Analysis of transcriptional response in zebrafish eleutheroembryos exposed to climbazole: Signaling pathways and potential biomarkers
Abstract
Climbazole is an antifungal active ingredient used in personal care products. After application this chemical reaches the aquatic environment and may pose a risk to fish. In the present study, we measured the transcriptional effects of essential genes related to a wide range of signaling pathways on zebrafish eleutheroembryos exposed to climbazole at environmentally relevant and predicted worst-case environmental concentrations, and explored the potential biomarkers via partial least squares discriminant analysis. Transcription analysis covering up to 73 genes revealed significant down-regulation of circadian rhythm- and steroidogenesis-related genes in zebrafish embryos and larvae after exposure to environmentally relevant concentrations of climbazole. This topical antifungal agent also modulated the transcripts of genes involved in inflammation, oxidative stress, oocyte maturation, and sexual differentiation at predicted worst-case environmental concentrations. In addition, mprα, igf3, nr1d1, nr1d2b, cyp19a1a, vtg1, il-1β, and il-8 were chosen as potential biomarkers in embryonic zebrafish following exposure to climbazole. These findings can help us understand the remarkable transcriptional response to climbazole in the early life stage of zebrafish. Future research should elucidate whether the transcriptional modulation translates into metabolic phenotypes associated with the corresponding signaling pathways. Environ Toxicol Chem 2019;38:794–805. © 2019 SETAC
INTRODUCTION
Azole fungicides are a class of antifungal chemicals with at least one 5-membered heterocyclic ring containing 2 or 3 nitrogen atoms in their structures. The antifungal activities of azole fungicides are based on their inhibition of synthesis of the essential membrane component ergosterol through high-affinity binding to the haem of the enzyme sterol 14α-demethylase by the N-3 (imidazole) or N-4 (triazole) substituent of the azole ring (Joseph-Horne and Hollomon 1997). Unfortunately, azole fungicides were shown to modulate the other cytochrome P450 (CYP450) enzymes such as aromatase and disturb the balance of androgens and estrogens (Zarn et al. 2003). For example, brain cyp19b messenger ribonucleic acid (mRNA) expression and serum 17β-estradiol (E2) concentrations in male goldfish were remarkably reduced when exposed to ketoconazole (Yan et al. 2013). Ketoconazole also exerted inhibitory effects on the transcription and concentration level of yolk precursor vitellogenin (Zhang et al. 2008; Ankley et al. 2012). Reports about the toxicological effect of azole fungicides have mainly focused on the endocrine system of adult teleosts; however, the impact on embryonic development remains unclear.
Apart from the endocrine system, other signaling pathways (e.g., inflammation, oxidative stress, and circadian rhythm) could be perturbed by environmental pollutants. Glucocorticoids exhibited stronger induction of immune system-related genes (Willi et al. 2018). Several key metabolites involved in the tricarboxylic acid cycle were shown to be inhibited by propiconazole; this indicated the induction of oxidative stress (Melvin et al. 2018). Progestins and corticosteroids can regulate the locomotor behavior and the transcriptional levels of core clock genes in zebrafish (Zhao et al. 2018). This suggests that the circadian rhythm system is a target of environmental steroid hormones. Previous studies have shown that azole fungicides may act as hormone-like compounds by altering the CYP450 enzymes involved in steroidogenesis (Hinfray et al. 2006; Gyllenhammar et al. 2009). Therefore, the question arises as to whether the immune system, oxidative stress, the circadian rhythm network, and other major signaling pathways are modulated by azole fungicides. Recent studies showed that adverse effects such as reproductive dysfunction occurred even in medaka fish females when exposed to azole fungicides at environmentally relevant concentrations (Chu et al. 2016). Thus the issues about the significant signaling pathways affected by different concentrations of azole fungicides, including environmentally relevant concentrations, should be systemically elucidated.
Climbazole, one of the azole fungicides, is widely used as an antifungal active ingredient in personal care products such as shampoos and lotions. The annual usage of climbazole in China was calculated to be 345 tons in 2011 (Zhang et al. 2015a). After use, climbazole enters the aquatic environment via direct or indirect discharge of wastewater. Our previous study revealed that climbazole was detected at the mean and maximum concentrations of 74.8 and 530 ng/L in surface water of the Dongjiang River in Guangdong Province, China (Chen and Ying 2015). The residues of climbazole could cause adverse effects on aquatic organisms. Acute toxicity of climbazole toward zebrafish (Danio rerio) for embryo mortality was reported with a median effect concentration of 8.20 mg/L at 48 h (Richter et al. 2013). Nevertheless, the research regarding the chronic effect (e.g., transcriptional alternation of key genes) of climbazole on aquatic organisms is quite limited.
