The effects of bifenthrin and temperature on the endocrinology of juvenile Chinook salmon
Abstract
The San Francisco Bay delta (USA) is experiencing seasonally warmer waters attributable to climate change and receives rainstorm runoff containing pyrethroid pesticides. Chinook salmon (Oncorhynchus tshawytscha) inhabit the affected waterways from hatch through smoltification, and thus juvenile fish may experience both pyrethroid and warmer water exposures. The effects of higher temperatures and pesticide exposure on presmolt Chinook are unknown. To improve understanding of the potential interaction between temperature and pesticide exposure on salmonid development, juvenile alevin and fry were reared in 11, 16.4, and 19 °C freshwater for 11 d and 2 wk, respectively, and exposed to nominal concentrations of 0, 0.15, and 1.5 µg/L bifenthrin for the final 96 h of rearing. Estradiol-17β (E2), testosterone, triiodothyronine, and thyroxine levels were measured in whole-body homogenates using hormone-specific enzyme-linked immunosorbent assays. Brain gonadotropin-releasing hormone receptor (GnRH2), dopamine receptor 2A, and growth hormone 1 (GH1) mRNA levels were measured using quantitative PCR. Results showed significantly decreased survival and condition factors observed with increasing temperature in alevin. Alevin thyroid hormones increased significantly with temperature, but fry thyroid hormones trended toward a decrease at lower temperatures with increasing bifenthrin exposure. There were significant reductions in fry testosterone and E2 at 11 °C with increasing bifenthrin treatments and significant changes in GnRH2 and GH1 gene expression in both alevin and fry, indicating potential disruption of hormonal and signaling pathways. Environ Toxicol Chem 2019;38:852–861. © 2019 SETAC
INTRODUCTION
Global climate change is causing surface water temperatures to rise in coastal California (USA) as well as increasing the intensity of rainfall events, specifically in the San Francisco Bay delta in northern California (Wagner et al. 2011). The highly urbanized area around the Bay delta is a significant source of pesticides associated with storm event–induced runoff loads into the waterways (Weston and Lydy 2010). Pyrethroids have been frequently detected in surface waters in the Bay delta watershed, and of all the pyrethroids detected, bifenthrin is often the predominant compound, with concentrations up to 3.79 µg/L (Siepmann and Holm 2000; Weston and Lydy 2010). Bifenthrin use has been steadily increasing in the state of California, with approximately 370 000 lb reported use in 2015 for both agricultural and urban sectors (California Department of Pesticide Regulation 2015). The high frequency of detection of bifenthrin in surface water and sediments raises concerns of its effect on nontarget aquatic organisms. Bifenthrin is acutely toxic to insects through binding to sodium channels and prolonging neuronal depolarization, which leads to fatal invertebrate neurotoxicity (Bradberry et al. 2005).
Bifenthrin has been shown to elicit estrogenic effects in ng/L concentrations, causing the inappropriate production of downstream sex steroids and expression of egg yolk proteins in male and juvenile fish associated with the endocrine pathway (Wang et al. 2007; Brander et al. 2016, 2012; Forsgren et al. 2013; Crago and Schlenk 2015). In teleost fish, bifenthrin has been shown to affect the dopaminergic pathway, which plays a vital role in the regulation of estrogen homeostasis (Crago and Schlenk 2015; Bertotto et al. 2018). Studies with zebrafish have demonstrated that bifenthrin may alter endocrine responses depending on the developmental timing of neuroendocrine pathways in fish (Bertotto et al. 2018). The exact mechanism by which bifenthrin affects endocrine pathways in fish is unclear, particularly at different developmental time points.
In addition to increased exposure to contaminants, euryhaline organisms that reside in the Bay delta, such as the endangered Chinook salmon (Oncorhynchus tshawytscha), are exposed to increasing annual and seasonal temperatures that cause increased thermal and osmotic stress (Hasenbein et al. 2013). Surface water exhibits the highest temperature increase in the summer months, reaching temperatures over 25 °C according to models using past monitoring data (Wagner et al. 2011; Cloern et al. 2011); and this coincides with development and growth of juvenile salmonid populations (Kammerer and Heppell 2013). Temperature can also influence the growth and rearing time of salmonids within the San Francisco Bay delta and alter migratory behaviors (Carter 2005).
