on IK1
Phenanthrene and retene affected all studied ventricular ion currents except IK1, but retene caused the effects at lower concentrations than phenanthrene. Under exposure of 10 µM phenanthrene or 1 µM retene, the peak density of INa was increased by 12 and 17%, respectively (Figure 4A and B). The effects of the highest concentrations (30 µM phenanthrene, 10 µM retene) were slightly less and statistically nonsignificant (Figure 4B).
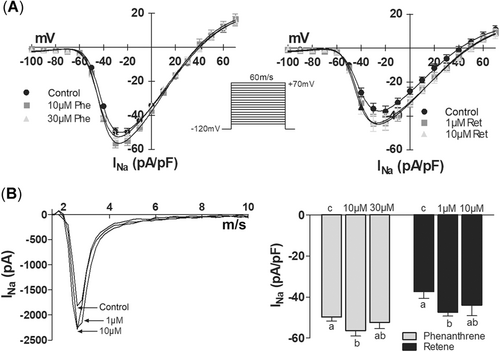
Phenanthrene and retene increase the fast Na+ current (INa) in rainbow trout ventricular cardiomyocytes. (A) Current–voltage relationship of INa in the absence and presence of phenanthrene (left) and retene (right). The stimulus protocol is shown between the graphs. (B) Effects of phenanthrene (10, 30 μM) and retene (1, 10 μM) on the peak density of INa. The results are means ± SEM of 12 to 14 myocytes from at least 3 animals. Groups denoted by the same letter do not differ significantly from each other.
After getting electrical access to the cell, there was a clear increase in the amplitude of ICaL attributable to the buffering of intracellular free Ca2+ by EGTA of the pipette solution (removal of Ca2+-dependent inactivation of ICaL). Then, the current stabilized and enabled the recording of drug effects on ICaL (Figure 5A and B). Phenanthrene reduced ICaL, but the effect was statistically significant only at the highest concentration tested, 30 µM (Figure 5C). Retene diminished ICaL at 1 and 10 µM (Figure 5C).
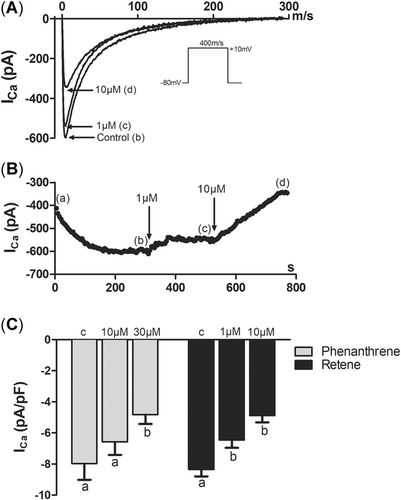
Phenanthrene and retene attenuate the L-type Ca2+ current (ICaL) in rainbow trout ventricular cardiomyocytes. (A) A representative experiment on ICaL. Immediately after gaining access into the cell, ICaL starts to increase because of the buffering of intracellular Ca2+ by EGTA (pipette solution is perfusing the cell from the inside). Then, ICaL stabilizes (b), and the cell is cumulatively exposed to 1 (c) and 10 (d) μM retene. (B) Fast time-based tracings of ICaL under control conditions and in the presence of 1 and 10 μM retene at the positions shown by letters b and c and of panel A. The stimulus pulse is shown below the tracings. (C) Effects of phenanthrene (10, 30 μM) and retene (1, 10 μM) on the peak density of ICaL. The results are means ± SEM of 12 to 16 myocytes from at least 3 animals. Groups denoted by the same letter do not differ significantly from each other.
Whereas phenanthrene attenuated IKr at 10 and 30 µM, retene was effective even at the lowest test concentration (0.1 µM; Figure 6). Both phenanthrene and retene decreased the IKr tail currents at all voltages, where the tail current was activated (Figure 6C and D). The maximum inhibition of IKr tail at +40 mV was 79.3 and 59.2% for phenanthrene and retene, respectively. During the depolarizing prepulse, phenanthrene and retene inhibited IKr (IKractiv) in the voltage range between 0 and +20 mV but did not have any effect at +40 and +60 mV (Figure 6E and F). This suggests that there is a phenanthrene- and retene-resistant current underlying IKr, probably the slow component of the delayed rectifier K+ current, IKs. Neither of the PAHs had an effect on the background inward rectifier, IK1 (Supplemental Data, Figure S1A and B).
