Polychlorinated biphenyl tissue-concentration thresholds for survival, growth, and reproduction in fish
Abstract
Polychlorinated biphenyls (PCBs) have left a legacy of environmental contamination. Even though they were banned from production and active use in the 1970s, they persist in the environment and still have the potential to impact aquatic life. Our objective was to identify data from controlled laboratory studies of PCB-related adverse effects in fish and to conduct a meta-analysis on mortality, growth, and reproductive (MGR) threshold responses. For each endpoint type, we compiled data on the lowest-observed-adverse effect concentration (LOAEC) and the degree of effect at the LOAEC as a percentage of control. The LOAECs were expressed as tissue concentrations, so the term lowest-observed-adverse-effect residue concentration (LOAER) was used to represent PCB exposures. The lower limit of applicability was set at 0.1 μg/g total PCB tissue concentration, below which adverse MGR effects in fish were not supported by the data. Sensitivity distributions identifying the probability of adverse effects in fish populations or communities predicted that 25% of fish species would be impacted between 0.1 and 7.5 μg/g. Concentration–response threshold regressions were developed from the MGR datasets. For example, a 1 μg/g total PCB tissue concentration would predict effects of 17% mortality, 15% growth, and 39% reproductive. The analysis determined the degree of adverse response, with uncertainty estimates, expected across a broad range of PCB tissue exposure concentrations in fish. Data generated from MGR endpoints were combined to determine an approach for overall effect thresholds for PCB-related injury in fish. The MGR datasets included only laboratory data; however, responses were compared with field-observed effects. The present review provides a comprehensive assessment of PCB-induced injury in fish utilizing a data-inclusive approach. Environ Toxicol Chem 2019;38:712–736. Published 2018 Wiley Periodicals Inc. on behalf of SETAC. This article is a US government work and, as such, is in the public domain in the United States of America.
INTRODUCTION
Polychlorinated biphenyls (PCBs) are organic chemicals that have a biphenyl structure and varying degrees of chlorination (Figure 1). They were used in many industrial applications because of their properties of resistance to degradation, high flash point, and high heat capacity. These chemical properties made PCBs ideal for use in electrical components, including heat transfer fluids, transformers, and capacitors. Industrial applications of PCBs grew during the mid-20th century, with production in the United States reaching 32 million pounds by 1957 and nearly 80 million pounds by 1980 (Cairns et al. 1986). Manufacture of PCBs in the United States was under the trade name Aroclor®; they were manufactured and marketed in Europe and Asia under the trade names Clophen® and Kanachlor®, respectively. Synthesis of PCBs occurred by bubbling chlorine gas through a solution of biphenyl, resulting in mixtures that varied in congener composition and were classified according to the degree of chlorine substitution on the biphenyl ring. Mixtures of PCBs were marketed and sold based on the percentage of chlorine content. For example, Aroclor 1242 contained 42% chlorine by weight, and Aroclor 1254 contained 54% chlorine by weight. Each of the commercial mixtures of PCBs sold in the United States had different combinations of the possible 209 individual PCB congeners.
The recognition that PCBs were of environmental concern became widespread after Jensen (1966) published the first description of PCB contamination in an aquatic food web. Jensen observed trophic-level magnification of PCB concentrations in Baltic salmon (Salmo salar) and white-tailed eagles (Haliaeetus albicilla) in the Baltic Sea (Jensen 1966, 1972). Over the next 5 decades it became evident that environmental contamination by PCBs was a global issue. Numerous reviews have summarized the persistence, movement, and bioaccumulation properties of PCBs in the environment (e.g., Waid 1986; Tanabe 1988; Voogt et al. 1990). Based on persistence in the environment, PCBs were included in the United Nations Environmental Programme Stockholm Convention (2001) list of persistent organic pollutants (POPs) and later in 2004 by the Organisation for Economic Co-operation and Development. The goals of these designations were to have global agreement on the management and phase-out of the listed POPs, including PCBs. Even though PCBs had been banned from production in the United States in 1979, PCB-containing electrical transformers and capacitors in use at that time were approved to remain in-place or “grandfathered” into continued use. It was estimated that approximately 96% of all manufactured PCBs worldwide (∼1.2 million tons) were either in the environment (31% of total production) or landstocked (65% of total production) in continued use or unsecured landfills (Tanabe 1988). Thus, the potential for continued release of PCBs into both aquatic and terrestrial environments continued over the next decades.
