Uptake of hydrophobic organic compounds, including organochlorine pesticides, polybrominated diphenyl ethers, and perfluoroalkyl acids in fish and blue crabs of the lower Passaic River, New Jersey, USA
Abstract
The bioavailability and bioaccumulation of sedimentary hydrophobic organic compounds (HOCs) is of concern at contaminated sites. Passive samplers have emerged as a promising tool to measure the bioavailability of sedimentary HOCs and possibly to estimate their bioaccumulation. We thus analyzed HOCs including organochlorine pesticides (OCPs), polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs), and polychlorinated dibenzo-p-dioxins/furans (PCDD/Fs) in sediment, porewater, and river water using low-density polyethylene passive samplers and in 11 different finfish species and blue crab from the lower Passaic River. In addition, perfluorinated alkyl acids (PFAAs) were measured in grab water samples, sediment, and fish. Best predictors of bioaccumulation in biota were either porewater concentrations (for PCBs and OCPs) or sediment organic carbon (PBDEs and PFAAs), including black carbon (OCPs, PCBs, and some PCDD/F congeners)–normalized concentrations. Measured lipid-based concentrations of the majority of HOCs exceeded the chemicals’ activities in porewater by at least 2-fold, suggesting dietary uptake. Trophic magnification factors were >1 for moderately hydrophobic analytes (log octanol–water partitioning coefficient [KOW] = 6.5–8.2) with low metabolic transformation rates (<0.01 d−1), including longer alkyl chain PFAAs. For analytes with lower (4.5–6.5) and higher (>8.2) KOWs, metabolic transformation was more important in reducing trophic magnification. Environ Toxicol Chem 2019;38:872–882. © 2019 SETAC
INTRODUCTION
Rivers in highly urbanized/industrialized areas are impacted by various classes of chemicals, often resulting in adverse ecological effects, such as habitat destruction, and various physiological and reproductive disorders in the river fauna. Once in the ecosystem, pollutants can accumulate in biota from the surrounding water, sediments, and/or porewater depending on the physicochemical properties of the pollutants, feeding habits, trophic position, and metabolic rates. The significance of bioaccumulation in fish and the accompanying ecological and human health risks have been highlighted in studies performed by the US Environmental Protection Agency (1992a, 1992b). Typically, organic chemicals are prioritized with respect to their persistence, bioaccumulation potential, and toxicity (Gobas et al. 2009). Recently, the use of chemical properties to predict the bioaccumulation potential of a chemical has been questioned (Borgå et al. 2012).
In our recent work (Khairy et al. 2014), we assessed the biomagnification of polychlorinated biphenyls (PCBs) and polychlorinated dibenzo-p-dioxin/dibenzofurans (PCDD/Fs) in fish and crabs collected from the Passaic River estuary. In general, the dominant exposure pathways for legacy hydrophobic organic contaminants (HOCs) in biota were porewater and sediments rather than the river water. In the present study, we aimed to expand on these results by investigating a wider range of contaminants with more diverse structures and properties. Targeted compounds include organochlorine pesticides (OCPs), polybrominated diphenyl ethers (PBDEs), and perfluorinated alkyl acids (PFAAs), including perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA). Because PFAAs and some OCPs display much greater aqueous solubility than the legacy HOCs, it is unclear whether sediment-bound contamination remains a primary exposure pathway to biota in the river. These compounds also cover a wide range of resistance to metabolism (US Environmental Protection Agency 2016a), which should, in conjunction with varying degrees of lipophilicity, affect their uptake and magnification in the food web.
Although recent work established the presence of PBDEs at relatively high concentrations in all of the environmental compartments of the lower Passaic River and deduced diffusive fluxes of PBDEs from sediment to overlying water (Khairy and Lohmann 2017), little is known regarding their uptake sources and bioaccumulation potential.
Unlike legacy HOCs, PFAAs are ionic, amphiphilic compounds (Key et al. 1997) and thus have different physicochemical properties. They have been globally detected in all environmental compartments (Labadie and Chevreuil 2011; Loi et al. 2011; Fang et al. 2014). They are persistent and tend to bioaccumulate (PFAAs with a longer fluorinated carbon chain; Conder et al. 2008), undergo long-range transport, and cause adverse ecological and health effects (Lau et al. 2007). Anionic PFAAs are proteinophilic and generally found at highest concentrations in blood and liver (Becker et al. 2010) in contrast to the lipophilic legacy pollutants.
Studying the bioaccumulation potential of HOCs requires the ability to measure the freely dissolved fraction in sediments (porewater) and/or the water column, where bioavailability is expected to increase with an increase in the dissolved concentrations (You et al. 2006). Passive samplers, such as low-density polyethylene (LDPE) sheets, have emerged as an inexpensive and effective technique for measuring the freely dissolved concentrations of HOCs. Traditionally, geochemical approaches have been used to predict porewater concentrations (sediment concentrations and organic carbon content) because of the difficulties associated with the measurement of the dissolved fraction in sediment's porewater directly. However, since the development of passive sampling devices, estimation of porewater concentrations has become feasible (Fernandez et al. 2009; Ghosh et al. 2014; Khairy and Lohmann 2017).