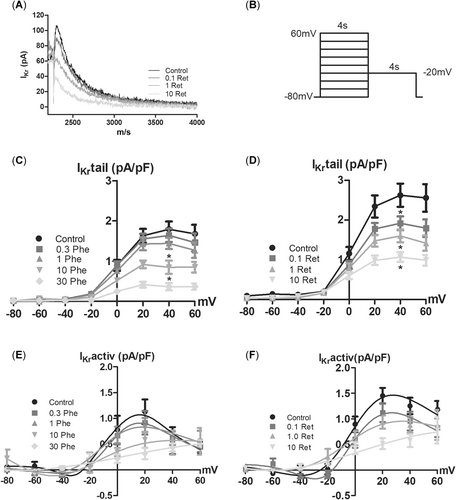
Phenanthrene and retene reduce the rapid component of the delayed rectifier K+ current (IKr) in rainbow trout ventricular cardiomyocytes. (A) A representative experiment showing the inhibitory effect of retene on IKr. The 2-step stimulus protocol is shown on the right. (C, D) Current–voltage relationship of the IKr tail current (IKr, tail during the repolarizing pulse at –20 mV) in the absence and presence of phenanthrene (C) and retene (D). (E, F). Current–voltage relationship of the IKr during the depolarizing pulses from +80 to –60 mV in the absence and presence of phenanthrene (E) and retene (F). The results are means ± SEM of 11 to 12 cells from at least 3 animals. An asterisk indicates statistically significant difference from control.
DISCUSSION
Effects on AP
Both 3-ring PAHs affected the ventricular AP of the rainbow trout heart, but retene was a much stronger AP modifier than phenanthrene. Phenanthrene did not change Vrest or AMP, consistent with the findings from bluefin tuna cardiomyocytes (Brette et al. 2017); Vrest is maintained by the IK1, which remained untouched by phenanthrene. Phenanthrene had only minor effects on APD; APD was slightly reduced at the zero-voltage level but remained unchanged at the 50% repolarization level. In this respect, rainbow trout clearly differs from bluefin tuna, where phenanthrene lengthened ventricular APD (Brette et al. 2017). The APD is regulated by a delicate balance between influx of Ca2+ via ICaL and efflux of K+ via IKr, IKs, and IK1 (Grant 2009). Because the resistance of the sarcolemma at the AP plateau is high (Ca2+ and K+ fluxes are small), small changes in the amplitude and activation/inactivation rate of Ca2+ and K+ currents will affect APD (Zaza 2010). Shortening of AP at the zero-voltage level suggests that in the early plateau ICaL is reduced more than IKr by phenanthrene. The results of the present study show a small but clear increase in +dv/dt in rainbow trout with 10 and 30 µM phenanthrene. Phenanthrene also steepened the rate of repolarization (–dv/dt). These are novel actions of PAHs on the AP of the fish heart. Collectively, the present findings show that phenanthrene has partly different effects on cardiac APs in rainbow trout and bluefin tuna.
In contrast to phenanthrene, retene strongly shortened APD, and the effect occurred at the lower drug concentrations (1 and 10 µM). The strong shortening of APD suggests that the relative effect of retene on ICaL is stronger than its effect on IKr, the main repolarizing current. The effects of retene differ from those of phenanthrene in that AMP and overshoot were enhanced by retene but not by phenanthrene. This difference may be associated with slightly stronger stimulation of INa by retene. Notably, the highest retene concentration (10 µM) attenuated excitability of ventricular myocytes as critical depolarization was increased. In vivo, the decrease in excitability could cause interruptions in AP propagation between ventricular myocytes (Vornanen 2016).