Environmental contamination concerns increased through the end of the 20th century as more information came to light on the toxicity of PCBs to humans and ecosystems. The PCBs were designated as human carcinogens and a better understanding of the different potencies of individual PCB congeners was formed during this time (e.g., Safe et al. 1985; Safe 1994, 1990). Congeners were observed to have different toxicities and modes of action, based on the chlorination pattern on the biphenyl rings (Safe et al. 1985; Safe 1994, 1990). A small set of the PCB congeners (∼18 congeners) with lateral chlorines and non-ortho-chloro-substitution or mono-ortho-chloro-substitution patterns were found to bind to the aryl hydrocarbon receptor (AhR) and elicit dioxin-like toxicity (summarized in Van den Berg et al. 1998). All vertebrates possess homologous forms of the AhR and have varying degrees of sensitivity to dioxins and dioxin-like PCBs. Other modes of action of PCBs include neurotoxicity, endocrine disruption, and carcinogenicity (Safe et al. 1985; Safe 1994, 1990). Toxicity thresholds for commercial PCB mixtures and individual PCB congeners in wildlife have been developed over the past 20 yr (Giesy and Kannan 1998; Kannan et al. 2000; Su et al. 2014).
The goal of the present study was to evaluate PCB-induced adverse effects data in fish and to develop tissue concentration–based effects thresholds. Assessment of the risk posed to fish populations by PCB exposure has been essential for natural resources management. Federal programs, such as the Natural Resource Damage Assessment and Restoration (NRDAR) Program administered under the Department of the Interior and the National Oceanic and Atmospheric Administration or the Superfund Program administered by the US Environmental Protection Agency, routinely evaluate contaminant-related effects using metrics of survival, growth, and reproduction. Thus, our review and analysis focused on survival, growth, and reproduction, effects designated as injuries in prospective and retrospective risk assessments. We accomplished this aim in 2 steps: first, a comprehensive review of the fish PCB effects literature; and second, development of a data-inclusive approach for analysis of PCB exposure–effect/injury relationships across all fish species tested.
MATERIALS AND METHODS
Data identification
Literature review
A comprehensive literature search was conducted to initiate this analysis on the effects of PCBs on fish. We focused on the endpoints of mortality, growth, and reproduction (MGR). Multiple databases were searched including Google Scholar (Google), Web of Science (Thomson Reuters), and SCOPUS (Elsevier). We excluded studies that focused on human health or other nonfish species, as well as studies that evaluated only PCB exposure and bioaccumulation. The primary literature was the preferred source of all data; however, previous reviews on the effects of PCBs on fish (Monosson 2000; Meador et al. 2002) gave critical insights into data selection and evaluation and provided a check to ensure that critical studies were included in the present meta-analysis.
Data inclusion criteria
Six criteria were developed to identify appropriate data for our meta-analysis (Figure 2), based on standards for determination of injury under the NRDAR framework (as laid out in 43 CFR Part 11.62; US Department of the Interior 2009) and the guidelines set forth by Rosenberger (1987).
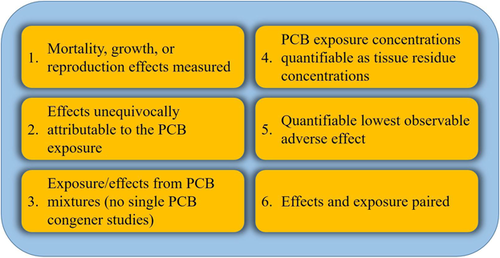
The first criterion required studies to evaluate MGR effects. The MGR endpoints provide practical and well-defined data that are useful in decision making (US Environmental Protection Agency 2003). Increased mortality and decreased reproductive capacity are clearly deleterious to fish populations and are considered injurious to fish (see 43 CFR Part 11.62; US Department of the Interior 2009). Growth impairment (reduction) in fish also has a substantial impact on populations, because smaller fish are more susceptible to loss through predation, do not compete for resources as well, and can indicate reduced fitness of the population (Groh et al. 2015).
The second criterion required that the effects be unequivocally attributable to PCB exposure. For inclusion, studies must have shown that fish exposed to PCBs exhibited adverse effects relative to a control group, thus restricting our primary analysis to laboratory studies.
The third criterion required that fish be exposed to PCB mixtures, as occurs in the environment. Although all PCBs are structurally similar, the number and orientation of chlorines influence toxicity, both empirically and mechanistically (Safe et al. 1985; Safe 1990, 1994). Laboratory studies of individual congeners, although mechanistically informative, do not represent how PCBs are likely to be encountered in the environment. Moreover, data from single PCB congener studies are not readily translated from a laboratory study to a field exposure (Giesy and Kannan 1998). In addition, studies that only included the most potent dioxin-like PCBs (PCBs 77, 81, 126, and 169; Giesy and Kannan 1998) were excluded. Fish toxicity studies using only dioxin-like PCBs ignore the potential for impacts from other PCB modes of action. To facilitate the use of multiple PCB mixtures, the dose metric of total PCBs was used.