The original equilibrium partitioning theory (Di Toro et al. 1991) linked porewater concentrations of HOCs to their accumulation in the lipid of organisms. Because LDPE samplers similarly accumulate HOCs from porewater, recent studies indicated that LDPE concentrations of HOCs (at equilibrium) can be used to predict their bioaccumulation (Friedman et al. 2009). In fact, recent work by Jahnke et al. (2014) suggested that passive sampling can be used to estimate the bioaccumulation potential of HOCs and indicated that the majority of studies resulted in tissue concentrations below the equilibrium concentration with porewater. Being able to rely on passive samplers to predict (maximum) bioaccumulation in aqueous food webs would certainly be very useful for the assessment of contaminated sites.
In the present study, we investigated the bioaccumulation of OCPs and PBDEs in 11 fish species and Callinectes sapidus (blue crab), collected from the fresh-brackish portion of the Passaic River estuary. Data on PFAAs for only 8 fish species were included in the present study. To that end, we calculated the bioaccumulation factors (BAFs) for OCPs, PBDEs, and PFAAs; compared the accumulated amounts of HOCs in LDPE and in the biota; and investigated the best predictors for HOCs and PFAAs in biota using sediment geochemistry and LDPE (active water samples for PFAAs). Because sampling of biotic and abiotic compartments was done at the same time intervals and all of the samples were analyzed in the same laboratory, we present a uniquely consistent set of BAFs. Our objectives were to 1) assess the sources of contaminants accumulated by the biota (sediments, porewater, diet, or overlying water), 2) assess the suitability of using LDPE to predict the bioaccumulation of HOCs in biota, and 3) derive BAFs and assess the trophic magnification (or dilution) of target analytes.
MATERIALS AND METHODS
Sample collection, extraction, analysis for OCPs and PBDEs
A detailed description of the sampling of biota and sediments, preparation and deployment of the LDPE in the river water (for OCPs and PBDEs), the stable isotope analysis in the biota tissues, the determination of porewater concentrations using an LDPE tumbling experiment, and the uncertainty analysis can be found in Supplemental Data, Figures S1, S2, and S3, and Table S1, as well as in the Supplemental Data text and in Khairy et al. (2016, 2014) and Khairy and Lohmann (2017). More information about the extraction of all matrices, instrumental analysis, quality assurance, and the determination of porewater concentrations is available in the Supplemental Data (Tables S2–S6) and is briefly summarized.
Finfish specimens (n = 350 representing 10 species) including benthic feeders (C. sapidus, Anguilla rostrata, Fundulus heteroclitus, Hybognathus regius, Morone americana), benthopelagic taxa (Fundulus diaphanus, Lepomis spp., Esox americanus, and juvenile Morone saxatilis), and pelagic species (Menidia menidia and Dorosoma cepedianum) and 7 C. sapidus (blue crab) specimens were collected during August to November 2011 at 3 locations in the Passaic River estuary (Supplemental Data, Figure S1). For marine transients, fish collections were restricted to the extent practical to young-of-year that had a recent history of exposure to contaminants in the system. See Supplemental Data, Table S1, for more details on the biota samples.
Sediment samples were collected from 18 different locations (Supplemental Data, Figure S2) along the lower Passaic River during September to November 2011 from the mudflats at low tide. A detailed description of the sampling methodology and sampling locations can be found in Khairy et al. (2016). Total organic carbon and black carbon contents in the sediments were determined as detailed in Gustafsson et al. (1997).
The LDPE passive samplers were fastened to an anchored rope and suspended in the water column approximately 1 to 2 m below the surface at 6 different locations along the lower Passaic River (Supplemental Data, Figure S1) to sample the freely dissolved OCPs and PBDEs.
Freely dissolved porewater concentrations were determined by shaking (equilibrating) LDPE passive sampling sheets (25 μm thick) with sediment–water (containing sodium azide as a biocide) slurry for 9 wk according to the method detailed in Lohmann et al. (2005).
Biota and sediment samples were extracted and subjected to cleanup according to the methods detailed elsewhere (Khairy et al. 2016, 2014). The LDPE (deployed in both the river water and porewater) was cold-extracted twice (24 h each) in dichloromethane followed by hexane and concentrated, and it was not subjected to any further cleanup steps.
Collection of water samples, extraction, and analysis of all samples for PFAAs
We used the same sediment and biota samples that were collected for OCPs and PBDEs. Water samples (∼20 cm below the surface) were collected in precleaned (inner walls were cleaned with basic methanol followed by methanol and left overnight to dry) 1-L polypropylene bottles with polypropylene lids at 8 different locations along the lower segment of the Passaic River (Supplemental Data, Figure S3). Samples were kept in an ice bath until they were preserved in a freezer for analysis. Water samples (400 mL each) were analyzed for PFAAs (perfluorohexanoic acid [PFHxA], perfluorododecanoic acid [PFDoA], perfluorobutanesulfonic acid [PFBS], perfluorohexanesulfonic acid, and PFOS) in the US Food and Drug Administration's Laboratory in Maryland according to the method detailed in Young et al. (2013). Sediments and fish samples were analyzed at the University of Rhode Island. Extraction and cleanup were done according to the US Environmental Protection Agency's (2011) method for the analysis of PFAAs (see Supplemental Data, Tables S2 and S3, for more information). Measurements of PFAAs were made in 8 fish species (M. saxatilis [29–33 cm], A. rostrata [110 cm], M. americana [8–16 cm], F. diaphanus, H. regius, M. menidia, D. cepedianum, and F. heteroclitus) but not in the blue crabs because of a lack of extra tissue after PBDE and OCP analysis.