Effects on cardiac ion currents
The Na+ current (INa) is active during the upstroke of the AP, causing depolarization of the sarcolemma by fast and large influx of Na+. Both phenanthrene and retene increased the peak INa density in the ventricular myocytes of rainbow trout. Retene was more potent than phenanthrene at enhancing INa, which is in line with its larger effect on +dv/dt and overshoot of the AP. At the level of intact tissue the larger INa means a faster propagation of AP in the ventricular wall. To our knowledge, there are no earlier data on the effects of PAHs on fish cardiac INa. However, in bluefin tuna ventricular myocytes, phenanthrene did not affect the upstroke velocity of the AP, thus suggesting species-specific differences in PAH modulation of INa.
Currents ICaL and IKr are the main determinants of the long AP plateau. They are counteracting currents because ICaL is depolarizing and IKr repolarizing. The net outcome of the inhibition of these currents can be seen as changes in APD. Notably, both PAHs caused shortening of APD, but retene was much more powerful than phenanthrene. Strong shortening of APD by retene indicates that the net charge influx via ICaL is inhibited more than the K+ efflux via IKr. The final phase 3 repolarization is accelerated by the background inward rectifier IK1. The increase in the rate of –dV/dt by PAHs is probably attributable to the resistance of IK1 to retene and phenanthrene whereby the uninhibited IK1 overwhelms the reduced ICaL.
The lowering of Ca2+ influx in phenanthrene-treated rainbow trout cardiac myocytes is in line with previous research showing that phenanthrene decreased Ca2+ transients in ventricular myocytes of scombrid fishes, even though scombrids may be more dependent on intracellular Ca2+ stores for contractile activation (Brette et al. 2017). The magnitude of ICaL inhibition by phenanthrene seems to differ between tuna and rainbow trout: whereas 5 µM phenanthrene decreased ICaL by approximately 30% and 25 µM phenanthrene by approximately 75% in bluefin tuna, in rainbow trout 10 and 30 µM phenanthrene attenuated ICaL only by approximately 20 and approximately 40%, respectively (Brette et al. 2017). On the other hand, in tuna the reduction of ICaL should be partly compensated by the prolonged AP plateau. Brette et al. (2017) did not report whether the rundown of ICaL was taken into account, which might have also affected their results. In rainbow trout, retene was more potent than phenanthrene at attenuating ICaL because the same reduction of ICaL by approximately 20 and approximately 40% was achieved with lower concentrations of retene of 1 and 10 µM, respectively.
The strong shortening of APD and inhibition of ICaL by retene are expected to have a strong reducing effect on the intracellular free Ca2+ concentration. In trout ventricular myocytes, the activation of contraction is largely dependent on the sarcolemmal Ca2+ influx during the AP plateau because approximately two-thirds of the activator Ca2+ is estimated to come from the extracellular space (Vornanen et al. 2002b). Inhibition of ICaL and shortening of the plateau means that Ca2+ influx is smaller and there is less time for Ca2+ entry. In the intact ventricle, this should appear as reduced force of contraction. Indeed, exposure to PAHs or oil reduces atrial and ventricular contraction and diminishes cardiac stroke volume in larval fish (Incardona et al. 2013; Jung et al. 2013; Edmunds et al. 2015; Esbaugh et al. 2016; Sørhus et al. 2016; Khursigara et al. 2017; Perrichon et al. 2018). In rainbow trout yolk sac larvae, retene causes pericardial and yolk sac edemas (Billiard et al. 1999; Scott et al. 2011; Vehniäinen et al. 2016), phenomena often seen with PAH and oil exposures and proposed to be caused by reduced cardiac output (Incardona and Scholz 2016). Taken together, inhibition of ICaL and shortening of the AP plateau would compromise contractility and cardiac output of the heart with the outcome of reduced physical performance level and fitness of the fish. These effects would be particularly strong under the intoxication by retene.
The IKr channels are notorious for their susceptibility to inhibition by low concentrations of various small-molecule compounds (Sanguinetti and Tristani-Firouzi 2006). The wide pore cavity of the channel allows access of small molecules to the pore (Vandenberg et al. 2001). Therefore, it is no surprise that also PAHs can block these channels in fish cardiac myocytes. In rainbow trout ventricular myocytes, 10 and 30 µM phenanthrene reduced IKr by 43 and 75%, respectively. This is slightly less than the inhibition in bluefin tuna, where 5 and 25 µM phenanthrene decreased IKr by 60 and more than 85%, respectively (Brette et al. 2017). In rainbow trout, the effect of retene on IKr was more pronounced because 10 µM retene caused a 60% reduction in IKr (Supplemental Data, Figure S2).