Criterion 4 required that PCB exposure concentrations be represented as quantifiable tissue residue concentrations. It is well established that PCBs bioaccumulate in tissues (Veith et al. 1983, 1979; Safe 1994; Meador et al. 2011), and the use of a tissue residue approach as an exposure metric provides the most direct connection to the observed effects (McElroy et al. 2011). This criterion required studies to measure PCBs in exposed fishes or provide sufficient data to allow tissue concentrations to be calculated. Quantification and conversion steps for estimation of PCB tissue residues are described in detail in the PCB exposure concentration selection and translations section).
Fifth, PCB-induced effects must be quantifiable for inclusion in this analysis. This criterion required that biological responses in PCB-exposed fish be measurably different from control or lower exposure organisms. Ideal data presented exposed fish effects that were statistically different from effects in control fish. This approach provided scientifically defensible determination of effects. In each case, the lowest-observed-effect concentration was selected for inclusion. Within this analysis, the effect terminology used was lowest-observed-adverse-effect residue concentration (LOAER), to more accurately reflect the type of data being collected (further described in the Data selection and translation section). The LOAER provided a definitive measure of adverse outcome from each study, that is, a single concentration for which statistically significant, PCB-related toxic effects were observed, presumably within the linear portion of the dose–response curve.
The sixth criterion required that the LOAER effect levels be paired with an exposure concentration to provide a single point for comparison across studies.
Data selection and translation
Data from studies meeting the 6 selection criteria were compiled. The primary data extracted from each study were individual LOAERs for MGR and the PCB tissue concentrations at which each of those effects occurred. Each paired set of primary data (tissue concentration +LOAER expressed as percentage effect) was referred to as a datapoint, within the context of the present review. Ancillary data were also collected for each datapoint, including PCB mixture type, fish species, and fish life history information (specifically temperature, salinity, and fish life stage). Other study data collected included route and duration of PCB exposure. All extracted data were converted to standardized units (e.g., μg/g for PCB tissue concentrations) to facilitate metadata analysis. Conversions for each type of data followed set procedures (described in the Effect data selection and translation section). Original and converted values specific to each datapoint are given in the Supplemental Data (Tables S1–S3). Abbreviations and information for each mixture are also given in Supplemental Data (SI Table PCB).
Effect data selection and translation
The MGR data were quantified using effect-specific endpoints. Mortality effects were defined by changes in survival between PCB-exposed and control groups. Studies with measurements of hatchability and larval survival were included among mortality studies. Hatchability compared the number of surviving, viable larval fish with the initial number of eggs. Growth effects were defined by change in weight or length in PCB-exposed treatments relative to control groups. Only reduced growth data (i.e., fish at LOAER significantly smaller than control fish) were included in our analysis, because decreased growth is generally accepted as an adverse effect. Growth data were excluded in cases in which size-selective mortality, (i.e., smaller fish dying first) may have influenced growth calculations (Seelye and Mac 1981; Garrido et al. 2015). For reproduction effects, only reproductive impairment in adult fish was included in the analysis under this category. Often studies on reproduction reported effects on eggs or larval fish (i.e., hatchability, larval survival; Hansen et al. 1973; Schimmel et al. 1974; Freeman and Idler 1975; Hugla and Thome 1999); those endpoints were more accurately characterized as mortality responses and were included in the mortality dataset. For adult fish, reproductive endpoints included egg production (fecundity), fertilization rate, and number of spawns.
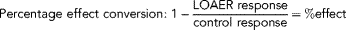 (1)
(1)PCB exposure concentration selection and translations
Concentration data at the LOAER were most commonly reported as micrograms (μg) of total PCBs/gram (g) of whole-body wet weight. The LOAER concentration data were standardized to this reporting metric using the protocols described below.