The present study aimed to assess the adverse impact on transcriptional profiles in zebrafish eleutheroembryos exposed to environmentally relevant and predicted worst-case environmental concentrations of climbazole. By analyzing the transcriptional levels of key genes in different signaling pathways before and after climbazole exposure, we can screen the differentially expressed genes and signaling pathways in zebrafish embryos and larvae, and propose the potential biomarkers by partial least squares discriminant analysis. The results obtained can help in a better understanding of the potential transcriptional effect of climbazole on the early development of zebrafish, and direct further investigation on metabolic phenotypes associated with the signaling pathways.
MATERIALS AND METHODS
Experimental design of embryo exposures
Basic information on climbazole, the supplier sources of chemicals, and the maintenance of zebrafish can be found in Supplemental Data, Table S1 and Text S1.
Zebrafish embryos were obtained from spawning adults placed in groups of 6 males and 3 females in tanks overnight. The spawning was stimulated by the onset of light on the following morning. The embryos were collected and examined under an inverted, biological microscope (Optec) within 2 h of natural spawning. Healthy, developing embryos at 2- to 3-h postfertilization (hpf) were selected for the subsequent exposure experiment. This trial consisted of 2 concentration groups (100 and 10 000 ng/L) for climbazole and a solvent control group (0.001%, v/v dimethyl sulfoxide [DMSO]). The concentration group of 100 ng/L was considered as the environmentally relevant concentration (low dose); the concentration group of 10 000 ng/L represented a predicted worst-case environmental concentration (high dose), and was lower than the 10% effective concentration of 1070 µg/L based on the mortality of zebrafish embryos (Richter et al. 2013). The high-dose concentration was used to: 1) simulate unexpected scenarios such as the direct discharge of wastewater, and 2) find more potential influences of climbazole on the transcriptional response of key genes in signaling pathways in zebrafish eleutheroembryos. The concentration of DMSO was constant among all treatment groups. The embryo culture medium, containing different concentrations of climbazole, was used as exposure solutions. The embryos (n = 100) were randomly distributed into glass beakers containing 100 mL of proper exposure solutions (0, 100, and 10 000 ng/L climbazole). All beakers were incubated in a semi-static system at 27 ± 1 °C during a 14:10-h, light:dark photoperiod for 14 d. Each concentration group was set in triplicate. Every 24 h unfertilized and coagulated eggs were removed and one-half of the exposure solution was renewed. The exposure solution samples at the beginning of exposure (day 0) and before water renewal (day 1) were collected for chemical analysis to determine whether the concentration of climbazole was constant during the exposure period. At different developmental stages (2-, 7-, and 14-d postfertilization [dpf]), 20 eleutheroembryos per replicate were randomly gathered, pooled, and preserved in RNA later (Wolact) at –80 °C before RNA analysis. Water quality parameters such as temperature, pH, and dissolved oxygen were recorded daily using a Yellow Springs Instrument Pro Plus multiparameter water quality device.
Transcriptional expression analysis
Ribonucleic acid isolation, complementary deoxyribonucleic acid (cDNA) synthesis, and real-time quantitative polymerase chain reaction (RT-qPCR) were performed according to a previous study with a little modification (Liang et al. 2015). Total RNA was extracted from eleutheroembryo samples using TRIzol reagent (Invitrogen), as described in the user manual. The quality of total RNA was determined by the electrophoresis on agarose gels stained with GelRed (Biotium; Supplemental Data, Figure S1). The concentration of RNA was detected by a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). The ratio of absorbance at 260 and 280 nm (A260/A280)―used to assess the RNA purity―ranged from 1.8 to 2.0 for all RNA samples in the present study. The first-strand cDNA was synthesized from 3 μg of total RNA using ReverTra Ace® qPCR RT Master Mix with genomic (g) DNA remover (Toyobo) in a total volume of 50 μL, as described by the manufacturer. Before RT-qPCR each cDNA sample was diluted with nuclease-free water to 200 μL and stored at –20 °C.
The qPCR analysis was conducted on the CFX Connect™ Real-Time PCR Detection System (Bio-Rad) using the THUNDERBIRD SYBR® qPCR Mix (Toyobo) in a final volume of 20 μL containing 2.5 μL of cDNA samples, 10 μL of THUNDERBIRD SYBR qPCR Mix, 0.4 μL of each primer (10 μM), and 6.7 μL of nuclease-free water. Based on the reports, primer sequences of the selected genes involved in different signaling pathways (e.g., circadian rhythm, inflammation, oxidative stress, hormone receptors, steroidogenesis, oocyte maturation, sexual differentiation, and embryo development) are listed in Supplemental Data, Table S2. The house-keeping gene β-actin was chosen as an internal standard for normalization because of its high stability in gene expression (Willi et al. 2018). The qPCR protocol utilized was denaturation at 95 °C for 60 s, 40 cycles of 95 °C for 15 s, and 60 °C for 60 s followed by a melting curve analysis post-run from 65 to 95 °C that was used to confirm the specificity of selected primers and absence of primer dimers.
