Rethinking microplastics as a diverse contaminant suite
Microplastics are not microplastics are not microplastics, just like pesticides are not pesticides are not pesticides. “Microplastics,” like other classes of chemical contaminants, is a catch-all term for a variety of unique chemical compounds. Yet, many scientific publications, policy reports, and media articles present microplastics as if they are simply a single compound or type of material.
Such simple communications have consequences, leading to simplified studies and protocols that may be inadequate to inform us of the sources and fate of microplastics, as well as their biological and ecological implications. For example, studying the fate and effects of one plastic type with a specific shape and size does not tell us the fate and effects of microplastics in general. Moreover, not recognizing the diversity of materials in a microplastics sample may overlook the complexity necessary to inform robust quality analysis and quality control (QA/QC) needed in sampling and analytical measurement techniques. For instance, some methods are better at recovering specific sizes, shapes, or types of microplastics.
Simplifying microplastics as a single compound has also led to confusion around the need for new policies and strategies to reduce future emissions of microplastics. For example, some policymakers and scientists are under the impression that banning microbeads from rinse-off personal care products has eliminated future releases of microplastics in general to the environment. In reality, such bans eliminate only one source of the diverse and complex emerging global contaminant suite that is “microplastics.” This can be compared to banning one specific use of a pesticide (e.g., in the home), leaving the market full of other applications of diverse pesticides that need to continue to be assessed for environmental persistence, bioavailability, and toxicity.
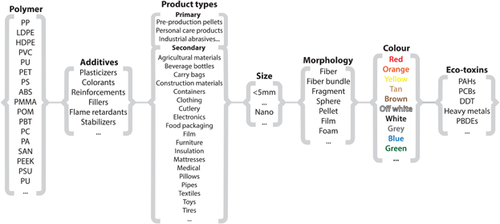
In our Focus article, we make the case that it is necessary to rethink microplastics (plastic particles <5 mm in size) and consider them a suite or class of contaminants, in the same way we do for pesticides, trace metals, or flame retardants. Microplastics are diverse; they come from many different product types; incorporate a broad range of sizes, colors, and morphologies; are composed of various polymers; and include a broad array of chemical additives (Figure 1 and Textboxes 1 and 2). This diversity is important to consider, and thinking of them like we do other classes of contaminants may help us advance methods for sampling and analysis and help us better understand the sources from which they enter the environment; their fate in water, sediment, and organisms; their toxicity; and relevant policies for mitigation.
RETHINKING MICROPLASTICS TO INCORPORATE THEIR DIVERSITY
Just like pesticides are made of diverse molecules, have varying molecular structures, and can be used for a variety of applications (e.g., fungicides, herbicides), microplastics are made from diverse molecules, have varying molecular structures, and come from products with various applications (e.g., tires, textiles, and packaging). What is unique to pesticides and other chemical contaminants is that microplastics are particles, comprising different sizes, shapes, and colors. Microplastic particles are not simply “microplastic” but a diverse suite of contaminants that we refer to as “microplastics.”
Diverse product types
As a contaminant class, microplastics come from a large diversity of product types and are generally classified as either primary or secondary. Primary microplastics are manufactured to be <5 mm in size. They include preproduction pellets used to make plastic products and microbeads used as abrasives for industrial purposes or in personal care products. Secondary microplastics are small pieces of plastic which are not produced intentionally but instead are the result of the breakup and fragmentation of larger plastic items via biological, physical, and chemical processes. Secondary microplastics can form during product use (e.g., microfibers shed from clothing during washing or tire wear particles) or once released into the environment (e.g., via fragmentation). Fragmentation is mediated by the polymer type and environmental conditions, which can be highly variable (Sivan 2011; Gewert et al. 2015). Microplastics can be a by-product of many plastic products, including construction materials, agricultural materials, furniture, clothing, and food packaging (Figure 1).
Diverse sizes
Microplastics encompass a broad range of sizes. Most often, they are defined as any plastic particle <5 mm in one dimension as defined by the National Oceanographic and Atmospheric Association (Figure 1). Others argue for size categorization that matches the metric system (e.g., 1–999 μm are microplastics). However, there is generally no lower limit, and nano-sized plastics (<0.1 μm) are often included in this definition. The way that discrete sizes and ranges of size are reported among researchers varies. For example, researchers may use multiple grades of sieves to size-fractionate samples, and thus their categorization is defined by the sizes of their sieves used during sample preparation.