Salmonids are commonly found in estuaries during smoltification—the physiological and morphological transition of fish from freshwater to salt water. However, there has been little research on salmonids exposed to increasing temperatures in earlier juvenile stages. Salmonids advance through the alevin, fry, and parr juvenile life stages; and parr typically undergo smoltification after a year. In the present study, we used winter-run Chinook salmon, which spawn in the winter months and develop from the alevin through the fry stage as surface water temperatures seasonally increase. In addition, spring- and fall-run Chinook spawn as surface water temperatures increase through the spring and summer months in the Bay delta watershed. Spawning is dependent of timing of adult Chinook returning to freshwater, and this largely depends on whether adults are fall-, winter-, spring-, or summer-run Chinook. Returning adults consistently spawn around the time of year of their own hatch. Increasing temperature is a cue for juvenile salmonids to migrate downstream and smolt (Brauer 1982). Because of warming surface waters, juvenile salmon may experience the environmental cue to smolt at earlier stages. Smoltification is a hormone-driven process that involves alterations in cortisol, growth hormones, pituitary hormones, sex steroids, and thyroid hormones (McCormick 1996; Ban 2004). The thyroid hormones triiodothyronine and thyroxine increase during smoltification through stimulation of the hypothalamus–pituitary–thyroid (HPT) axis, which can be regulated by environmental cues such as salinity, temperature, and photoperiod (Dickhoff et al. 1978; Blanton and Specker 2007). The feedback of the hypothalamus–pituitary–gonadal (HPG) axis and the production of sex steroids are regulated by the dopaminergic system in teleost fishes (Levavi-Sivan et al. 2004; Dufour et al. 2005). Dopaminergic neurons innervate the hypothalamus and decrease the release of gonadotropin-releasing hormone (GnRH), which furthers reduces the release of gonadotropins and subsequently sex steroid hormones from the gonads (Levavi-Sivan et al. 2004). The potential impacts of thermal stress coupled with contaminant exposure on early salmonid life stages are largely unknown.
The overall objective of the present study was to investigate the effects of pyrethroid exposure and increasing water temperatures on the survival and endocrinology of juvenile Chinook salmon through HPG and HPT axis endpoints. Chinook salmon were used because they are vital components of the complex ecosystem within the San Francisco Bay delta. The present study will help explain the effects bifenthrin may have on the endocrinology of early life stages and evaluate the potential impacts climate change can have on salmonid survival and restoration. We hypothesize that bifenthrin can negatively affect the sex steroid, dopaminergic, and thyroid hormone pathways and that the adverse effects of bifenthrin may be exacerbated by the stress of increasing temperatures.
METHODS
Test organisms
Alevin (29 d posthatch [dph]) and fry (68 dph) Chinook salmon were obtained from the California Department of Fish and Wildlife's Feather River Fish Hatchery. Alevin (mass = 0.288 ± 0.037 g) were acclimated to laboratory conditions for 3 d in static systems at 12 °C on a 14:10-h light: dark cycle. Fry (mass = 0.887 ± 0.184 g) were also obtained from the Feather River Hatchery and acclimated to laboratory conditions for 1 wk in flow-through Living Stream systems (Frigid Units). Fry were fed BioClark's Starter (Bio-Oregon) twice a day ad libitum. All fish were handled and treated in accordance with the approved Institutional Animal Care and Use Committee protocols at the University of California, Riverside (animal use protocol 20130010).
Experimental design
Alevin exposures
After laboratory acclimation, 216 alevin were divided randomly into individual 8-L static glass aquaria (2 replicates per treatment, n = 16 individuals per tank) and subjected to 3 temperature regimes for 11 d: 11, 16.4, and 19 °C. Water was chilled or heated to the respective temperature with external heating and chilling units. Alevin were exposed for 11 d because we wanted to ensure that the fish retained their yolk sacs in this short life stage for the duration of the exposure. In the final 96 h (4 d) of the temperature exposure, fish were also simultaneously coexposed to bifenthrin (purity >98%; Chem Service) in concentrations of 0, 0.15, and 1.5 μg/L with ethanol as the vehicle control. Bifenthrin concentrations were chosen from a range of reported measured concentrations during runoff events and have been used in previous studies (Forsgren et al. 2013; Crago and Schlenk 2015). Alevin were given 6 d to acclimate to each temperature regime and then exposed to bifenthrin for 96 h to simulate exposure from an intense pesticide runoff event and increased surface water temperatures throughout the next century. The low 11 °C temperature represents the current temperature during spawning, 16.4 °C is the springtime water temperature, and 19 °C is the minimum predicted end-of-century temperature in the Bay delta (Wagner et al. 2011). Bifenthrin stock solutions were prepared to administer 1 mL of solution per 1 L of tank water. A 50% water change was conducted daily to refresh the bifenthrin treatment. Fish were starved during the final 96 h of exposure to prevent bifenthrin sorption to food and organic matter. Fish were then euthanized using 0.3 g/L MS222 (Sigma-Aldrich), and tissues were harvested, snap-frozen in liquid nitrogen, and stored at –80 °C. Mortality was recorded throughout the exposure, and the average masses and fork lengths were measured at the end of the treatment.
Fry exposures
After laboratory acclimation, 144 fry were divided randomly into individual 20-L static glass aquaria (2 replicates per treatment, n = 8 individuals per tank) and then exposed to 3 temperature regimes for 14 d; 11, 16.4, and 19 °C. As with the alevin exposures, the fry were also exposed to 0, 0.15, and 1.5 μg/L bifenthrin with ethanol as the vehicle control for the final 96 h of the temperature exposure. Water changes of 50% were carried out daily during exposure. Fish were then euthanized using MS222, tissues were harvested as described, and masses and fork length were taken at the beginning and end of the experiment.