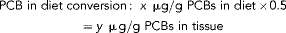 (2)
(2)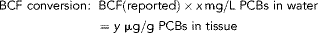 (3)
(3)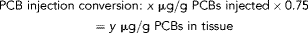 (4)
(4)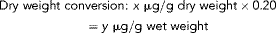 (5)
(5)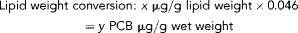 (6)
(6)Total PCBs were used as the metric for data standardization. Most included studies used total PCBs to report concentrations. Total PCBs were also often used in injury assessment or risk assessment cases (e.g., DeVault et al. 2001; Blazer et al. 2006). The detailed congener information required to calculate toxic equivalency factor (TEQ)-based exposure estimates were not available for most of the studies. An extensive evaluation of total PCBs versus TEQs in terms of concentration–response (see the Supplemental Data, total PCB vs TEQ) suggested that total PCBs provided the best approach for the development of PCB injury thresholds for this dataset.
Data analysis
Sensitivity distribution development
Probabilistic sensitivity distributions were developed separately for the MGR LOAER datasets. Probabilistic sensitivity distributions, similar to chemical toxicity distributions and species sensitivity distributions (SSDs), were applied to our current datasets to develop a probabilistic injury assessment for PCBs. This allowed us to identify the percentage of species expected to display significant mortality, growth, or reproductive impact at any given total PCB concentration. The use of LOAERs anchored these distributions to measured effect concentrations. We recognize that the included species and PCB mixtures may not represent the full range of sensitivities. This approach simply represents the best available information we have at this time, which can be updated with new information as it becomes available.
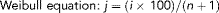 (7)
(7)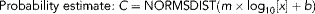 (8)
(8)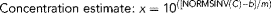 (9)
(9)PCB concentration: Effect regressions
The next step in the meta-analysis was the direct evaluation of the relationship between PCB tissue concentrations and effects. Log-transformed concentrations were compared with their associated percentage effects at the LOAER using linear regression. Given the range of LOAERs across studies, this produced PCB concentration–effect relationships across a wide range of potential concentrations. Each dataset (MGR) was evaluated to determine whether it fit the statistical assumptions required for linear regression, including normality and homogeneity of variance. Values with studentized-deleted residuals > 3 were identified as potential outliers, and specifically evaluated further. Regression calculations, statistics, and graphing were conducted in SigmaPlot (Ver 13.1; Systat Software).
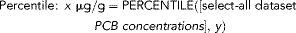 (10)
(10)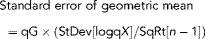 (11)
(11)Linear regressions along with applicability statistics were regenerated using the quintile data from each dataset (MGR). For all the regressions, the significance (p < 0.05) and the coefficient of determination (r2) were calculated by comparing percentage effect and log PCB tissue concentrations (LOAER values).
PCB concentration: Effect regression threshold calculation and prediction development
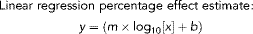 (12)
(12)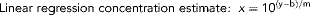 (13)
(13)Applicability limits within our analysis were defined based on the input data. We established a lower cutoff of 0.1 μg/g for the subsequent analysis of LOAER values. The lowest LOAERs within the MGR datasets occurred in the range of 0.1 μg/g, but not below. Furthermore, background PCB concentrations, at and below the 0.1 μg/g level, were observed in control fish without reported MGR effects. The PCB concentrations below this 0.1 μg/g cutoff are not expected to elicit adverse MGR effects based on our review of the literature. The upper limit for prediction of MGR effects based on PCB tissue concentration was set at 3000 μg/g, based on the average molecular weight of PCBs and the corresponding threshold for mortality from narcosis alone. In other words, if PCB concentrations were to reach this level in tissue, all fish would be expected to die from the nonspecific narcotic mode of action. This cutoff was more theoretical than practical because the data clearly showed that mortality occurred well below 3000 μg/g. Values above or below these limits were identified within tables and figures to denote that they were beyond practical applicability of the models.
PCB concentration: Effect threshold uncertainty analysis
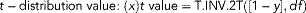 (14)
(14)The probability (y), in decimal percent, and degrees of freedom (df) were used to calculate a t value.
Evaluating testing parameters across individual studies
We compared ancillary information within datasets to test our assumption that including all available data provided robust and generally applicable estimates of PCB effects in fish. Previous injury assessments have often limited data selection based on species of concern, life history characteristics (e.g., temperature and salinity), or the mixture of PCBs deemed to be present in the geographic area. This limits the amount of data available to make assessments, often to only 1 or 2 studies. Although limited data prevented meaningful multivariate analysis, 5 ancillary variables were evaluated: PCB mixture, species, life stage, salinity tolerance, and temperature preference. Analysis of the PCB mixture was of particular interest given that 13 different PCB mixtures were included in our study and it has been well established that different mixtures of PCBs have different potencies (Tillitt et al. 1991; Harris et al. 1993). Twenty different fish species were included. The other ancillary data were evaluated as binary variables: life stage (early life stage [ELS] or juvenile/adult), salinity tolerance (fresh or saltwater), and temperature preference (cold or warm water).