Defining how microplastics are described in relation to size is a prevalent topic of discussion among researchers in the field. Historically, researchers predominantly sampled microplastics using manta trawls with a 333-μm mesh net, but methods are evolving toward using smaller mesh sizes or collecting bulk water (Barrows et al. 2017). In addition, researchers are beginning to expand their analytical techniques to incorporate those that can detect and identify smaller and smaller microparticles. As a result, the sizes of microplastics reported in the literature are becoming more diverse, incorporating a broader range that is dictated by the lower limit defined by our sampling or analytical methodologies.
Diverse shapes and colors
Microplastics come in many shapes and colors. The shape of a microplastic is often used to assign it to a common category, which helps inform the source (Helm 2017). Generally, researchers use somewhere between 4 and 7 different categories defined by shape or morphology, which include fiber, fiber bundle, fragment, sphere (or bead), pellet, film, and foam (Figure 1). To help with source apportionment, we know that certain shapes are generally shed from different products. This provides clues related to where microplastics in nature may originate. For example, fibers and fiber bundles tend to shed from clothing, upholstery, or carpet; pellets are generally associated with industrial feedstock; spheres may be microbeads from personal care products or industrial scrubbers; and foam often comes from expanded polystyrene foam products such as insulation or food packaging. Detailed descriptions and images of each of these shapes can be found in Textbox 1.
TEXTBOX 1: Fibers may have clean-cut ends (a) or fraying (b). Fiber bundles (c) are in a tightly wound mass that cannot be untangled. Fragments are rigid (d,e) and irregular (f). Spheres (g) are round with a smooth surface. Pellets (h) are typically rounded or cylindrical. Films (i) are flat, thin, and malleable. Foams (j) are soft and compressible
| TEXTBOX 1: Fibers may have clean-cut ends (a) or fraying (b). Fiber bundles (c) are in a tightly wound mass that cannot be untangled. Fragments are rigid (d,e) and irregular (f). Spheres (g) are round with a smooth surface. Pellets (h) are typically rounded or cylindrical. Films (i) are flat, thin, and malleable. Foams (j) are soft and compressible. | |
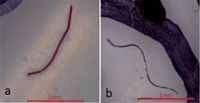 |
Fibers are flexible, with equal thickness throughout and ends that are clean-cut, pointed, or fraying. Typically, they are tensile and resistant to breakage. The durability varies among materials and their state of degradation. Fibers are present in a range of colors, which may be inconsistent across one particle due to bleaching. |
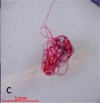 |
Fiber bundles comprise 20 or more individual fibers tightly wound in a mass that cannot be untangled. Fiber bundles should only be classified as such when it is too challenging to quantify individual fibers or when untangling the mass may lead to breakage of individual fibers. Fibers present in bundles should be consistent in appearance (i.e. color, thickness, surface texture). |
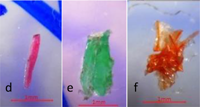 |
Fragments have a rigid structure and sometimes irregular shape. They can be round, subround, angular, or subangular. They are not always equally thick throughout and can appear twisted or curled. Shavings, droplets, and seams from plastic manufacturing fit within this category. Fragments can be any color or combination of colors. |
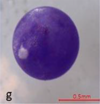 |
Spheres are round in shape with smooth surfaces. Spheres may also be present as hemispheres, possibly the result of breakage during manufacturing, use, or weathering. They typically range in size between 100 µm and 2 mm. |
 |
Pellets (sometimes called “nurdles”) are similar to spheres but tend to be larger, generally ranging between 3 and 5 mm. Pellets are often rounded or cylindrical in shape. Both spheres and pellets can be any color. |
 |
Films are flat, thin, and malleable. Films can fold or crease but do not break apart easily. Films are typically partially or fully transparent and are found in a range of colors. |
 |
Foams are soft, compressible, and cloud-like. They are usually white and/or opaque but can be any color. |
Diverse polymer types
Microplastics are composed of a diverse suite of polymer types just like pesticides are composed of a diverse suite of molecular structures. All plastic polymers consist of repeating monomers, which form the backbone of the polymer. This backbone structure is the fundamental difference between polymer types, informing a plastic's physical and chemical properties (Textbox 2). The most produced and consumed polymer types are polypropylene, low-density polyethylene (LDPE), high-density polyethylene (HDPE), polyvinyl chloride (PVC), polyurethane, polyethylene terephthalate (PET; also known as polyester), and polystyrene (PlasticsEurope 2017). This diversity of polymers is necessary to fulfill the many applications of plastics. For example, LDPE is too flimsy to be used in water bottles, and thus PET is used instead. Also, LDPE is often used in single-use shopping bags, food packaging, and film. In addition to bottles, PET is produced in fiber form to make synthetic clothing.