In mammalian heart, blockade of IKr by many drugs is shown to be proarrhythmic and able to induce chaotic ventricular tachycardia, torsades de pointes (Vandenberg et al. 2001). However, if both IKr and ICaL are inhibited simultaneously and at similar drug concentrations, the effect is antiarrhythmic, even when drugs prolong, shorten, or triangulate ventricular APs (Kramer et al. 2013; Obejero-Paz et al. 2015). A typical example is verapamil, a useful human cardiovascular medicine, which inhibits human IKr and ICaL at similar concentrations (Shetuan et al. 1999; Kang et al. 2012). Because PAHs inhibit both IKr and ICaL at similar micromolar concentrations, they should not be proarrhythmic in fish ventricle. However, IKr and ICaL are essential components of the cardiac pacemaker, which determines the rate and rhythm of the heartbeat (Schram et al. 2002). Half-maximal inhibition of IKr by E-4031 is known to reduce the beating rate of rainbow trout sinoatrial preparations, and therefore inhibition of IKr might explain the PAH-induced bradycardia of larval fish (Haverinen and Vornanen 2007). Inhibition of ICaL is likely to affect impulse generation and conduction of the nodal tissues (sinoatrial pacemaker and atrioventricular canal) because ICaL is the main determinant for the rate of AP upstroke and impulse conduction (INa is absent or small in nodal cells; Schram et al. 2002). Inhibition of ICaL might therefore appear as atrioventricular block and ventricular bradycardia, phenomena seen in larval fish exposed to PAHs or oil (Incardona et al. 2004, 2005, 2009, 2011; Zeltser et al. 2004; Perrichon et al. 2016, 2018). However, care must be taken when applying results from mature fish to embryos or larval fish.
Because retene is quite hydrophobic (logKOW ~6), the actual concentrations in the test chamber most probably were lower than nominal. It must also be borne in mind that retene is quickly metabolized by cytochrome P450 (CYP1A) in fish, and this may lower the concentration of parent retene that reaches cardiac myocytes in vivo (Hawkins et al. 2002). In nature, however, fish are exposed to PAH mixtures that frequently contain CYP1A inhibitors, which in turn decrease the metabolism of PAHs and thus increase the concentration of parent compounds (Hawkins et al. 2002).
Retene is an AhR agonist, and it disturbs cardiovascular development in fish via activating AhR and altering transcription (Scott et al. 2011; Vehniäinen et al. 2016). The present study shows that in addition to this transcriptional route, retene has a direct effect on cardiac function via modulating voltage-gated ion channel activity. Because normal cardiac function is important for cardiovascular development (Glickman and Yelon 2002; Incardona et al. 2015), as well as the development of other tissues and organs (Incardona et al. 2004), retene may cause developmental defects also independently of the AhR. The ability to modulate the activity of cardiac ion channels also means that in addition to early-life stages, retene may be cardiotoxic to juveniles and adults.
CONCLUSION
The 3-ring PAHs phenanthrene and retene differentially modified ventricular APs in rainbow trout cardiomyocytes. Retene was more potent and strongly reduced the duration of ventricular AP. Although phenanthrene and retene had qualitatively similar effects on ion currents, phenanthrene only slightly affected AP duration, probably because of its weaker inhibition of IKr and ICa in comparison to retene. Furthermore, the effects of phenanthrene on ventricular AP differed from those reported earlier for the marine warm-water scombrid fish bluefin tuna. The present results suggest that different PAHs may have different direct effects on cardiac function and that these effects may be partly species-specific. This further complicates the environmental risk assessment of PAHs.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.4530.
Acknowledgment
The authors thank A. Kervinen for technical assistance. Kontiolahti fish farm is acknowledged for the donation of the fish. The present study was supported by the Academy of Finland (projects 285296, 294066, and 319284, to E.-R. Vehniäinen). The fish used in the experiments fall under the laboratory animal permission of ESAVI/2832/04.10.07/2015.
Open Research
Data Accessibility
Data are available from the authors ([email protected]).