Chemical analysis
Climbazole in water samples was extracted using solid-phase extraction according to our earlier study with some adjustments (Chen et al. 2012). The detailed procedure of chemical analysis of climbazole in water samples is described in Supplemental Data, Text S2 and Tables S3 and S4. The recovery, method detection limit, and method quantification limit of climbazole were 96.1% and 2.50 and 8.33 ng/L. This indicates that the analytical method was sensitive with satisfactory extraction efficiency. In addition, climbazole was not detected in blank samples.
Data processing and statistical analysis
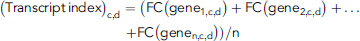 (1)
(1)The fold changes in the relative transcriptional expression levels of target genes to the solvent control were analyzed by the delta-delta comparative threshold cycle (2–ΔΔCt) method (Livak and Schmittgen 2001), whereas the relative transcriptional expression levels of target genes to the solvent control were expressed as log2(2–ΔΔCt). The significance of differences between the solvent control and climbazole-treated zebrafish eleutheroembryos at transcript levels was investigated by a two-tailed independent sample t test that was performed in SPSS software. Volcano plots, Venn diagrams, and histograms were constructed with GraphPad Prism software, whereas hierarchical clustering heat maps were created using Hemi software (Deng et al. 2014). The differences in mRNA expression induced by climbazole-treated groups and the potential biomarkers were analyzed by the partial least squares discriminant analysis and variable importance values, and were run in SIMCA-P software. All data are shown as mean ± standard deviation. Differences with p < 0.05 were deemed statistically significant.
RESULTS AND DISCUSSION
Change of chemical parameters in the exposure system
The concentrations of climbazole were determined in the water samples at the beginning of exposure (day 0) and before water renewal (day 1), whereas the concentrations of water quality parameters were analyzed during the exposure period. Climbazole was not found in the DMSO control groups. For the treatment with 100 ng/L climbazole exposure, the average measured concentrations of climbazole were 96.1 and 96.8 ng/L at days 0 and 1. For the 10 000 ng/L climbazole exposure group, average concentration measurements were 8080 and 8820 ng/L at days 0 and 1 (Supplemental Data, Table S5). These findings indicate that the concentrations of climbazole were steady during the exposure period. The temperature, pH, and dissolved oxygen values were in the range of 26.3 to 28.1 °C, 7.7 to 8.2, and 6.1 to 7.4 mg/L, respectively (Supplemental Data, Table S6). This suggests that an optimum and stabilized aquatic environment was provided for the raising of early zebrafish embryos.
No significant differences in embryo survival were observed among the solvent control and low- and high-dose treatment groups during the exposure trial (data not shown). The survival rates were more than 95% in all control and climbazole-treated groups. Richter et al. (2013) reported that the toxicity of climbazole toward zebrafish for embryo mortality was moderate, with a median effect concentration of 8.20 mg/L. However, climbazole was not acutely toxic to zebrafish embryos at environmentally relevant concentrations.
Transcriptional alteration of circadian rhythm, inflammation, and oxidative stress in embryonic zebrafish
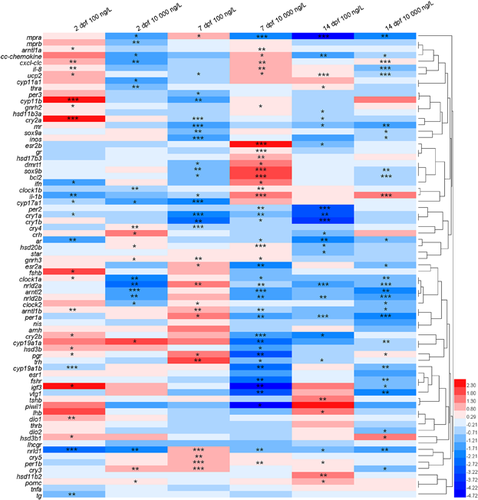
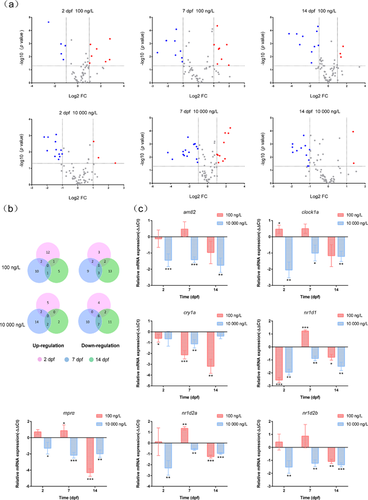