Although the polymers listed in the previous paragraph are the most commonly used, countless others exist. Plastics are divided into 2 families: thermoplastics and thermosets. Thermoplastics can be melted when heated and hardened when cooled. They include many of the plastics described in the previous paragraph (PET, polypropylene, polystyrene, LDPE, HDPE, polyurethane, PVC) and others, such as acrylonitrile butadiene styrene, polymethyl methacrylate, polyoxymethylene, polybutylene terephthalate, polycarbonate, polyamides, elastomers, styrene-acrylonitrile, polyether ether ketone, fluoropolymers, and polyarylsulfone. Thermoset plastics undergo a chemical change when heated. They include polyurethane, epoxy resins, acrylic, urea-formaldehyde, vinyl esters, and phenolic resins. Thus, microplastics are not comprised of one material; instead, they come from a complex group of hundreds of chemically unique substances.
| TEXTBOX 2: Some examples of different chemical structures of polymers associated with common plastic types. | |
| Polyethylene terephthalate (PET) | 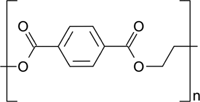 |
| Polypropylene (PP) | 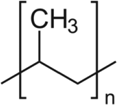 |
| Polystyrene (PS) | 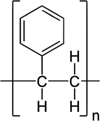 |
| Polyethylene (PE) | 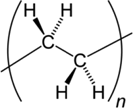 |
| Polyvinyl chloride (PVC) | 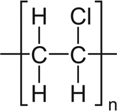 |
| Polyurethane (PU) | 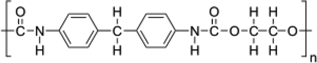 |
Diverse chemical cocktails
For some applications, chemical contaminants like pesticides or flame retardants are used in mixtures of various different chemical congeners. For microplastics, they are always found in the environment as a mixture or diverse suite of chemicals. Although plastic is often described as an inert material because of its bulky molecular structure, every piece of plastic contains a complex chemical cocktail of monomers, oligomers, and additives. In addition, chemical additives are added to the polymers during production, sometimes accounting for a large proportion of the overall weight (e.g., phthalates, which are used to alter the properties of plastics, can comprise up to 50% of a PVC product's total weight). There are several categories of additives, including plasticizers, colorants, reinforcements or fillers, flame retardants, and stabilizers. Plasticizers, such as phthalates, alter plastic from a hard, glassy material into a soft, rubbery material. Colorants are used to color the plastic product. Reinforcements or fillers enhance the mechanical properties of plastic, such as the strength of the material. Flame retardants are used for specific applications, such as building materials and electronics. Stabilizers increase the longevity and stability of an end product.
When found in nature, the complex mixture of chemicals associated with microplastics also includes sorbed pollutants. Microplastics accumulate organic chemicals and trace metals from the surrounding environment (Rochman 2015). For example, microplastics are known to sorb persistent organic pollutants, such as polycyclic aromatic hydrocarbons, polychlorinated biphenyls, and DDT, as well as trace metals (e.g., copper and lead). The type and amount of chemicals a piece of plastic accumulates depends on the physical and chemical properties of the polymer, such as diffusivity, crystallinity, hydrophobicity, and surface area (Rochman 2015).
RETHINKING MICROPLASTICS TO ADVANCE RESEARCH AND POLICY
The complexity of chemical classes, like pesticides or flame retardants, informs how we measure and manage them. Likewise, the immense diversity in microplastic types, morphologies, sizes, color, and chemical mixtures should inform how we measure microplastics and how we use data to inform policy. The methods used to monitor microplastics in the environment should be developed with careful consideration of this diversity. For example, different methods will perform better for capturing or analyzing certain sizes or densities of microplastics. Diversity should also be considered when studies are designed to test hypotheses about the sources, fate, and effects on organisms and ecosystems. Finally, these differences are important to consider when making decisions relevant to mitigation. Some strategies will be very effective for some microplastics but less relevant to others. Rethinking microplastics as a contaminant suite, like other contaminant suites, to include the diversity described in the present report will inform Methods for sampling, quantifying, and reporting.