Quality assurance
Procedural blanks, field blanks (LDPE), matrix spikes, and duplicate samples (20% of the total samples) were included with each sample batch and carried throughout the analytical procedure in a manner identical to the samples. We detected BDE-47 in the blanks (10–20% of the least detected concentration in the different matrices). Similarly, detected PFAAs in the blank samples were 12–25% of the sample concentrations; and accordingly, correction of samples for the blank detects was performed.
Recoveries of the surrogate standards in the biota, LDPE, and sediment samples generally ranged from 65 to 102% and from 72 to 104% for OCPs and PBDEs, respectively. Matrix spikes recoveries (Supplemental Data, Table S4) ranged from 94 to 104% for OCPs and PBDEs with RSD <10%. Reproducibility of the results ranged from 7.0 to 17% and from 5.0 to 18.5% for OCPs and PBDEs, respectively.
Recoveries of PFAAs in the matrix spikes (Supplemental Data, Table S5) were 87 to 133% for the majority of the target PFAAs and <55% for C13- to C18-perfluorocarboxylic acid and C10-perfluorosulfonate. Accordingly, these PFAAs were excluded from the discussion. Recoveries of the surrogate standards ranged from 57 to 120% (Supplemental Data, Table S5). The limit of detection (LOD) corresponded to a signal-to-noise ratio of 3 (Supplemental Data, Table S6). Variability between duplicates was <20%.
Physicochemical properties
Octanol–water partitioning coefficient (KOW) values of OCPs were obtained from Schenker et al. (2005). The KOW values for PBDEs were obtained from Yue and Li (2013). Polyethylene–water partitioning coefficients for OCPs and PBDEs were obtained from Lohmann (2012). Lipid–water partitioning coefficients (KLIP-W) were estimated from KOW values according to the method of Endo et al. (2011). Organic carbon–water partitioning coefficient (KOC) were calculated as shown in Xia (1998).
For PFAAs, KOC values were obtained from Higgins and Luthy (2007). Missing values were obtained by correlating the available values against those obtained from Labadie and Chevreuil (2011). Protein–water partitioning coefficients (KPW) were obtained from Kelly et al. (2009). Missing values were obtained from correlating the available values against KOCs (n = 8; R2 = 0.98); KOW values were obtained from Wang et al. (2011). Value of KPW rather than KLIP-W were used with PFAAs because these compounds bind to proteins rather than to general lipids. All of the physicochemical properties used in the present study are given in Supplemental Data, Table S7.
Estimated compound-specific metabolic biotransformation rates (kmtrs) normalized to 10 g tissue weight were obtained from the BCFBAF function in EPI Suite 4.11 (US Environmental Protection Agency 2016b).
Calculation of BAFs and trophic magnification factors and predicting biota concentrations
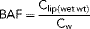 (1)
(1)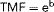 (2)
(2)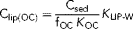 (3)
(3)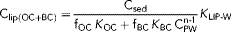 (4)
(4)In Equation 4, fBC is the fraction of black carbon in the sediment and KBC is the black carbon–water partitioning coefficient. We used site-specific KBC values in the present study.
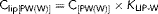 (5)
(5)For PFAAs, protein-based concentrations were predicted only from sediment organic carbon and from river water using Equations 3 and 5. In both equations, KLIP-W was replaced with KPW. Concentrations of PFAAs in the samples were normalized to a generic total protein content (25%) assigned for fish tissues, as shown in Kelly et al. (2009) and references therein.
Statistical analysis
Statistical tests (analysis of variance, t test, and correlations) were performed with SigmaPlot (Ver 11) and regression analysis with IBM SPSS, Ver 25.
RESULTS
Uncertainty analysis
Uncertainties associated with the estimated porewater and river water concentrations (Supplemental Data, Equation S4) from LDPE ranged from 41 to 65% and from 40 to 64%, respectively. Uncertainties associated with predicted tissue concentrations from sediment organic carbon were the lowest (22–58%), followed by predicted concentrations from porewater (41–65%), river water (40–80%), and sediment organic carbon + black carbon (28–88%).