Bifenthrin water chemistry
Water samples were taken from bifenthrin-treated fry tanks at 0 and 96 h after bifenthrin exposure. Water samples at time 0 were taken immediately after the first volume of bifenthrin was added. The samples were collected in 1-L amber glass bottles and stored at 4 °C, then processed within 2 wk of sampling. Extractions were performed in glass separatory funnels with the individual water sample after the addition of 10 g of burned NaCl. Methylene chloride (ThermoFischer Scientific; 50 mL) was added to the funnel and shaken for 2.5 min. After phase separation, the methylene chloride layer was filtered through a layer of dried Na2SO4 into a round-bottom flask. The same extraction was repeated 3 consecutive times, and the combined extract was dried on a Rotevaporator to approximately 1 mL. The flask was rinsed with hexane and the sample further dried under N2 to dryness, and the final sample was reconstituted in 1 mL of hexane. Samples were run on a GC/MS (5973N/6890N; Agilent Technologies), and a 7-point standard curve was used to determine concentrations. Polychlorinated biphenyl 206 was used as the recovery surrogate and added to each sample before extraction.
Hormone quantification
Competitive enzyme-linked immunosorbent assays (ELISAs) were used to determine the concentrations of triiodothyronine, thyroxine, estradiol (E2), and testosterone in whole-body extracts of alevin and fry. Testosterone and E2 kits were sourced from Cayman Chemical, and triiodothyronine and thyroxine kits were sourced from Genway Biotech. Whole alevin and fry were homogenized in a lysis buffer with 1 μM phenylmethylsulfonyl fluoride and centrifuged at 12 000 g at 4 °C, and the supernatant was used for the triiodothyronine and thyroxine ELISAs. Homogenate supernatant (250 μL) was extracted for sex steroid hormones 3 times with methylene chloride. Samples were dried under N2 and reconstituted with the E2 ELISA buffer. All buffers and reagents were prepared with ultrapure water and mixed according to the respective kit protocols. Standard curves for calculating hormone levels, provided with the kits, were run for each analysis. Plate absorbances were detected on a Spectra Max Plus 385 microplate reader (Molecular Devices) at 450 nm for the thyroid hormone ELISAs and 405 nm for the E2 and testosterone ELISAs.
Gene expression
Real-time quantitative PCR (qPCR) was used to assess the expression of growth hormone 1 (GH1), gonadotropin releasing hormone 2 receptor (GnRH2), and dopamine receptor 2a (DR2a) in fry brain (the same individuals used for hormone analysis) and a subset of alevin head tissues. Genes were selected because of the role of each receptor in the development and smoltification of salmonids through the HPG, growth axis, and dopaminergic system, respectively. The expression of deiodinase 2 (DIO2) in fry brain was also assessed using qPCR, to further characterize the stressor effects to the thyroid axis observed in fry. Total RNA was extracted using the RNeasy mini extraction kit (Qiagen), and brain tissue was extracted with Trizol and chloroform to aid in the extraction of RNA from lipid tissue. The quality and concentration of RNA were checked using a Nanodrop ND-1000 (ThermoFischer Scientific), and ratios of 280/260 and 260/230 were all between 1.85 and 2.1. The integrity of RNA was confirmed by the presence of distinct 28S and 18S bands on 1% agarose gels. Complementary DNA (cDNA) was synthesized with 1 µg of total RNA using the Reverse Transcription System (Promega) with random primers according to the kit protocol to result in 20 µL of cDNA. Quantitative PCR was run using primers designed for rainbow trout (Oncorhynchus mykiss) from Integrated DNA Technologies (PrimerQuest tool; Supplemental Data, Table S1), and SSO Advanced Universal SYBR Green Supermix (BioRad) was used as the quantification dye. Protocols were optimized for primer concentration, and 1 µL of cDNA was used per well. Samples were run on a CFX Connect Real-Time PCR Detection System (BioRad) with the following protocol: 95 °C for 3 min and then 40 cycles of 10 s at 95 °C and 30 s at 56 °C. A melting curve was performed after the PCR protocol with continuous fluorescent measurement. The PCR products were run on an agarose electrophoresis gel to confirm target amplicons. Elongation factor 1α (EF1α) was used as the housekeeping gene in fry, and both EF1α and 18S were used as housekeeping genes in alevin. Percent efficiency curves were calculated for all primers using a 5-point serial dilution: DIO2 (97%), EF1α (98.9%), DR2A (97.8%), GH1 (98.1%), GnRH2 (97.9%). Fold change was calculated using the 2–ΔΔCt method.