In the present study, the transcriptional profiles of 73 target genes involved in diverse biological and physiological pathways in zebrafish eleutheroembryos were examined under varying exposure times and levels. The changes in transcription of all target genes (n = 73) in zebrafish embryos and larvae before and after climbazole exposure are depicted in a visualization heat map (Figure 1). Differentially expressed genes were identified by the criteria of |log2(FC)| > 1 and p < 0.05. Fifteen, 26, and 15 genes exhibited divergent transcriptional expression in zebrafish eleutheroembryos at 2, 7, and 14 dpf after exposure to high-dose climbazole, whereas only 12, 16, and 13 dissimilar genes were found at 2, 7, and 14 dpf in the low-dose treatment (Figure 2A and Supplemental Data, Table S7). This implies that higher exposure concentrations of climbazole could lead to more interference in gene transcription. For the low- and high-dose climbazole-treated groups, the proportions of contrasting genes were 21.9 and 35.6% (n = 73) at 7 dpf―higher than the proportions at 2 and 14 dpf (Supplemental Data, Table S7). These findings contend that the transcriptional expression of genes in 7-dpf zebrafish embryos was the most susceptible to climbazole. In addition, a Volcano plot was utilized to quickly identify significant up-regulation or down-regulation of transcription of all target genes among different climbazole-treated and control groups. Higher ratios of statistically significant down-regulated genes can be seen when compared with up-regulated genes in all treated groups (Figure 2A). At all sampling times (2, 7, and 14 dpf), the expression of cry1a mRNA was significantly down-regulated after exposure to 100 ng/L climbazole, whereas the transcriptional levels of mprα, clockα, arntl2, nr1d1, nr1d2a, and nr1d2b genes were remarkably decreased after exposure to 10 000 ng/L climbazole (Figure 2B,C). Certain genes such as mprα, nr1d1, and nr1d2a were significantly up- and down-regulated at 7 dpf after low- and high-dose exposure, respectively. Distinctly opposite results that occurred among different concentrations of exposed groups are often seen in previous studies (Zucchi et al. 2011; Han et al. 2017) but the mechanism is unknown. For example, an earlier report indicated that p-nonylphenol can induce the extracellular signal-regulated kinase cell-signaling pathway at low and high doses but inhibit it at a certain middle dose (Bulayeva and Watson 2004).
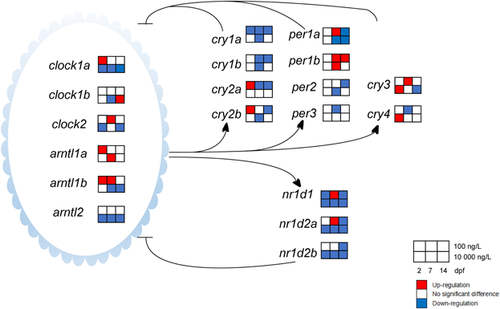
It is known that cry1a, clock1a, arntl2, nr1d1, nr1d2a, and nr1d2b are involved in the regulation of circadian rhythms (Albrecht 2004; Vatine et al. 2011; Zhao et al. 2018). An unexpected mechanism that climbazole could disturb the circadian rhythm in zebrafish eleutheroembryos was identified. Circadian rhythms are controlled by an endogenous circadian clock that exerts its regulatory role through a positive/negative feedback loop driving recurrent rhythms in mRNA and protein levels of 6 clock gene families (clock, arntl, per, cry, nr1d, and ror; Vatine et al. 2011). For the negative feedback loop, the Clock/Arntl heterodimer activates the expression of per and cry, after which Per and Cry proteins translocate to the nucleus, interact with the Clock/Arntl heterodimer, and thereby inhibit the transcriptional activation driven by the Clock/Arntl heterodimer (Albrecht 2004). In addition, the Clock/Arntl1 heterodimer also activates 2 subfamilies of nuclear hormone receptors―Rev-erbs including Rev-erbα (encoded by nr1d1) and Rev-erbβ (encoded by nr1d2), and Rors including Rorα (encoded by rora), Rorβ (encoded by rorb), and Rorγ (encoded by rorc). These receptors then repress or activate the transcription of their downstream genes directly or indirectly. This constitutes the positive feedback loop of circadian regulation (Albrecht 2004). Thus the positive and negative feedback loops are co-regulated by the Clock/Arntl heterodimer, which plays a pivotal role in rhythm generation.