Concentration of organic pollutants
OCPs
Concentrations of OCPs in the biota samples are given in Figure 1A–C and Supplemental Data, Table S8. In all species, the following descending order was observed: dichlorodiphenyltrichloroethanes (DDTs; 900–21 000 ng/g lipid) > chlordanes (840–4700 μg/g lipid) > hexachlorobenzene (HCB; 11–330 ng/g lipid) > α-endosulfan (<LOD–290 ng/g lipid) > HCHs (8.0–83 ng/g lipid). Concentrations of HCB, α-endosulfan, and DDTs were 2.0 to 10 times higher in the blue crab samples (Figure 1A) than in the other biota, as was previously observed for PAHs, PCBs, and PCDD/Fs (Khairy et al. 2014). Average concentrations of HCB in the benthopelagic (including the top predator) and pelagic (164 and 200 ng/g lipid, respectively) species were 3.0-fold higher than in the benthic species (70 ng/g lipid). Similarly, the average concentration of DDTs in the benthopelagic species (9000 ng/g lipid) was 3.0- to 4.0-fold higher than in benthic (3000 ng/g lipid) and pelagic (2000 ng/g lipid) species (Figure 1A).
The dominant pesticide in the sediment samples was DDTs (24-52 ng/g dry wt; Supplemental Data, Table S9), followed by chlordanes (12–35 ng/g dry wt) and α-endosulfan (1.0–11 ng/g dry wt). In the porewater (Supplemental Data, Table S10) and surface water samples (Supplemental Data, Table S11) HCHs (2.6–9.0 and 1.4–3.1 ng/L, respectively) dominated, followed by α-endosulfan (0.20–5.7 and <LOD–0.90 ng/L) and chlordanes (0.30–1.1 and 0.16–0.48 ng/L).
PBDEs
Concentrations of Σ12PBDEs ranged from 120 (F. heteroclitus) to 2500 ng/g lipid (Callinectes sapidus) in the biota (Supplemental Data, Table S12), from 1.0 to 11 ng/g dry weight in sediments (Supplemental Data, Table S13), from 19 to 40 pg/L in porewater (Supplemental Data, Table S14), and from 12 to 31 pg/L in surface water (Supplemental Data, Table S15). All biotic, sediment, porewater, and surface water samples were dominated by BDE-47 and BDE-99 (Figure 1D–F). Other congeners that were detected included BDE-183 in sediments and BDE-100 and BDE-49 in the biota, sediments, and porewater (estimated by LDPE).
PFAAs
Concentrations of PFAAs ranged from 130 ng/g wet weight (F. heteroclitus) to 350 ng/g wet weight (Anguilla rostrate; Supplemental Data, Table S16). All samples were dominated by PFOS (52–76% of the total PFAAs; Figure 1G), followed by PFDoA, perfluoroundecanoic acid (PFUnDA), PFOA (4.0–10% each), and PFHxA. Pentafluorobenzoic acid (PFBA), perfluoro-n-pentanoic acid (PFPeA), and perfluoroheptanesulfonic acid (PFHpS) were below LOD in all samples. In all samples, concentrations of perfluorinated sulfonic acids (PFSAs) were significantly (t test, p < 0.05) higher than perfluorinated carboxylic acids (PFCAs).
In the sediment (Figure 1H) samples, PFAAs ranged from 8.0 ng/g dry weight (km 3.7) to 15 ng/g dry weight (km 24; Supplemental Data, Table S17), with PFOS being the dominant compound (42% of total concentrations). The longer chain PFCAs (PFDoA, 35%; PFUnDA, 11%), PFOA (5.0%), and perfluorodecanoic acid (PFDA; 4.0%) were easily detected in sediment, probably related to their greater affinity for particles and hence accumulation in sediments (sorption to organic carbon) because of increasing hydrophobicity with increasing number of CF2 groups (Ahrens et al. 2010). In all samples, PFBA, PFPeA, and PFBS were below LOD, whereas perfluoroheptanoic acid (PFHpA) and PFHpS were below LOD in the majority of samples.
Concentrations of PFAAs in the surface water ranged from 44 (km 3.7) to 120 ng/L (km 22.4; Supplemental Data, Table S17). Similar to results for sediments and biota, PFOS dominated in surface water samples, followed by PFOA and PFHxA (Figure 1I).
Tissue concentrations versus abiotic compartments
The LDPE concentrations of HOCs in river water (CPE(RW)) and porewater (CPE(PW)) at equilibrium (nanograms per kilogram PE), organic carbon-normalized sediment concentrations (Csed(OC); nanograms per kilogram organic carbon), black carbon-normalized sediment concentrations (Csed(BC); nanograms per kilogram black carbon), and organic carbon+black carbon-normalized sediment concentrations (Csed(OC+BC)) were compared with lipid-normalized concentrations (Clip) of the target analytes in the food web of the lower Passaic River (Supplemental Data, Figure S4). For PFAAs, the comparison was only made with Csed(OC). Significant linear relationships were observed between log measured Clip and log CPE(RW) (Supplemental Data, Figure S4A), log CPE(PW) (Supplemental Data, Figure S4B), and log sediment (Supplemental Data, Figure S4C–E) concentrations (including PFAAs). Coefficients of determination (R2) ranged from 0.79 to 0.82, and the slopes of the best fits were insignificantly different from 1 (slopes = 1.2–1.3, p < 0.001).