Average percentage effect and geomean PCB tissue concentration, along with SEs, were calculated for each subgroup of ancillary variables within the MGR datasets. Differences among subgroups within each variable were evaluated using an analysis of variance (ANOVA) with post hoc testing. Data from the binary variables (life stage, salinity, and temperature) were used to generate linear regressions for each subgroup, and a concentration–effect regression was generated for each individual PCB mixture and fish species with sufficient data (n ≥ 3).
Application of metadata analysis to evaluate cumulative effects
We developed the cumulative largest effect model (CLEM) to integrate simultaneous adverse MGR effects expected at a given exposure. The CLEM uses the most sensitive MGR effect at a given concentration. The CLEM additively combines mortality and growth given that fish exposed to PCBs experience mortality monotonically with concentration, whereas those that survive have reduced growth (Nebeker et al. 1974; DeFoe et al. 1978; Mauck et al. 1978; Bengtsson 1980; Mayer et al. 1985; Thomas and Wofford 1993; Fisher et al. 1994; Black et al. 1998a; Orn et al. 1998; Gutjahr-Gobell et al. 1999). The largest cumulative effects, either reproduction (R) or the additive mortality and growth (M+G) effect rates, were used to generate the CLEM. The CLEM prevents double counting of any single effect. Furthermore, the reproductive dataset was used independently, based on the fact that reproductive effects of PCBs on fish generally occurred at lower concentrations than effects on growth and mortality, along with the idea that reproductive effects will generally occur through different pathways than mortality and growth-related effects. Moreover, reproductive effects limit populations through decreases in natality, whereas mortality and growth are ultimately measures of loss to the juvenile/adult within a population. Independent analysis of each of these adverse effects fails to consider the influence that each of these outcomes exerts on a population simultaneously. Therefore, we developed the CLEM for the evaluation of potential effects of PCB exposures on fish populations across a wide range of exposures, as well as through multiple, co-occurring mechanisms.
The CLEM calculations were derived from reproduction and mortality + growth dataset regressions. The mortality and growth regression percentage effect responses were added at designated concentration intervals (0.01, 0.1, 1, 10, 100, and 1000 μg/g). The CLEM concentration–response regression was then based on the most sensitive endpoint, either mortality + growth or reproduction percentage effects, at each designated exposure concentration. The CLEM regression identified cumulative concentration-effects across the range of expected PCB concentrations, following procedures previously described (Equations 12 and 13).
Analysis of field-derived PCB effects data
Field-derived PCB effects data in fish were compared with the laboratory-based data in an effort to corroborate the toxicity threshold estimates. Thus field data were vetted and compiled according to the same criteria developed for laboratory data, with the following caveats. Criteria 1, 3, 4, and 6 were used without modification. Effects evaluated from the field studies (i.e., mortality, growth, reproduction) were in concordance with laboratory effects data. The studies included measured tissue concentrations of PCB mixtures. Each study included both effects and concentration data. Criterion 2 (effects solely from PCBs) and criterion 5 (quantifiable lowest observable effect) required modification. The presence of non-PCB contaminants and abiotic/biotic differences at study locations make unequivocal PCB attribution of effects and establishing noneffected reference population effects difficult. Study data were included if PCBs were reported as the primary contaminant driver of effects. Percentage effect was determined on a case-by-case basis. The designated reference sites were not necessarily PCB free, and in some cases field data followed a gradient of exposure/responses. To establish percentage effect responses, exposed fish were compared with responses in fish from a designated reference site within the study, or in some cases between substantial and nonsubstantial effects, as identified by study authors (see details in the Supplemental Data, Field Data). For all field studies, information was collected on the specific field location, PCB mixture (if available), fish life history (species, temperature, salinity, and life stage), whole-body tissue concentrations, percentage effect, and specific endpoint measured.
Vetted individual and average field responses (geomean LOAER, average percentage effect) for MGR were compared with laboratory-generated LOAER regressions.
Statistics
Statistics were calculated using Microsoft Excel and SigmaPlot (Ver 13.1; Systat Software). Excel was used to calculate all individual data translations. This included means, geometric means, SEs, quintiles (both tissue concentration and percentage effect), and uncertainty factors. Normality, regression, and ANOVA were calculated using SigmaPlot. Significance was set at 95% (p < 0.05) for all analyses. All normality was evaluated using Kolmogorov–Smirnov with Lillifors correction. Each regression was evaluated to determine whether it fit the statistical assumptions required for linear regression, including normality, and homogeneity variance (measured as equal variance). For ANOVA, normality (Kolmogorov–Smirnov with Lillifors correction) and equal variance (Brown–Forsythe) were evaluated. If data did not meet normality requirements, an alternate ANOVA approach was used, Kruskal–Wallis one-way ANOVA on ranks, to provide the assessment. Post hoc testing approaches included Tukey's test for pairwise comparisons for data with normal distribution or Dunn's test for non-normal data in which ranks were evaluated.