Climbazole significantly altered the circadian rhythm network in zebrafish eleutheroembryos. Transcripts of the key gene cry1a showed a time-dependent down-regulation in response to 100 ng/L climbazole exposure. Cryptochrome 1a is a light-induced gene that can prevent the heterodimerization of Clock and Arntl, and hinder their ability to transactivate from E-box enhancer elements (Tamai et al. 2007). These findings indicated that long-term exposure to climbazole might lengthen the periods of biological rhythms of zebrafish and cause a phase delay of circadian rhythms. Furthermore, significant down-regulation of most circadian-related genes was found at different stages (Figure 3), with the maximum fold change up to 5.6 times relative to the solvent control. The key circadian clock gene expression pattern induced by climbazole was remarkably different from the pattern for progestins (e.g., progesterone, norethisterone acetate, and levonorgestrel) and corticosteroids (e.g., dexamethasone, fludrocortisone acetate, cortisol, and betamethasone); however, it was similar to the pattern for estrogens (e.g., 17α-ethinylestradiol and estrone) and androgens (e.g., testosterone and androsterone; Zhao et al. 2018). These determinations implied the similar effects on circadian clock function between climbazole and estrogens/androgens. The progesterone and glucocorticoid receptors (encoded by pgr and gr) have been reported to directly participate in the regulation of circadian rhythms, whereas the estrogen and androgen receptors do not (Zhao et al. 2018). The results of the correlation analysis showed a significant correlation between the transcript index of progestin/glucocorticoid receptors and circadian rhythm (R2 = 0.87; p < 0.01; Table 1). Therefore, the transcriptional alterations of pgr and gr in response to climbazole exposure in the present study could lead to a stronger effect on the circadian rhythm network (Figure 1).
| Transcript index | CRS | HRS | GFL | ER | PGR | SD | OM | CR | OS | IM |
|---|---|---|---|---|---|---|---|---|---|---|
| CRSb | − | − | − | − | − | − | − | − | − | − |
| HRSc | 0.11 | − | − | − | − | − | − | − | − | − |
| GFLd | 0.74* | 0.28 | − | − | − | − | − | − | − | − |
| ERe | 3.02 × 10−3 | 0.04 | 0.06 | − | − | − | − | − | − | − |
| PGRf | 9.80 × 10−5 | 0.80* | 0.06 | 0.01 | − | − | − | − | − | − |
| SDg | 0.01 | 0.05 | 0.06 | 0.14 | 0.25 | − | − | − | − | − |
| OMh | 0.84* | 0.21 | 0.94** | 0.08 | 0.02 | 0.01 | − | − | − | − |
| CRi | 0.03 | 0.92** | 0.21 | 0.04 | 0.87** | 0.15 | 0.14 | − | − | − |
| OSj | 0.03 | 0.06 | 0.03 | 0.40 | 0.21 | 0.34 | 0.04 | 0.12 | − | − |
| IMk | 1.07 × 10−4 | 0.01 | 0.01 | 0.14 | 0.08 | 0.026 | 5.97 × 10−5 | 0.06 | 0.68* | − |
- a Values and asterisks represent correlation coefficients and significant differences.
- b Cytochrome P450 enzyme-related steroidogenesis genes cyp19a1a, cyp19a1b, cyp17a1, and cyp11b.
- c Hydroxysteroid dehydrogenase-related steroidogenesis genes hsd20b, hsd17b3, hsd11b2, and hsd3b1.
- d GnRH/FSH/LH signaling pathway genes gnrh2, gnrh3, fshβ, and lhβ.
- e Estrogen receptor genes esr1, esr2a, esr2b, and vtg1.
- f Progestin/glucocorticoid receptor genes pgr and gr.
- g Sexual differentiation genes sox9a and ar.
- h Oocyte maturation genes mprα, hsd20b, and igf3.
- i Circadian rhythm genes clock1a, arntl2, nr1d1, nr1d2a, nr1d2b, per1a, per1b, cry1a, and cry1b.
- j Oxidative stress genes bcl2 and ucp2.
- k Inflammation genes il-1β, il-8, cxcl-clc, and cc-chemokine.
- *Significant at p < 0.05.
- **Significant at p < 0.01.
The circadian system could interface with several physiological processes in such a way that the modulation of these genes might have a considerable impact on the signaling pathways, including those involved in inflammation and oxidative stress (Bollinger et al. 2010; Fanjul-Moles and Lopez-Riquelme 2016). The physiological connection between the circadian clock and chronic inflammatory diseases has been identified (Keller et al. 2009; Bollinger et al. 2010; Narasimamurthy et al. 2012). For example, Hashiramoto et al. (2010) have shown that a mammalian circadian clock gene cry could inhibit the transactivation of the tumor necrosis factor α (TNF-α) gene. Furthermore, the lack of cry gene function in arthritic mice resulted in the up-regulation of concentrations of inflammatory cytokines (TNF-α, IL-1β, and IL-6) in serum (Hashiramoto et al. 2010). Although the significant down-regulation of cry1a mRNA was seen (Figure 2C), there was no alternation in tnf-α mRNA level during the exposure duration. The transcripts of il-1β, il-8, cxcl-clc, and cc-chemokine genes involved in inflammation were significantly raised at 7 dpf after high-dose climbazole exposure (Supplemental Data, Figure S2). However, the transcript index of inflammation did not reveal a significant correlation with that of circadian rhythm (R2 = 0.06; p > 0.05; Table 1). This indicates that the induction of inflammatory response in the present study was not directly mediated through the circadian clock pathway.