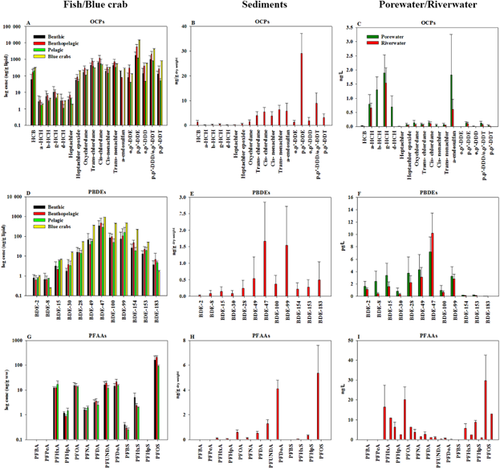
Values of Clip for all HOCs were predicted from Equations 3 through 5 using the organic carbon (Clip(OC)) and the organic carbon+black carbon models (Clip(OC+BC)), porewater (Clip(PW)), and river water (Clip(RW)) LDPE (Figure 2) and compared to measured concentrations. Similarly, protein-based tissue concentrations of PFAAs were predicted from Clip(OC) and Clip(RW) based on measured river water concentrations. To facilitate the comparison between HOCs and PFAAs, measured (in biota) and predicted protein-based concentrations of PFAAs from sediment and river water were normalized to the lipid content. This step was done by converting the predicted protein-based concentrations (from abiotic compartments) to equivalent wet weight concentrations and then normalizing to the measured lipid content in tissues. In addition to the regression lines, a factor difference of ±10 (represented by the dashed red lines) was used to evaluate the predictive ability of the abiotic compartments. This factor was previously used to evaluate the ability of using passive samplers as a surrogate for bioaccumulation of HOCs (Joyce et al. 2016). Points within the dashed lines indicate similarities in the affinities of geochemical natural sorbents (organic carbon and black carbon), lipid, and LDPE to the organic pollutants. Mono- through trichlorinated dioxins and furans and HCHs were excluded from the comparison because of their high biotransformation potential and/or uncertainties in the physicochemical properties.
Highly significant linear relationships (all regression parameters are shown in Figure 2) were observed between log measured Clip and predicted log Clip(OC) (Figure 2A), Clip(OC+BC) (Figure 2B), and Clip(PW) for HOCs only (Figure 2C) and Clip(RW) (Figure 2D) for all species. In general, PCDD/Fs were less well predicted than other classes of pollutants.
Jahnke et al. (2014) used passive samplers to derive the chemical activity of several PCBs and HCB in sediment and biota and to investigate the possibility of using passive porewater samplers as a metric for the thermodynamic potential for the bioaccumulation of HOCs. According to their results and other cited studies, measured Clip/predicted Clip values from porewater were either below or near their equilibrium benchmark values (0.5–2.0). In the present study, 2,3,7,78-tetrachlorodibenzo-p-dioxin; 2,3,7,8-tetrachlorodibenzofuran; PCBs 52, 101, 105, 118, 128, 138, 153, 180, and 187; BDEs 28, 49, 47, 100, and 154; HCB; chlordanes; and DDTs in the majority of the fish and blue crab samples showed values greater than the equilibrium benchmark based on porewater (>2).
Bioaccumulation and biomagnification of HOCs and PFAAs
Calculated BAFs for OCPs, PBDEs (both in liters per kilogram lipids), and PFAAs (in liters per kilogram wet weight) are given in the Supplemental Data (Tables S18–S20). Average BAFs ranged from 4.1 × 103 (α-HCH) to 7.7 × 107 (o,p′-dichlorodiphenyldichloroethylene [DDE]), 7.1 × 105 (BDE-2) to 5.3 × 108 (BDE-154), and 4.8 × 102 (PFBS) to 1.1 × 105 (PFDoA) for OCPs, PBDEs, and PFAAs, respectively. Calculated BAFs for PBDEs were the highest (Supplemental Data, Table S18), followed by OCPs (Supplemental Data, Table S19) and PFAAs (Supplemental Data, Table S20). In addition, PBDE BAFs were higher than those previously calculated for PAHs in the same samples (Khairy et al. 2014) but lower than BAFs previously calculated for PCDD/Fs (4.0 × 103–6.0 × 108) and PCBs (2.0 × 103–2.0 × 109; Khairy et al. 2014). Reported BAF values for OCPs and PBDEs in the present study were higher than values previously reported for freshwater fish from China (2.0 × 103–8.0 × 105 and 2.0 × 102–3.2 × 106 for OCPs and PBDEs, respectively; Wu et al. 2008; Wang et al. 2012) and for lake trout in Lake Michigan (Streets et al. 2006). Calculated BAFs for PFAAs were within the range of values calculated for PFAAs in the literature (1.2 × 101–1.0 × 105; Kwadijk et al. 2010; Labadie and Chevreuil 2011).
Trophic levels of the fish species were calculated based on the levels of nitrogen isotopes in the tissues (Khairy et al. 2014). Blue crabs were excluded because of their relatively high detected concentrations of OCPs and PBDEs despite their low trophic position, which is probably attributable to accidental sediment ingestion. Calculated TMFs were significantly greater than 1 (p < 0.05) for o,p′-dichlorodiphenyldichlorethane (4.1), HCB (3.9), o,p′-DDE (3.6), p,p′-DDE (3.1), p,p′-DDT (2.1), heptachlor (2.0), oxychlordane (1.8), BDE-47 (3.1), BDE-154 (2.5), Σ12PBDEs (2.4), and BDE-100 (2.1; Supplemental Data, Table S21).