Methods for sampling, quantifying, and reporting
Microplastics are increasingly being added to government lists as priority contaminants to monitor because of their ubiquity in the environment and concerns regarding negative impacts. As monitoring strategies are designed for microplastics, researchers and environmental managers can take lessons from best practices for other contaminant suites to determine the best practices for sampling, quantifying, and reporting microplastics in environmental studies. The field of environmental chemistry has been establishing protocols and best practices for monitoring and evaluating concentrations of contaminants in the environment for decades. These best practices reduce procedural contamination, increase recovery, and have good QA/QC that provide robust and trustworthy results. We recommend common analytical practices for chemicals that should be considered during sample collection, sample preparation, sample analysis, and data reporting for microplastics (Figure 2).
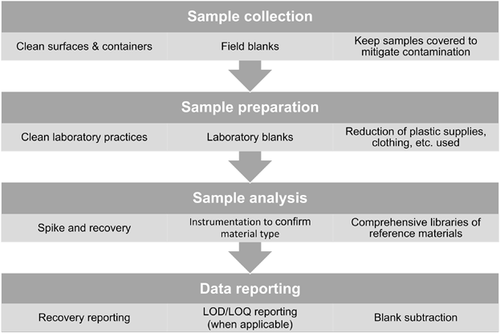
To quantify chemicals in the environment, care should be taken to reduce procedural or cross-contamination during sampling, preparation, and analysis. To reduce procedural and cross-contamination of microplastics in samples (e.g., from dust or clothing worn during sampling), appropriate (e.g., glass) and precleaned (e.g., rinsed 3 times with filtered water) storage containers should be used during sample collection as a criterion for good QA/QC. During sample preparation (e.g., extraction, cleanup), clean techniques should also be required to reduce cross-contamination and procedural contamination. These include washing tools between samples, covering samples with aluminum foil as often as possible to protect from dust, and cleaning the working space prior to use. Researchers should also consider personal protective equipment (gloves, natural fiber laboratory coats, etc.), air filters that remove dust (e.g., high-efficiency particulate air filters), and clean rooms or cabinets. Training and proficiency testing of laboratory personnel are also important to ensure sample quality and limit contamination during sample handling.
To account for any procedural and cross-contamination and to measure the effectiveness of the sample extraction and/or cleanup, field blanks, laboratory blanks, and spike recoveries should be included, as they are for chemical analysis. Before using an extraction and cleanup method, a spike-and-recovery exercise should be performed to quantify the recovery of different sizes and types of microplastics with the methods that will be used. Importantly, researchers should collect field and laboratory blanks to account for procedural contamination during sampling and laboratory protocols. The levels in the blank can be subtracted from environmental samples or at least reported in the results section. When subtracted, they can be subtracted by color/category or color/chemical identifier, in the same way chemicals are subtracted by congener when analyzing flame retardants or pesticides.
The establishment of universal criteria for identifying diverse microplastics to material type and for reporting data is needed. For small size ranges (i.e., <1 mm) and some morphologies (e.g., fiber), visual identification becomes less accurate and a chemical identifier is necessary to tell if something is truly plastic (Lenz et al. 2015). Comprehensive libraries of reference materials for microplastics, encompassing pure polymers, plastic products, and environmental samples, for common analytical methods, such as Raman spectroscopy and Fourier-transform infrared spectroscopy, will help make chemical characterization of polymers standardized, faster, and more accurate. When reporting microplastics in samples, it is important to report the count, shape, color, size, and material type (if possible). It would also be prudent to report results from recovery tests and the limit of detection related to particle size or mass determined by extraction and analysis methods.
Source of microplastics
Microplastics enter the environment via diverse sources and pathways (Figure 3), as described previously. Because microplastics are small and often the weathered remnants of their original product, it can be difficult to trace them back to their source. Still, it is useful to try to source-apportion them (i.e., trace microplastics back to their original products) by examining their size, color, shape, and polymer type (Helm 2017). These features of microplastics can serve as clues for determining what product they came from. For example, the spherical shape of microbeads identified as polyethylene via spectroscopy generally infers an origination from personal care products. Microplastics with a cylindrical or oval shape, which are roughly 3 to 5 mm in size, are generally industrial pellets. Finally, colorful polyester microfibers likely originated from synthetic clothing or other textiles.