Statistics
Differences between tank replicates were analyzed using a t test, and no significant differences between replicates at each treatment were observed. All data were tested for normality using a Shapiro-Wilk test, and Levene's tests were run to determine homogeneity of variance. Because the data were not normal, generalized linear models fitted to a Poisson distribution were used to determine the significance of the hormone and qPCR data for both alevin and fry. A Tukey's post hoc test was run to compare the significance between treatment groups if p < 0.05 for each variable and for the interaction of bifenthrin and temperature. Multivariate survival tests were run on the survival data to determine significance between survival curves of each treatment. All statistical analyses were conducted in R studio.
RESULTS
Bifenthrin water chemistry
Measured bifenthrin and recovery values are reported as averages of 0- and 96-h samples (Table 1). There were approximately 200% higher measured concentrations of bifenthrin in the 0.15 μg/L nominal treatment between 0 and 96 h. In the 1.5-μg/L treatment, measured values were 75% higher than nominal concentrations. Recovery values were calculated from PCB 206 and ranged between 118 and 137%.
| Nominal bifenthrin water concentration (μg/L) | Average measured bifenthrin ± SD (μg/L) | % Recovery ± SD |
|---|---|---|
| 0 | <LOD | 131.0 ± 25.83 |
| 0.15 | 0.4749 ± 0.1835 | 118.2 ± 34.65 |
| 1.5 | 2.63 ± 1.751 | 137.1 ± 22.99 |
- a n = 12 for bifenthrin aquaria and n = 4 aquaria for vehicle controls.
- LOD = limit of detection; SD = standard deviation.
Survival and condition factor
For all comparisons 11 °C and the ethanol vehicle controls were used as an optimal control. Alevin survival significantly decreased by 34.1% at the 19 °C exposure compared to the optimal 11 °C controls (Supplemental Data, Figure S1A). At 16.4 °C, the 1.5-μg/L bifenthrin treatment was associated with a 23.5% decrease in alevin survival compared to the vehicle control. Although bifenthrin and temperature did not significantly impact the survival of fry, there was a trend toward a decrease in fry survival after the 1.5-μg/L bifenthrin exposure in all temperature treatments (Supplemental Data, Figure S1B).
Alevin condition factor significantly decreased by an average of 7.7% in all 19 °C exposures regardless of bifenthrin exposure when compared to 11 °C exposures (Figure 1A). Similarly, fry condition factor significantly decreased with exposure to 19 °C compared to the 11 °C exposures (11 and 12%, respectively). There was also a significant decrease (9.5%) in condition factor in fry exposed to 11 °C and 1.5 μg/L bifenthrin compared to the vehicle controls (Figure 1B).

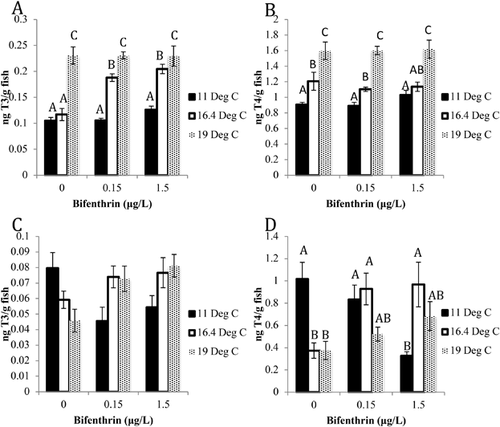
Thyroid hormone measurements
Whole-body triiodothyronine and thyroxine levels followed the same patterns in alevin after bifenthrin and temperature exposure, increasing by an average of 105 and 70.5%, respectively, from all 19 °C exposures compared to the 11 °C–treated fish (Figure 2A,B). In addition, there were 77.3 and 67.7% increases in the concentrations of triiodothyronine in alevin at the 16.4 °C temperatures treated with 0.15 and 1.5 μg/L bifenthrin compared to the controls. Fry triiodothyronine levels trended toward an increase, and thyroxine levels significantly increased (p < 0.05) at the 16.4 and 19 °C exposures with increasing bifenthrin exposure (Figure 2C,D). In contrast, triiodothyronine levels in the 11 °C–exposed fry showed a decreased trend, and thyroxine levels significantly decreased (p < 0.05) with increasing bifenthrin concentrations. Levels of triiodothyronine showed a decreased trend, and thyroxine significantly decreased (p < 0.05) with increasing temperature exposures in the vehicle control–treated fry.
Steroid hormone measurements
Testosterone in alevin treated at 19 °C was significantly increased 1.7-fold compared to the controls (Figure 3A). Temperature also significantly increased testosterone levels 2.7- and 4.4-fold in 16.4 °C exposures following treatment with 0.15 and 1.5 μg/L, respectively, compared to the 11 °C–exposed alevin in the same bifenthrin treatment. In addition, testosterone levels were significantly increased in the 19 °C exposures in the 0.15 and 1.5 μg/L bifenthrin treatments when compared to the 11 °C alevin (3.5- and 6.7-fold, respectively). Bifenthrin significantly elevated alevin testosterone levels 1.0-fold at the 19 °C and 1.5 μg/L bifenthrin treatment compared to the vehicle controls. There was also a significant temperature-dependent increase in E2 levels in alevin in the 16.4- and 19 °C–exposed fish in all bifenthrin treatments (79 and 187% average increases, respectively; Figure 3C). However, bifenthrin did not have a significant effect on alevin E2 levels.