RESULTS
The evaluation of the available literature on adverse effects of PCBs on fish found 31 laboratory MGR studies that fit the acceptability criteria. This resulted in 55 datapoints for LOAER, divided among mortality (30 datapoints; Table 1), growth (17 datapoints; Table 2), and reproduction (8 datapoints; Table 3). The 55 datapoints represented 16 PCB mixtures and 22 species. The individual MGR datasets also included varied species and PCB mixtures (Table 4). The different types of PCB mixtures evaluated (10 for mortality, 10 for growth, 7 for reproduction) represent most of the common commercial mixtures (see SI Table PCBs for PCB mixture descriptions and abbreviations). The number of fish species tested for PCB effects (mortality, 16; growth, 11; reproduction, 6) was relatively large, given the limited number of species usually used in effect-driven ecotoxicology. The MGR PCB tissue concentration data required log-transformation to meet conditions of normality. The geometric mean LOAER values (±SE) were 49.4 ± 8.9, 19.3 ± 4.2, and 9.8 ± 4.7 μg/g, for MGR, respectively. In addition, the 20th percentile quintile boundary concentrations were calculated (Table 4). Percentage effect values for the LOAERs were normally distributed for MGR. The mean percentage effects were 54.8 ± 4.4, 26.5 ± 3.8, and 50 ± 5.7%, for mortality, growth, and reproduction, respectively.
| PCB | Common name | Scientific name | Life stage | Temp. | Saline | Exp. (d) | Route | Concn. μg/g | Concn. code | Effect % | Quint | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1260 | Barbel | Barbus barbus | ELS | Warm | Fresh | 75 | par | 0.26 | D | 92 | —a | Hugla and Thome 1999 |
| A1254 | Tilapia | Oreochromis niloticus | ELS | Warm | Fresh | 15 | di | 0.75 | Fd | 57 | Q1 | Coimbra and Reis-Henriques 2007 |
| A1254 | Lake trout | Salvelinus namaycush | ELS | Cold | Fresh | 176 | aq/di | 1.53 | M | 32 | Q1 | Berlin et al. 1981 |
| A1254 | Atlantic croaker | Micropogonias undulates | Adult | Warm | Salt | 17 | di | 2.1 | Fd | 44 | Q1 | Thomas and Wofford 1993 |
| PCB-ORN | Zebrafish | Danio rerio | Adult | Warm | Fresh | 91 | di | 2.7 | M | 18 | Q1 | Orn et al. 1998 |
| A1254 | Sheepshead minnow | Cyprinodon variegates | ELS | Warm | Salt | 28 | par | 5.1 | m | 22. | Q1 | Hansen et al. 1973 |
| A1254 | Sheepshead minnow | Cyprinodon variegates | ELS | Warm | Salt | 28 | aq | 5.1 | cf | 30 | Q1 | Schimmel et al. 1974 |
| Aro-FIS | Atlantic salmon | Salmo salar | ELS | Cold | Fresh | 195 | aq | 6 | m | 5.8 | Q2 | Fisher et al. 1994 |
| PCB-BLA | Mummichog | Fundulus heteroclitus | Adult | Warm | Salt | 56 | di | 6.65 | fd | 13 | Q2 | Gutjahr-Gobell et al. 1999 |
| A1254 | Pinfish | Lagodon rhomboids | ELS | Warm | Salt | 14 | aq | 14 | m | 66 | Q2 | Hansen et al. 1971 |
| PCB-BLA | Mummichog | Fundulus heteroclitus | Adult | Warm | Salt | 40 | inj | 14.25 | ip | 54 | Q2 | Black et al. 1998a |
| Clo A50 | Eurasian minnow | Phoxinus phoxinus | Adult | Warm | Fresh | 300 | di | 15 | m | 28 | Q2 | Bengtsson 1980 |
| A1254 | Common sole | Solea solea | ELS | Cold | Salt | 40 | aq | 17.2 | li | 50 | Q2 | Foekema et al. 2014 |