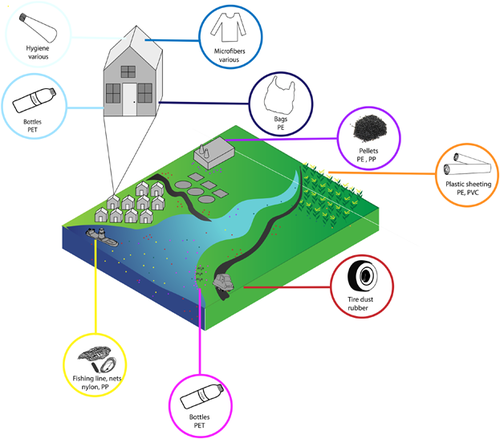
These differences across microplastic particles may also help determine how and from where microplastics entered the environment. For example, storm water runoff includes litter, abrasion from car tires, and road paint. Wastewater effluent includes microbeads from personal care products and microfibers from textiles. Agricultural runoff may incorporate microplastics degraded from greenhouse films, plastic mulch, irrigation systems, and planters. Other sources include industrial spills and runoff from industrial processes, leakage from end-of-life vehicle yards, recycling facilities, landfills, and fishing gear. Thus, rethinking microplastics to incorporate their diversity will help identify dominant sources, which is ultimately linked to mitigation.
The fate of microplastics
The fate of microplastics in the environment seemingly has no boundaries, and they have become ubiquitous in ecosystems globally. The type, size, shape, and color of microplastics are important factors that inform their fate in the environment and in biota (Figure 4). The density, related to polymer type, will inform the fate of a microplastic particle in aquatic systems and in the atmosphere. In aquatic systems, denser plastics, such as PET or PVC, are expected to sink and are likely more common in sediments than lighter plastics such as polyethylene or polypropylene, which are expected to float. In the atmosphere, denser plastics are less likely to be picked up by wind and thus should disperse less readily. Other factors such as the shape, size, or presence of a biofilm may also alter the fate of microplastics in the environment.
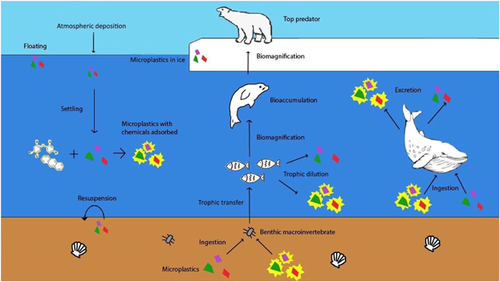
The size of a microplastic particle may inform how microplastics are transported and where they ultimately end up. Because microplastics comprise various sizes, they can be ingested by all animals, from the smallest zooplankton to large predatory fish and birds. For certain sizes (<150 μm), microplastics are expected to be able to leave the gut and enter cells, organs, and other tissues in living organisms (Lusher et al. 2017). As the size of microplastics decreases, the potential for transfer outside of the gut is expected to increase. This transfer, or translocation, may facilitate bioaccumulation or even biomagnification in food webs.
Size, in addition to shape, may also affect the retention time in biota. For example, fibers may become entangled within the intestinal tract and be retained in the organism for a longer duration of time. Finally, the color may affect whether an animal chooses to ingest microplastics or not. Some research suggests that microplastics with specific colors are preferentially selected by some organisms.
The toxicity of microplastics
Many researchers debate whether exposure to microplastics will have a negative or neutral effect on organisms. The relevant literature provides evidence supporting both sides of this argument (Foley et al. 2018). In many studies, exposure to microplastics has been found to negatively affect an organism's gene expression, survival, or reproductive output. However, in other studies, biological effects from microplastics are not detected. This discrepancy may be partly a result of the great diversity of physical and chemical characteristics of the microplastics to which organisms are being exposed.
The extent to which microplastics cause harm to an organism likely depends on polymer type, size, shape, and associated chemical mixtures. This is not unexpected given evidence demonstrated by numerous laboratory studies using different combinations of these characteristics. Some polymer types are thought to be more harmful than others based on their constituent monomers or chemical additives (Lithner et al. 2011). Some polymers may be carcinogenic or mutagenic based on their chemical ingredients (e.g., PVC, polyurethane), whereas others are thought to be more inert (e.g., polyethylene, polypropylene). Further, microplastics with high surface area to volume ratios (small, elongated, or irregularly shaped) have a higher sorption capacity, which may lead to the accumulation of harmful chemicals that may be transferred to organisms that ingest microplastics (Rochman 2015). There is some evidence that suggests that the physical characteristics of microplastics, such as size, may impact toxicity. Small microplastics (e.g., nanoplastics) are of particular concern for toxicity because of their potential ability to transfer between the tissues and cells of organisms (Lusher et al. 2017). Presently, we have a limited understanding of whether demonstrated effects are attributable to the chemical or physical characteristics of the particles or, more likely, both.