DISCUSSION
Comparing tissue concentrations to sediment, river water, and porewater concentrations
In the present study, significant linear relationships were observed between log CPE(PW), CPE(RW), Csed(OC), and Csed(OC+BC) and log Clip in biota. Similar relationships were previously observed for PCBs (Friedman et al. 2009; Joyce et al. 2015; Joyce et al. 2016) and PBDEs (Joyce et al. 2015) when tissue concentrations were compared to LDPE concentrations adjusted for equilibrium. Coefficients of determination (R2) reported in the present study (for all classes of HOCs + PFAAs in sediments) were within the range previously observed for PCBs (0.59–0.94; Friedman et al. 2009; Joyce et al. 2015; Joyce et al. 2016) and DDTs (0.72) but higher than the value for PBDEs (0.59; Joyce et al. 2015).
In contrast, the slopes of the regression lines in the present study (1.2–1.3, p < 0.001) were higher than those observed in the previous studies for PCBs, PBDEs, and DDTs (0.74–1.0; Friedman et al. 2009; Joyce et al. 2015; Joyce et al. 2016). The slopes we observed indicated that accumulated amounts of HOCs in tissues exceeded those predicted from LDPE or sediment geochemistry. This implies that biotransformation of most HOCs did not play a significant role in the biota collected from the lower Passaic River. These results suggest that the linear relationships with abiotic compartments could be used to predict the bioaccumulation potential in the fish. Best predictions of Clip for HOCs and PFAAs were obtained from Csed(OC+BC) and/or CPE(PW) (Figure 2), as demonstrated by higher R2, lower SE, and slopes closer to 1.
All OCPs and PFAAs and the majority of PBDE, PCDD/F, and PCB congeners occurred within a factor of 10 of measurements, although lipid-based concentrations were overestimated for many congeners. Importantly, PFAAs were not separated from the other HOCs, and the regression parameters with and without PFAAS did not differ (Figure 2). This possibly implies a similar affinity of HOCs and PFAAs to organic carbon. Moreover, tissue concentrations of PFOA through PFDoA, PFOS, and PBDEs were well predicted from Csed(OC) within a factor difference ranging from 1.0 to 2.8 (Figure 2A).
Lipid-normalized concentrations of OCPs and PCBs were similarly better predicted using the CPE(PW) and Csed(OC+BC) models (Figure 2B,C) within a factor difference of 1.0 to 4.0. Higher factor differences (measured/predicted > 2.0) were observed for some PCB congeners (PCBs 28, 52, 101, 153, 138, and 180, which are known to be strongly bioaccumulative) and OCPs such as chlordanes and DDTs, presumably attributable to their uptake from food and their biomagnification potential. As for OCs, regression parameters for the measured versus predicted tissue concentrations from river water were similar with and without adding PFAAs (Figure 2D), which again indicates a similar lipid–water partitioning behavior for HOCs and the more polar PFAAs. However, many PFAAs were underestimated by river water, with factor differences (measured/predicted) >10. Only the more soluble PFHxA and PFHpA were better predicted from river water (factor difference 1.5–2.5) than sedimentary organic carbon, Csed(OC). Unfortunately, concentrations of PFAAs were not measured in porewater, and the predictive ability of CPE(PW) and Csed(OC+BC) cannot be examined. However, based on regression output of Csed(OC) and CPE(RW), we assume that the uptake of PFAAs probably occurred from porewater and diet similar to HOCs.
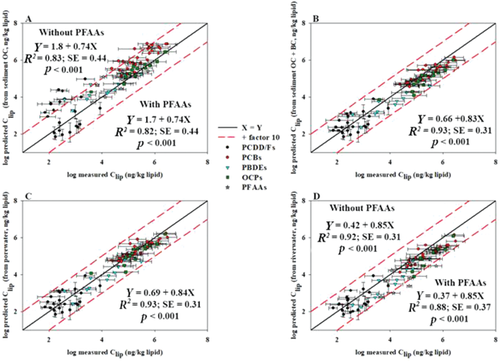
When comparing measured to predicted concentrations of the legacy pollutants, we did not account for the biotransformation rates in the biota. We assumed that biotransformation was negligible for several reasons. First, significant linear relationships were observed between calculated log BAFs and log KOWs (Figure 3), indicating either equilibrium or steady state for the HOCs. Second, as shown in Figure 3, log BAFs for all of the analytes (except BDE-99) were above the 1:1 line. In other words, calculated log BAFs were higher than their corresponding log KOWs, which is indicative of low or no biotransformation except for BDE-99. Third, lipid-based concentrations of HOCs were higher than sediment concentrations (normalized to organic carbon, black carbon, and organic carbon+black carbon) and LDPE concentrations (nanograms per kilogram PE; Supplemental Data, Figure S4). Finally, Wirgin et al. (2011) indicated that fish in the Hudson River, which is located in the same region as the Passaic River, have developed a mechanism to resist biotransformation attributable to evolutionary changes from exposure to legacy pollutants.