| A1254 | Spot | Leiostomus xanthurus | ELS | Warm | Salt | 20 | aq | 46 | m | 51 | Q3 | Hansen et al. 1971 |
| A1254 | Brook trout | Salvelinus fontinalis | ELS | Cold | Fresh | 21 | par | 77.9 | m | 92 | Q3 | Freeman and Idler 1975 |
| A1016 | Pinfish | Lagodon rhomboides | Adult | Warm | Salt | 42 | aq | 106 | m | 47 | Q3 | Hansen et al. 1974 |
| A1248 | Fathead minnow | Pimephales promelas | ELS | Warm | Fresh | 30 | aq | 120 | cf | 44 | Q3 | DeFoe et al. 1978 |
| Aro-MAY | Rainbow trout | Oncorhynchus mykiss | ELS | Cold | Fresh | 90 | aq | 120 | m | 31 | Q3 | Mayer et al. 1985 |
| A1254 | Brook trout | Salvelinus fontinalis | ELS | Cold | Fresh | 118 | aq | 125 | m | 18 | Q4 | Mauck et al. 1978 |
| A1248 | Fathead minnow | Pimephales promelas | ELS | Warm | Fresh | 30 | aq | 138 | cf | 25 | Q4 | Nebeker et al. 1974 |
| Clo A50 | Eurasian minnow | Phoxinus phoxinus | ELS | Warm | Fresh | 300 | par | 170 | m | 83 | Q4 | Bengtsson 1980 |
| A1016 | Sheepshead minnow | Cyprinodon variegates | ELS | Warm | Salt | 33 | aq | 200 | m | 82 | Q4 | Hansen et al. 1975 |
| Clo A50 | Goldfish | Carassius auratus | Adult | Warm | Fresh | 22 | aq | 250 | m | 50 | Q4 | Hattula and Karlog 1972 |
| A1260 | Fathead minnow | Pimephales promelas | ELS | Warm | Fresh | 30 | aq | 260 | cf | 69 | Q4 | DeFoe et al., 1978DeFoe, Veith, and Carlson 1978 |
| A1254 | Sheepshead minnow | Cyprinodon variegates | Adult | Warm | Salt | 28 | aq | 300 | cf | 95 | Q5 | Hansen et al. 1973 |
| A1016 | Sheepshead minnow | Cyprinodon variegates | Adult | Warm | Salt | 28 | aq | 365 | cf | 91 | Q5 | Hansen et al. 1975 |
| A1254 | Coho salmon | Oncorhynchus kisutch | ELS | Cold | Fresh | 260 | di | 645 | m | 100 | Q5 | Mayer et al. 1977 |
| A1242 | Fathead minnow | Pimephales promelas | ELS | Warm | Fresh | 240 | aq | 795 | cf | 100 | Q5 | Nebeker et al. 1974 |
| A1016 | Sheepshead minnow | Cyprinodon variegates | ELS | Warm | Salt | 28 | aq | 1100 | m | 88 | Q5 | Hansen et al. 1975 |
| A1254 | Fathead minnow | Pimephales promelas | ELS | Warm | Fresh | 240 | aq | 2950 | cf | 100 | Q5 | Nebeker et al. 1974 |
- a Sample met acceptability criteria but was determined to be an outlier and was not included in the analysis.
- PCB = mixture of polychlorinated biphenyls (see S1 Table PCB in the Supplemental Data for description); Life stage = adult (reproductive capable); ELS = early life stage including nonreproductive juveniles; Temp. = testing temperature range; Saline = salinity conditions used in testing; Exp (d) = duration of testing in days; Route = route of PCB exposure (di = dietary; inj = injection; aq = aqueous; par = parental transfer); Concn. = PCB tissue concentrations in μg/g in total PCB whole-body wet weight; Conc code = code defining method of determining PCB tissue concentrations (m = direct measure; d = measured dry weight converted; fd = dietary exposure converted; li = measured lipid weight, converted; ip = injected dose converted; cf = concentration determined from study reported bioconcentration factor (see Materials and Methods and Supplemental Data, Table S1 for further description); Quint = identifies 20th quintile for each data (Q1 = 0–20; Q2 = 20–40, Q3 = 40–60; Q4 = 60–80; Q5 = 80–100).