The circadian clock network was shown to regulate the redox system (Fanjul-Moles and Lopez-Riquelme 2016). The genes related to the production of reactive oxygen species (ROS) were studied. Uncoupling protein 2 (Ucp2, encoded by ucp2) was involved in the reduction of mitochondrial superoxide production (Giardina et al. 2008), whereas B-cell lymphoma 2 (Bcl2, encoded by bcl2) was localized at the sites of free radical generation and acted as an antioxidant to limit ROS production and protect cells from oxidative deaths (Hockenbery et al. 1993). Thus these 2 genes also play critical roles in reducing cell apoptosis (Hockenbery et al. 1993; Mao et al. 2017), which is a basic physiological process for regulation of cell populations (Schwartzman and Cidlowski 1993). In the present study, significant inhibition of these 2 genes was found in the embryos and larvae of zebrafish at 7 dpf after low-dose climbazole exposure (Supplemental Data, Figure S2). Reactive oxygen species-induced apoptosis is thought to contribute to abnormal development during embryogenesis (Yamashita 2003). However, no malformation was observed in climbazole-exposed zebrafish embryos and larvae. This evidence supported the hypothesis that climbazole has the potential to induce oxidative stress in zebrafish eleutheroembryos. In Table 1, the transcript index of oxidative stress was statistically related to that of inflammation (R2 = 0.68; p < 0.05) but not to that of circadian rhythm (R2 = 0.12; p > 0.05). It may be related to the nondirect effect of the circadian rhythm on the oxidative stress, which was linked with the inflammatory response (Forrester et al. 2018).
Transcriptional alteration of oocyte maturation, steroidogenesis, and sexual differentiation in embryonic zebrafish
In Figure 2C, the expression of mprα mRNA was clearly inhibited during exposure to high-dose climbazole. The membrane progestin receptor protein plays a vital role in the induction of oocyte maturation (Zhu et al. 2003; Tan et al. 2009), which reflects climbazole-induced perturbation on oogenesis. Two distinct membrane progestin receptor subtypes (mprα and mprβ) have been identified in fish (Thomas et al. 2004). The transcriptional down-regulation of mprα was found in response to high-dose climbazole exposure, with fold changes up to 0.44, 0.23, and 0.26 times relative to the solvent control at 2, 7, and 14 dpf, whereas the mprβ mRNA expression was only slightly decreased at 2 dpf (Figure 1). This discrepancy could be explained by the different function of these 2 mpr subtypes. Hanna and Zhu (2011) have reported that mprα is the long-sought-after nongenomic progestin receptor to induce meiosis resumption in follicle-enclosed zebrafish oocytes, whereas mprβ is an unlikely receptor for initiating final oocyte maturation. Moreover, high-dose climbazole significantly suppressed the mRNA expression of igf3, encoding the insulin-like growth factor 3 (Igf3), at 7 and 14 dpf (Supplemental Data, Figure S2). The Igf3 protein plays an important role in the ovarian functions of zebrafish, especially in oocyte maturation (Li et al. 2011). These findings suggest that climbazole could exert the inhibitory effect on oocyte maturation by controlling the expression of the membrane progestin receptor protein, which is in accordance with an earlier report (Ankley et al. 2002). Nevertheless, the expression of hsd20b mRNA was increased after exposure to high-dose climbazole at 2 and 7 dpf (Figure 1). It is well known that 20β-hydroxysteroid dehydrogenase (encoded by hsd20b) regulates the production of 17α, 20β-dihydroxy-4-pregnen-3-one, which is a natural maturation-inducing steroid for the induction of oocyte maturation in fish (Tokarz et al. 2013). This discrepancy could be explained by a negative feedback mechanism.