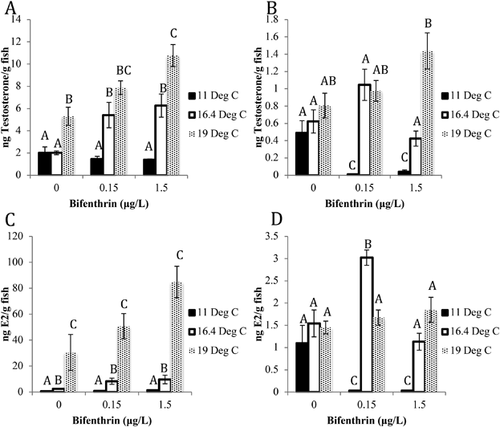
Fry testosterone was significantly decreased in the 11 °C treatment with 0.15 and 1.5 μg/L bifenthrin exposure (46.3- and 10.7-fold, respectively) compared to the control (Figure 3B). Similarly, fry E2 significantly decreased in the 11 °C treatment with exposure to 0.15 and 1.5 μg/L bifenthrin (34.5- and 36.4-fold, respectively) compared to the control (Figure 3D). There was a significant increase in E2 levels at the 16.4 and 19 °C temperatures with exposure to 0.15 μg/L (96.4- and 52.9-fold, respectively) and 1.5 μg/L (37.4- and 61.9-fold, respectively) bifenthrin when compared to 11 °C-exposed fry in the same bifenthrin exposures. There was also a significant increase in testosterone levels at the 16.4 and 19 °C temperatures with exposure to 0.15 μg/L (99.9- and 92.8-fold, respectively) and 1.5 μg/L bifenthrin (9.17- and 33.5-fold, respectively) when compared to fry at 11 °C exposed to the same bifenthrin concentrations.
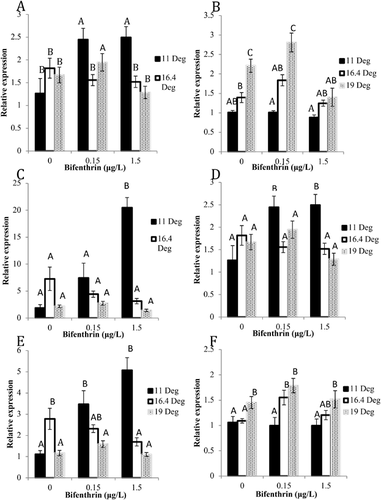
Relative expression of brain transcripts
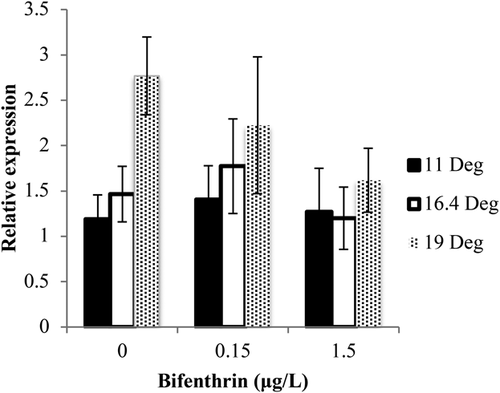
Bifenthrin significantly affected alevin GnRH2, GH1, and DR2A mRNA expression; however, temperature had a more significant effect on the expression of the same genes in fry. There was a significant increase in transcripts of GH1 (Figure 4D) and DR2A (Figure 4F) in fry exposed to 11 °C and 0.15 and 1.5 μg/L bifenthrin when compared to the control-treated fish in the same temperature. Expression of GnRH2 mRNA was significantly increased 10.0-fold in alevin exposed to 11 °C and 1.5 μg/L bifenthrin compared to controls (Figure 4A). Relative expression of the targeted genes did not significantly change in alevin exposed to 16.4 and 19 °C when treated with bifenthrin. In fry, there were 1.9- and 1.8-fold increases in GH1 in both the vehicle control– and 0.15 μg/L bifenthrin–exposed fish, respectively. Similar to alevin, both 0.15 and 1.5 μg/L bifenthrin significantly increased GnRH2 mRNA expression in fry at 11 °C compared to the controls (Figure 4B). The level of DIO2 mRNA was not significantly affected by treatments, but a trend of an increase was observed with increasing temperature in the ethanol control–treated fry (p = 0.117; Figure 5).