Comparison of porewater and food web chemical activities
In contrast to other studies (Lau et al. 2007 and references therein), measured Clip values of the majority of the HOCs in the present study were higher than the porewater's equilibrium benchmark set by Jahnke et al. (2014). The benchmark value was derived as a factor of 2 of the chemicals’ activities in porewater as estimated by passive samplers in sediment and biota directly. Possible reasons for these divergent results in the present study include 1) the structure of the food webs, 2) the relative importance of pelagic versus trophic coupling, and 3) relying on a different approach of comparing chemical activities in biota relative to abiotic compartments.
We rule out metabolism having a significant influence on these persistent chemicals, as we mentioned in the previous section. Similarly, trophic levels in the Swedish lake's food chain were even greater (assumed to reach a trophic level of up to 4.4) than in the Passaic River, where the top predator was at a trophic level of 4.0 (Khairy et al. 2014).
We argue that the exceedance of the porewater chemical benchmark observed in biota of the Passaic River is attributable to the strong benthic coupling, as we described previously (Khairy et al. 2014; Khairy and Lohmann 2017). In other words, the fish in the Swedish lake were likely strongly affected by water column concentrations (not measured), which we assume were much below porewater concentrations. In the Passaic River, differences between porewater and the water column were only 0.6 to 11. Lastly, we inferred chemical activities in biota by lipid-normalizing HOC concentrations, while we used passive samplers in porewater and the water column. This introduces more uncertainty, but the good agreement observed by numerous studies between lipid-normalized and passive sampler–derived HOC concentrations implies that this is of minor importance (Friedman et al. 2009; Friedman and Lohmann 2014; Joyce et al. 2016). Overall, the present results suggest that predicting the ecological risk to aquatic biota using measurements of porewater concentrations is not sufficient unless adjusted by the calculated BAFs in a benthically coupled system.
Bioaccumulation of the organic pollutants
Significant log BAF – log KOW (R2 = 0.80–0.91, p < 0.001) and log BAF – log KLIP-W (R2 = 0.87–0.94, p < 0.001) linear relationships were observed for PBDEs and OCPs in the biota using both river water (BAFRW; Figure 3A; Supplemental Data, Figure S5A) and porewater (BAFPW; Figure 3B; Supplemental Data, Figure S5B), indicating that the observed partitioning between lipids (in biota) and water can be approximated from octanol–water equilibrium partitioning. In our previous work, we indicated and quantified diffusive fluxes of PBDEs from the porewater to the overlying river water, which could explain the tight coupling of HOC concentrations and profiles in porewater and river water (Figure 1). Lower log BAFRW values were generally observed for BDE-99 than for the tri- through heptabrominated congeners (Figure 3A), possibly attributable to its biotransformation (metabolism) in fish species (Hu et al. 2010). Similar findings were previously observed in aquatic species collected from China (Wu et al. 2008) and in Lake Michigan's trout (Streets et al. 2006). Also, BDE-183 showed lower BAF values compared to penta and hexa congeners, probably attributable to the limited uptake induced by its large molecular size. However, calculated log BAFRW and BAFPW values for the majority of the fish samples were higher than their corresponding log KOW values (Figure 3) and log KLIP-W (Supplemental Data, Figure S5), indicating a possible dietary uptake. We argue that the exceedance of KOW-predicted BAFRW and BAFPW values is the result of the influence of the porewater on river water (via diffusive fluxes).
Calculated BAFs for PFAA increased significantly with increasing log KOW (Figure 3C) and alkyl chain length (Figure 3D; R2 = 0.60–0.65; SE = 0.42–0.45; p < 0.01). Log BAF of the longer chain PFUnDA, PFDoA, and PFOS (average 4.1, 4.4, and 3.7, respectively) were generally higher than the shorter chain PFAAs. In addition, values for PFOS were higher than all of the PFAAs other than the above-mentioned long chain ones, indicating a possible higher bioaccumulation potential for PFOS. Based on the Toxic Substance Control Act bioaccumulation criteria (Hong et al. 2015), only the longer chain PFAAs and PFOS appear to be bioaccumulative to very bioaccumulative in the fish species (3 ≤ log BAF < 5), which agrees well with other studies (Hong et al. 2015).
Biomagnification of pollutants in the food web
All of the HOCs (DDTs; HCB; oxychlordane; BDEs 47, 100, and 154; some PCB and PCDD/F congeners) that showed significant trophic magnification potential (TMF >1, p < 0.05) were the analytes that exceeded the equilibrium porewater benchmark value set by Jahnke et al. (2014), indicating the importance of dietary intake and strong benthic coupling in the Passaic River. All of the other investigated OCPs and PBDEs showed either insignificant trophic dilution (γ- and δ-HCHs) or trophic magnification. For PFAAs (Supplemental Data, Table S22), significant trophic magnification was only observed for PFUnDA (1.9), PFOS (1.8), PFDA (1.6), and PFDoA (1.5), as was observed in previous studies (Fang et al. 2014; Xu et al. 2014). However, calculated TMFs for PFAAs were generally lower than values calculated for OCPs and PBDEs.