Azole fungicides have been reported to disrupt the steroid biosynthesis in teleosts via interactions with cytochrome P450 genes (Ankley et al. 2007; Ankley et al. 2012). The mRNA expression levels of cyp17a1, encoding the cytochrome P450 17-hydroxylase/17,20-lyase, were significantly down-regulated in both climbazole-treated groups when compared with the control groups (Figure 4), which resulted in the inhibition of androstenedione production. Significantly, transcriptional suppression of the 2 aromatase genes cyp19a1a and cyp19a1b was also seen after high-dose climbazole exposure. These 2 isoforms of aromatase gene encode gonadal (cyp19a1a) and brain (cyp19a1b) aromatase with different regulation and functions (Goto-Kazeto et al. 2004). For the cyp19a1a, the inhibition of its mRNA expression will block the synthesis of estrogens such as estrone and E2 (Tokarz et al. 2013). The vtg1 transcript involved in regulating estrogen production was clearly restrained by high-dose climbazole treatment at 7 and 14 dpf (Supplemental Data, Figure S2). In addition, some previous reports have indicated that ketoconazole, one of the azole fungicides, obviously reduced E2 concentrations in serum, ovaries, and cells (Villeneuve et al. 2007; Ankley et al. 2012; Yan et al. 2013). The presence of high-dose climbazole could cause the reduction of estrogen levels in zebrafish eleutheroembryos, leading to potential endocrine disruption. It is known that a gonadotropin-releasing hormone (encoded by gnrh2 and gnrh3) can stimulate the expression and secretion of a follicle-stimulating hormone (encoded by fshβ) and a luteinizing hormone (encoded by lhβ) that have been shown to activate sex steroid (e.g., estrogens and androgens) synthesis and release (Yaron et al. 2003; Zhang et al. 2015b). Enhancement of gnrh2, gnrh3, fshβ, and lhβ transcription in the present study can be interpreted as a compensatory response (Figure 4). The cyp19a1b encodes brain aromatase that is responsible for not only the biosynthesis of estrogens but also for the modulation of serotonergic neurons in embryonic zebrafish (Caulier et al. 2015; Ulhaq and Kishida 2018). In other words, the down-regulation of cyp19a1b mRNA by climbazole reveals the possibility of reducing the serotonin levels and further affecting the development and functions of serotonergic neurons.
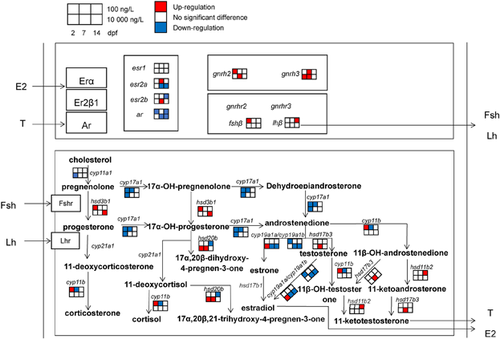
In Figure 4, not only cytochrome P450 genes but also hydroxysteroid dehydrogenase genes (e.g., hsd3b1, hsd11b2, and hsd17b3) that are also involved in steroidogenesis are modulated by climbazole. The hsd11b2 and hsd17b3 genes regulate the conversion from androstenedione to testosterone and from 11β-OH-androstenedione to 11-ketotestosterone (Tokarz et al. 2013). Up-regulation of transcription of these 2 androgen-related genes may promote the production of androgens and even affect the sexual development. Interestingly, the transcriptional levels of sox9a and ar genes, participants in the process of sex differentiation, were significantly reduced with the decreasing level of cyp19a1a mRNA at 14 dpf after high-dose climbazole exposure (Supplemental Data, Figure S2). This phenomenon was observed in an earlier report by the use of cyp19a1a mutant zebrafish (Yin et al. 2017). Prochloraz as an imidazole fungicide has been documented to cause irreversible masculinization of zebrafish during sexual differentiation (Baumann et al. 2015). Taken together, the masculinizing effect on gonadal development of aquatic animals may be induced by azole fungicides.
The relationship among 3 different signaling pathways (cytochrome P450 enzyme-related steroidogenesis, gonadotropin-releasing hormone [GnRH]/ follicle-stimulating hormone [FSH]/ luteinizing hormone [LH], and oocyte maturation) was analyzed by linear regression analysis (Table 1). These 3 signaling pathways displayed significant correlations (R2 = 0.74−0.94; p < 0.05) and were involved in the secretion of steroid hormones. To illustrate, oocyte maturation is mainly regulated by the release of a FSH and a LH (Thomas et al. 2004) that are stimulated by a GnRH (Yaron et al. 2003). Hence we can hypothesize that climbazole has the ability to disrupt the transcripts of important genes in the hypothalamic–pituitary–gonadal axis, leading to the modulation of oogenesis. Furthermore, the transcript index of hydroxysteroid, dehydrogenase-related steroidogenesis—but not of other steroidogenesis-related signaling pathways—was significantly correlated to that of circadian rhythm (R2 = 0.92; p < 0.05). Recent observations in the human ovary have suggested a potential relationship between the circadian clock and steroidogenesis (Chen et al. 2016). Taken together, the circadian clock and its modulation by climbazole may directly regulate the production of steroid hormones via hydroxysteroid dehydrogenases.