DISCUSSION
The San Francisco Bay delta is experiencing the effects of climate change as surface water is seasonally warming (Wagner et al. 2011). Juvenile endangered Chinook salmon (O. tshawytscha) inhabit affected waterways as alevin, fry, and parr. As a result of the enhanced intensity of storm events, bifenthrin concentrations have been observed in surface waters of the Bay delta comparable to those used in the present study, in particular the 0.15-μg/L treatment (Siepman and Holm 2000). Although bifenthrin is not acutely toxic to humans and other mammals, many studies have demonstrated that bifenthrin has adverse sublethal effects in fish (Brander et al. 2016). Thus, juvenile fish may experience increased seasonal temperatures during bifenthrin runoff events. Consequently, the effects of these combined stressors were investigated through controlled laboratory exposures of Chinook salmon at 2 developmental stages in the present study.
Survival and thyroid hormone effects
Increasing temperature without bifenthrin caused a significant decrease in alevin survival and condition factor. In fry, there was no significant mortality observed in any treatment, but there was a significant decrease in condition factor in fish exposed to 19 °C in the control and 0.15-μg/L bifenthrin treatments. Increased mortality, decreased fish health, and decreased growth with exposure to increasing temperatures attributable to climate change have been documented in juvenile teleost fish. Fish from cold and temperate waters experience faster development, leading to early hatching and increased metabolic vulnerability in juveniles when exposed to upper thermal limits (Pankhurst and Munday 2011). When juvenile Chinook salmon were exposed to 21 to 24 °C at the upper limit of their thermal tolerance, significantly lower condition factors and growth rates were observed (Marine and Cech 2004). A decrease in condition factor in both alevin and fry at the 19 °C temperature exposure in the present study suggests that the predicted end-of-century surface water temperatures in the Bay delta can negatively impact juvenile salmonid health and survival. In addition, bifenthrin decreased fry condition factor at the 1.5 μg/L concentration in salmon exposed to both 16.4 and 19 °C temperatures, indicating that bifenthrin may also impact the growth axis in fry during this period where rapid growth normally occurs and is critical for the successful survival and timing of salmonid smoltification.
The decrease in fry condition factor attributable to increasing temperature may be related to the trending decrease of both thyroid hormones. Thyroid hormones, specifically triiodothyronine, play a major role in rapid growth and development in juvenile salmonids (Tagawa and Hirano 1987; Farbridge and Leatherland 1992; Dickhoff et al. 1997). In alevin, triiodothyronine and thyroxine followed the pattern of increasing concentration after exposure to both increasing bifenthrin and temperature. Higher thyroxine levels and low triiodothyronine levels suggest that thyroxine is not converted into triiodothyronine by deiodinase, specifically in the fry stage. The hypothalamus of the brain signals the pituitary gland to release thyroid-stimulating hormone, which subsequently releases the precursory thyroxine to be converted by deiodinase to the biologically functional triiodothyronine (Ban 2004). We observed that alevin triiodothyronine and thyroxine increased with temperature, so bifenthrin and temperature may not be impacting deiodinase activity at this life stage because triiodothyronine and thyroxine were not measured in inverse concentrations. If we observed an increase in triiodothyronine and a decrease in thyroxine, this would suggest that deiodinase is converting thyroxine to triiodothyronine, which was observed as a trend at the high-temperature exposures in fry. There is evidence that larval fish use maternal thyroid hormones in growth and development until they develop their own thyroid follicles to produce thyroxine (Blanton and Specker 2007). Alevin may not be producing endogenous triiodothyronine and thyroxine at this stage, so healthy control fish may be using more of the maternal hormones for growth compared to the bifenthrin and high temperature–treated alevin. Less chemical and environmental stress may allow the alevin to grow and develop faster than stressed fish, and this is seen in the condition factor results.
To further investigate our thyroid hormone results, we measured the relative expression of DIO2 mRNA to determine if bifenthrin and/or temperature had an impact on fry. A trend toward an increase of deiodinase mRNA expression with increasing temperature was consistent with lower thyroxine levels at the 16.4 and 19 °C exposures compared to the 11 °C exposures. Expression of DIO2 also trended toward a decrease with increasing bifenthrin concentration at the 19 °C exposure, which also corresponds to the same decreasing trends observed in fry triiodothyronine and thyroxine. Thyroid hormone levels change throughout salmonid development, and thyroxine levels typically decrease as triiodothyronine levels increase during periods of rapid growth after yolk absorption as a result of the formation of thyroid follicles in older juvenile stages (Tagawa and Hirano 1987). However, studies with other teleost fish have found that increasing temperature does not directly affect deiodinase activity; rather, the levels of altered thyroid hormones are attributable to temperature impacts upstream of thyroid hormone conversion (Van Den Burg et al. 2003). Contrasting these results, a decrease in DIO2 was observed in zebrafish embryos exposed to nominal concentrations of 1, 3, and 10 μg/L of bifenthrin (Tu et al. 2016). The impacts of temperature and bifenthrin on DIO2 in fish are unclear and suggest multiple crosstalk hormonal targets that may undergo equally subtle changes. For example, increasing sublethal temperatures have been shown to promote the increase of thyrotropin-releasing hormone and to trigger crosstalk with cortisol pathways that can also regulate downstream thyroid hormone production in fish (Van Den Burg et al. 2003). Alternatively, molecular docking studies have indicated that bifenthrin can effectively bind to thyroid hormone receptors in zebrafish (Tu et al. 2016). Additional studies exploring crosstalk linkages with other hormonal systems involved in thermoregulation within poikilothermic organisms are necessary to identify the role of the HPT axis in bifenthrin toxicology.