For PFCAs, TMF values were relatively stable for C5 to C8 perfluorinated acids, then increased for C8 to C10, but decreased again for PFDoA, which is probably attributable to its molecular size, limiting its uptake. For PFSAs, PFOS showed the highest TMF value among all of the PFAAs, which agrees well with previous findings in other food webs worldwide (Conder et al. 2008).
Role of metabolic transformation
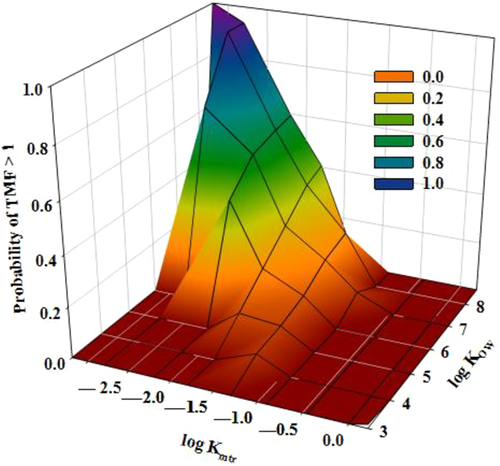
To investigate the relationships between hydrophobicity, metabolic transformation (kmtr), and TMFs, a conditional probability table was computed using TMFs and is presented graphically in Figure 4 based on cumulative frequencies. The figure indicates that the maximum probability of obtaining a TMF >1 was observed for HOCs having log KOW >7.5 and characterized by low metabolic transformation rates (kmtr ≤ 0.01 d−1). Starting from log KOW >6.5, high probability rates of TMFs >1 were observed. This range included dioxins, PCBs, PBDEs, OCPs, and the longer alkyl chain PFAAs (>C8). Exceptions were observed at lower log KOWs (4.5–6.5) for some OCPs and higher–molecular weight PAHs but still at low metabolic transformation rates (log kmtr ≤1.25 equivalent to kmtr ≤0.06 d−1), indicating that at these lower KOW ranges, there is an increase in the importance of kmtr in controlling trophic magnification. In addition, calculated TMFs for PCBs 189, 195, 206, and 209 (1.4–1.97) were higher than those calculated for octachlorodibenzodioxin, octachlorodibenzofuran, and BDE-183 (0.70–1.72), although all have log KOW values >8.0 (8.02–8.79). This can be explained because we know that the latter group has higher biotransformation rates (log kmtrs –1.96 to –1.46) than the former group (log kmtrs –3.04 to –3.30). Of all of the investigated HOCs with TMFs >1 (n = 59), only 4 had log kmtr >–1.5 (kmtr >0.032 d−1). All of these findings suggest that kmtr values are probably more important than hydrophobicity in explaining the biomagnification of HOCs. The uncertainty related to the estimated kmtr values could be substantial, however.
CONCLUSIONS
Although banned decades ago, contamination by legacy pollutants is widespread in the aquatic environment of the lower Passaic River. The present results indicate that CPE(PW) and CPE(RW) of HOCs and—for some HOCs and PFAAs—Csed(OC) can be used to estimate Clip in biota. In contrast to previous results, though, measured Clip values in the present study were above the porewater equilibrium benchmark concentration (0.5–2). The present results imply that in systems with strong benthic coupling, bioaccumulation needs to be considered to predict HOC and PFAA concentrations in top predators. Measured tissue concentrations of PFOA, perfluorononanoic acid, PFUnDA, PFDoA, and PFOS were 1.4 to 2.7 times higher than predicted tissue concentrations from sediment organic carbon. This agrees well with their bioaccumulative nature, as suggested by Toxic Substance Control Act bioaccumulation criteria (Hong et al. 2015), and suggests dietary uptake. Both hydrophobicity and metabolic transformations are needed to assess the biomagnification potential for both the lipophilic contaminants and PFAAs. Future work should confirm how useful chemical activities in sediment are for predicting the chemicals’ body burden in a given food web. This will always depend on the specifics of the ecosystem under consideration, including variations in the environmental conditions, food web characteristics, and physicochemical properties of sediments in the area under investigation. Yet the present study indicated that porewater concentrations from passive sampling for HOCs and/or sediment geochemistry for PBDEs and PFAAs were good predictors of lipid-normalized concentrations. There are additional factors, though, that will influence this predictive ability between sites and methods: first, variabilities arising from using different passive samplers and/or using unstandardized methods for the same passive sampler (Jonker et al. 2018); second, uncertainties associated with the use of partitioning coefficients in particular for compounds such as PFAAs that bind to proteins; and finally, expected differences from estimating porewater concentrations using in situ versus ex situ samplers. Accordingly, these factors should be addressed in future research.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.4354.
Acknowledgment
We acknowledge the Hudson River Foundation for funding this project (Hudson River Award HRF 2011-5), partial support by the National Institute of Environmental Health Sciences (grant P42ES027706) and SERDP (grant ER 2538), and M. Weinstein and K. Barrett (Manhattan College) for sampling support.