Potential biomarker genes and environmental relevance
Based on the supervised partial least squares discriminant analysis, potential biomarkers can be discriminated among the control groups and the treated groups (Hu et al. 2014). Model parameters (e.g., R2Y [proportion of explained variance] and Q2 [proportion of predicted variance]) were used to evaluate the fitting quality of partial least squares discriminant analysis. The results revealed that in the model of partial least squares discriminant analysis with good quality (R2Y > 0.65 and Q2 > 0.50), the climbazole-treated groups versus the control groups showed very distinct separation on the transcriptional expression of target genes (Supplemental Data, Table S8 and Figure S3). A variable importance plot loading from partial least squares discriminant analysis was then conducted for screening the important biomarkers that differentiated among the treated groups and the control groups (Figure 5). Genes with variable importance values greater than 1 were considered as the important biomarkers. One (mprα) and 5 genes (igf3, nr1d1, nr1d2b, cyp19a1a, and vtg1) were extracted as differential genes in 6 and 5 climbazole-treated groups, respectively. These findings were similar to the results that climbazole could cause the obvious dysfunctions of the circadian clock, oocyte maturation, and steroidogenesis in embryonic zebrafish at gene expression levels. The variable importance values of the inflammation-related genes (il-1β and il-8) were greater than 1 in all high-dose, climbazole-treated groups, which implied that the inflammatory response can be induced after high-dose climbazole exposure. Considered together with the transcriptional analysis, mprα, igf3, nr1d1, nr1d2b, cyp19a1a, vtg1, il-1β, and il-8 were selected as the potential biomarkers for climbazole exposure to zebrafish eleutheroembryos. However, metabolomic analysis should be conducted in the future to investigate whether these biomarkers are closely associated with differential metabolites.
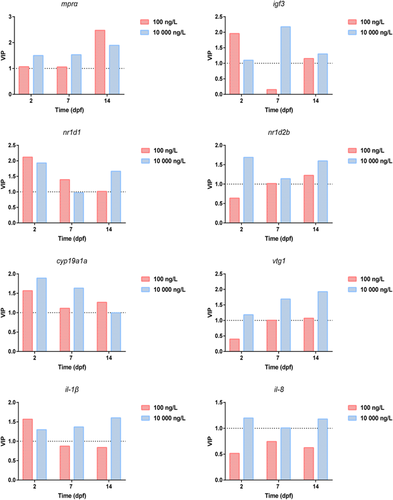
Climbazole is a widely used antifungal agent in personal care products. It has frequently been found in the aquatic environment, with a maximum concentration of up to 530 ng/L (Chen and Ying 2015). The presence of climbazole could cause adverse effects on aquatic organisms. Consequently, the ecotoxicity of climbazole has received increasing attention. Richter et al. (2013) have shown the high acute toxicity of climbazole to water lentil (Lemna minor) but moderate acute toxicity to zebrafish (D. rerio), with a median effect concentration of 8200 μg/L based on the embryo mortality. Nevertheless, changes in the transcriptional profiles of embryonic zebrafish induced by climbazole with environmentally relevant concentrations were found in the present study (Figure 1). In addition to the expected steroidogenic pathway, the circadian rhythm pathway in zebrafish embryos and larvae was interrupted by low-dose climbazole. Several pharmaceuticals such as diazepam, progesterone, and drospirenone have been found to modulate key gene expression in the circadian clock system of zebrafish (Oggier et al. 2010; Zhao et al. 2015). Considered collectively with our results, the circadian rhythm pathway might be a novel target of environmental pollutants in zebrafish. To our knowledge, the present study is the first to explore the transcriptional effects of climbazole on embryonic zebrafish by large-scale screening of important genes related to diverse biological and physiological pathways. Future studies should clarify whether the transcriptional alterations in signaling pathways translate into metabolic phenotypes and physiological effects.
CONCLUSIONS
The results in the present study showed that climbazole could regulate transcriptional profiles of important genes during the early development of zebrafish. The differential genes in embryonic zebrafish after high-dose climbazole exposure were greater in number than those in the low-dose, climbazole-treated groups. Significant transcriptional changes in circadian rhythm and steroidogenesis pathways occurred in zebrafish embryos and larvae exposed to environmental concentrations of climbazole. This suggests that the presence of climbazole in the natural aquatic environment could pose risks at gene expression levels to a certain extent. Based on the supervised partial least squares discriminant analysis and signaling pathway analysis, mprα, igf3, nr1d1, nr1d2b, cyp19a1a, vtg1, il-1β, and il-8 were selected as the potential biomarkers for climbazole exposure to embryonic zebrafish. These conclusions have implications in the risk assessment of climbazole in the aquatic environment and provide a glimpse of prospective studies on metabolic phenotypes associated with the signaling pathways.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.4355.
Acknowledgment
The present study was funded by the National Natural Science Foundation of China (21507163). We thank Y.-Q. Liang (Guangdong Ocean University), Y.-X. Jiang (Guangzhou Institute of Geochemistry, Chinese Academy of Sciences), W.-J. Shi (South China Normal University), and H.-C. Su (South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences) for technical assistance.
Data Accessibility
Data pertaining to this manuscript are deposited in figshare.