Sex steroid effects
The most notable response in the HPG axis endpoints examined in the present study was the significant decrease in E2 and testosterone at the 11 °C temperature with increasing concentrations of bifenthrin in fry. Aromatase (CYP19A1) converts testosterone to E2 in both fish brain (CYP19A1b) and gonadal tissues (CYP19A1a; Piferrer and Blázquez 2005), and studies have shown that aromatase enzyme activity is reduced by temperature in fish (Shen and Wang 2014). Although salmonid sex differentiation is genetically determined (rather than through temperature-dependent sex determination), higher temperature exposures can lead to masculinization in many other teleost species by decreasing aromatases (Piferrer and Blázquez 2005). However, fry E2 and testosterone followed the same pattern of decreasing levels at 11 °C and increasing levels at 16.4 and 19 °C with bifenthrin exposure in the present study, and we observed no significant differences in steroid levels in control fry in each temperature treatment. The decrease of the sex steroid hormones at optimal temperatures suggests upstream modulation of the HPG axis by bifenthrin in the sex steroid biosynthetic pathways because the activity of aromatases in fry does not appear to be affected by either temperature or bifenthrin, as evidenced by the same profiles of E2 and testosterone. Cholesterol-based hormones, such as pregnenolone and progesterone, are synthesized to E2 or testosterone in both the brain and gonads by a series of cytochrome P450s and hydroxysteroid dehydrogenase enzymes (Sanderson 2006), so it is possible that bifenthrin could target other components of steroid biosynthesis signaling upstream of E2 synthesis.
The stage-dependent effects of diminished sex steroids between fry and alevin may be attributed to the incomplete development of gonads at the earlier larval stage. Rainbow trout become sexually dimorphic at approximately 35 d postfertilization (Vizziano et al. 2007), which is comparable to the same alevin stage in Chinook salmon. Although salmonids are sexually differentiated at the alevin stage, sex steroid synthesis is localized to a group of somatic cells in the precursory gonads (Vizziano et al. 2007). The gonads in juvenile salmonids are still developing through smoltification; therefore, gonad and HPG development may be more sensitive to stress from temperature compared with older, mature fish. Life stage has been shown to be important with regard to the estrogenic effects of bifenthrin, with greater estrogenic activity noted in juveniles relative to embryos in zebrafish (Bertotto et al. 2018). Temperature increases the rate of development and growth of juvenile salmonids as long as there is ample food availability (Brett et al. 1969; Marine and Cech 2004); however, as juvenile fish approach their critical thermal maximum, nutrient conversion and basic physiological processes are impaired (Brett et al. 1969). If young salmonids are rapidly developing as a result of warmer temperatures, there could be a threshold where excessive temperatures lead to developmental impairment, specifically in the HPG pathways. The differences we observed in E2 and testosterone levels with exposure to bifenthrin may be attributable to incomplete development of the HPG pathway in earlier life stages, and differences observed as a result of temperature may be attributed to basic changes in the rate of development. Whatever the mechanism, it is clear that temperature impacted the stage-dependent effects of bifenthrin, which raises concern for how temperature will affect the toxic mechanisms of other potential endocrine-disrupting compounds in aquatic systems.