The diversity of microplastics, coupled with the gamut of potential toxicological associations, makes it difficult to generalize the effects of microplastics on organisms. While studies show a range of negative or neutral effects (Foley et al. 2018), it is often the case that laboratory studies expose organisms to pristine microplastic spheres and at high concentrations. This is an unrealistic scenario that is not representative of the diversity of microplastics wild populations of biota may encounter. Clearly, the complex nature of microplastics, similar to other contaminant classes, can make it difficult to design laboratory studies that capture environmentally relevant scenarios. However, careful consideration can be made with regard to the type, shape, and size of plastic being used, in addition to its chemical cocktail. With carefully designed laboratory studies, the effects of specific classes of microplastics will become clearer. After all, just as we should not be asking what are the effects of pesticides in general, we should not be asking what are the effects of “microplastics” in general. Instead, we should be asking about the effects of a range of types, shapes, and sizes of microplastics, just like we do for specific chemical congeners of pesticides.
Effective policies for mitigation
Given the range of sources of microplastic pollution, it is not surprising that few solutions have been implemented to address microplastic pollution at large. Although all mitigation strategies relevant to plastic debris are relevant to microplastics (because large plastics break up into small plastics), there are some solutions that can be designed specifically to tackle microplastics. For microplastics in personal care products, many countries (e.g., Canada, the United Kingdom, New Zealand, and the United States) have passed legislation to ban microbeads used in rinse-off products. For preproduction pellets, there is a voluntary industry initiative, Operation Clean Sweep, for companies to prevent emissions of their pellets or scrap and a law in California to control release of plastic pellets into the environment.
Despite increasing efforts to address plastic pollution for large plastic debris and few to address some sources of microplastics, the governance of microplastic pollution is challenging. The diversity of sources, morphologies, and chemical mixtures is an important consideration when attempting to prevent emissions of microplastics to the environment or to enact cleanup. Implementing effective mitigation strategies to address some of the main sources of secondary microplastics, such as the shedding of microfibers from textiles, the release of industrial abrasives, and tire wear particles from road runoff, requires specific and novel solutions.
Indeed, there is no one-size-fits-all solution to microplastic pollution, and diverse strategies should be considered that are relevant to specific types, sources, and pathways. For example, microfibers are among the most common types of microplastics found in environmental samples, and we know washing clothing is one source. As such, filters on washing machines may be a simple solution to prevent the release of microfibers into the environment. In addition, tire wear particles are estimated to be a large fraction of microplastics in storm water runoff that leads to the environment. If bioretention cells or rain gardens were added to storm drains, we may reduce the amount of tire wear particles that are entering urban watersheds. By designing research programs that consider the diversity of microplastics to inform sources, we can inform decision-makers of the most relevant sources of microplastics on which to focus.
CONCLUSION
As we move forward, we must appreciate the complexity of microplastics. They are diverse. They come from a multitude of sources; comprise different sizes, shapes, colors, and types of materials; and include a mixture of diverse chemicals. They migrate through nature via diverse pathways and affect biota and ecosystems in different ways. We suggest a change in our thinking: from one contaminant “microplastic” to a diverse suite of contaminants “microplastics.” If we want to truly understand microplastics the way we have come to understand pesticides and flame retardants, we must recognize this diversity in our study design, monitoring protocols, methods of analysis, assessments of toxicity, and our communication with policymakers and the public. Yes, governance and mitigation strategies to address microplastics are challenging, especially given that microplastics are not microplastics are not microplastics. The diversity of microplastics is an important consideration when attempting to prevent emissions to our global ocean, freshwater, and terrestrial ecosystems.
Acknowledgment
We thank Bear, the dog, for helping us randomize the order of all authors who contributed equally to the manuscript. We also thank Bear's owner, D. Luo, for aiding in Bear's author determination activity. We thank the Gordon and Betty Moore Foundation, 5Gyres, and the San Francisco Estuary Institute for providing hundreds of environmental samples to our laboratory that showcase the diversity of this complex emerging contaminant. The authors declare no conflicts of interest.