Dopamine receptor effects
Dopaminergic signaling is a critical regulator of gonadotropin release in the HPG axis. Dopamine has an inhibitory effect on GnRH hypothalamic neurons by binding directly to dopamine 2 receptors (DR2A) and thus prevents the release of GnRH and the cascade of downstream HPG axis signaling (Levavi-Sivan et al. 2004; Gopurappilly et al. 2013). In the present study, GnRH2 was chosen as an endpoint because of its role in the neuroendocrine system and in fish sensory behavior, and it is well characterized in salmonids (Wootton and Smith 2015). Overall, dopamine prevents release of gonadotropins and subsequently lowers E2 and testosterone levels (Dufour et al. 2005). Given the altered concentrations observed in the temperature and bifenthrin treatments, the role of the dopaminergic system was evaluated. Expression of DR2A mRNA was unchanged with bifenthrin exposure in fry at the higher temperature exposures but increased with bifenthrin exposure at 11 °C. In contrast, GnRH2 mRNA was lower in control fry at 11 °C compared to the higher temperatures where there was a significant increase with bifenthrin. Similar to fry, there was also a significant increase in alevin DR2A mRNA with bifenthrin exposure at 11 °C, indicating that bifenthrin has concentration-dependent effects on DR2A mRNA expression at optimal temperatures at both the alevin and fry life stages. Although DR2A expression was not significantly changed in alevin exposed to 19 °C in any bifenthrin treatment, E2 and testosterone levels significantly increased with increasing bifenthrin compared to the other temperatures. This suggests that negative feedback through DR2A interactions on hypothalamic neurons to reduce GnRH levels may not be occurring at lower temperatures but that disruption may be occurring at higher temperatures and with bifenthrin exposure. Ratios of dopamine and its metabolite 3,4-dihydroxyphenylacetic acid have been shown to increase in juvenile zebrafish exposed to bifenthrin (Bertotto et al. 2018). Although this did not correlate with significant changes in estradiol, it does indicate that bifenthrin can possibly impact dopamine production or metabolism. Another variable is the relationship between dopamine and DR2A expression. It is unclear whether DR2A undergoes autoregulation by dopamine or its metabolites. Thus, to assume a direct relationship between dopamine and its receptor is unfounded. In addition, control of the HPG axis by the dopaminergic system may not be fully developed in salmon alevin and fry compared with older juvenile-stage animals (Levavi-Sivan et al. 2004; Crago and Schlenk 2015). If the dopaminergic system was actively regulating the production of sex hormones in alevin through negative feedback, then we should observe an increase in dopamine, which would presumably increase DR2A as a result of an increase of GnRH to potentially lower the elevated sex hormone levels in the higher temperature exposures. However, this correlation was not observed, so the developing dopaminergic system may not be fully capable of regulating HPG feedback in alevin or temperature may be disrupting other HPG feedback processes. Clearly, additional study is needed to evaluate the stage-dependent regulation of the HPG axis in teleosts.
Growth hormone receptor effects
An additional hormonal pathway that regulates fish condition and/or growth is that controlled by growth hormone. Bifenthrin had concentration-dependent effects on GH1 mRNA expression at optimal temperatures at the alevin life stage. Growth hormone is critical to the development of juvenile fish and eventually to the smoltification of juvenile salmonids (Melamed et al. 1998; Nilsen et al. 2007). Growth hormone stimulates thyroid hormone activity in trout (MacLatchy and Eales 1990), and GnRH and sex hormones partially regulate growth hormone during sexual development in fish (Björnsson et al. 2002). Both decreasing thyroid hormones and increasing growth hormone levels may play a role in the regulation of growth hormone receptor in developing vertebrates by increasing growth hormone receptor expression (Schwartzbauer and Menon 1998). We observed that bifenthrin and temperature impact growth and expression of GH1 in alevin but that temperature only significantly increases triiodothyronine levels in alevin. If triiodothyronine was independently regulating GH1 expression, we would expect to see lowered GH1 expression in all 11 °C-treated fish. However, we did not see this correlation. In fry we observed an increase in GH1 expression with temperature, but triiodothyronine levels were lower in 11 °C bifenthrin-treated fish. This suggests that in the later life stage the increase in GH1 expression could be attributable to a subtle decrease in triiodothyronine and possibly to an increase in growth hormone. Especially in alevin, growth hormone may be a potential link in the crosstalk between HPG and HPT pathways because of the rapid growth at these earlier stages of overall development.
SUMMARY AND CONCLUSIONS
Although we observed lethality attributable to increasing temperature and bifenthrin in alevin, the results showed a range of adverse sublethal effects in both the alevin and fry life stages. Increasing temperature and bifenthrin concentrations may have negative impacts to juvenile salmon. Altered reproduction, viability, and growth were several of the adverse transgenerational effects observed from low (1 ng/L) bifenthrin and increased temperature exposures in Menidia beryllina (Decourten and Brander 2017). Similarly, we found that subchronic exposure to increasing temperature and bifenthrin can adversely affect growth as well as thyroid and estrogenic signaling pathways in developing juvenile salmonids. Although exposures to bifenthrin and elevated temperature do not cause direct mortality on all stages of juvenile salmonids (Weston et al. 2015; Crago and Schlenk 2015), the adverse sublethal effects on endocrine and reproductive pathways may have ongoing population-level effects (Baldwin et al. 2009; Forsgren et al. 2013). The present results indicated that climate change and contamination of human-made chemicals may affect endangered salmon populations and demonstrate the need to consider the developmental stage of fish when determining the risks of climate change and chemical stressors.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.4372.
Acknowledgment
The present study was supported by the Delta Stewardship Council Delta Science Program and the State and Federal Contractors Water Agency (SFCWA). The contents do not represent the official views or policies of the SFCWA. We thank D. Luu for help with sample preparation and analysis and J. Richards in the Jay Gan lab for help setting up analytical chemistry protocols.