| PCB | Common name | Scientific name | Life stage | Temp. | Saline | Exp (d) | Route | Concn. μg/g | Concn. code | Effect % | Quint | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCB-ORN | Zebrafish | Danio rerio | Adult | Warm | Fresh | 91 | di | 0.14 | M | 26 | Q1 | Orn et al. 1998 |
| A1254 | Atlantic croaker | Micropogonias undulates | Adult | Warm | Salt | 17 | di | 2.1 | Fd | 12 | Q1 | Thomas and Wofford 1993 |
| PCB-BLA | Mummichog | Fundulus heteroclitus | Adult | Warm | Salt | 40 | inj | 2.85 | Ip | 12.5 | Q1 | Black et al. 1998a |
| Aro-FIS | Atlantic salmon | Salmo salar | ELS | Cold | Fresh | 195 | aq | 3 | M | 16.9 | Q1 | Fisher et al. 1994 |
| A1254 | Atlantic croaker | Micropogonias undulates | ELS | Warm | Salt | 13 | par | 3.2 | M | 4.1 | Q2 | McCarthy et al. 2003 |
| PCB-BLA | Mummichog | Fundulus heteroclitus | Adult | Warm | Salt | 56 | di | 6.65 | Fd | 26 | Q2 | Gutjahr-Gobell et al. 1999 |
| A1242 | Channel catfish | Ictalurus punctatus | ELS | Warm | Fresh | 130 | di | 14.33 | M | 39 | Q2 | Hansen et al. 1976 |
| A1248 | Fathead minnow | Pimephales promelas | ELS | Warm | Fresh | 30 | aq | 36 | Cf | 20 | Q3 | DeFoe et al. 1978 |
| Aro-LEA | Coho salmon | Oncorhynchus kisutch | ELS | Cold | Fresh | 90 | di | 43 | ma | 25 | Q3 | Leatherland and Sonstegard 1978 |
| A1254 | Arctic charr | Salvelinus alpinus | ELS | Cold | Salt | 90 | di | 50 | fd | 33 | Q3 | Jorgensen et al. 2004 |
| K500 | Hybrid tilapia | Oreochromis sp. | Adult | Warm | Fresh | 84 | di | 50 | fd | 18 | Q3 | Shiau and Chen 1992 |
| A1248 | Fathead minnow | Pimephales promelas | ELS | Warm | Fresh | 30 | aq | 60 | cf | 50 | Q4 | Nebeker et al. 1974 |
| A1254 | Brook trout | Salvelinus fontinalis | ELS | Cold | Fresh | 48 | aq | 71 | m | 23 | Q4 | Mauck et al. 1978 |
| A1248 | Fathead minnow | Pimephales promelas | ELS | Warm | Fresh | 30 | aq | 120 | cf | 66 | Q5 | DeFoe et al. 1978 |
| Aro-MAY | Rainbow trout | Oncorhynchus mykiss | ELS | Cold | Fresh | 90 | aq | 120 | m | 11 | Q5 | Mayer et al. 1985 |
| A1254 | Rainbow trout | Oncorhynchus mykiss | ELS | Cold | Fresh | 365 | di | 150 | fd | 39 | Q5 | Cleland et al. 1988 |
| A1260 | Fathead minnow | Pimephales promelas | ELS | Warm | Fresh | 30 | aq | 260 | cf | 30 | Q5 | DeFoe et al. 1978 |
- a Measurement reported in companion study (Leatherland et al. 1979).
- PCB = mixture of polychlorinated biphenyls (see SI Table PCB in the Supplemental Data for description); Life stage = adult (reproductive capable); ELS = early life stage including nonreproductive juveniles; Temp. = testing temperature range; Saline = salinity conditions used in testing; Exp (d) = duration of testing in days; Route = route of PCB exposure (di = dietary; inj = injection; aq = aqueous; par = parental transfer); Concn. = PCB tissue concentrations in μg/g in total PCB whole-body wet weight; Concn. code = code defining method of determining PCB tissue concentrations (m = direct measure; d = measured dry weight converted; fd = dietary exposure converted; ip = injected dose converted; cf = concentration determined from study reported bioconcentration factor (see Materials and Methods and Supplemental Data, Table S2 for further description); Quint = identifies 20th percentile quintile for each data (Q1 = 0–20; Q2 = 20–40, Q3 = 40–60; Q4 = 60–80; Q5 = 80–100).
| PCB | Common name | Scientific name | Temp. | Saline | Exp (d) | Route | Concn. μg/g | Concn. code | Effect % | Quint | Endpoint | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCB-DAU | Zebrafish | Danio rerio | Warm | Fresh | 60 | di | 0.26 | Fd | 26 | Q1 | ↓ Fertilization rate | Daouk et al. 2011 |
| A1260 | Barbell | Barbus barbus | Warm | Fresh | 50 | di | 0.52 | D | 52 | Q1 | ↓ Eggs/kg♀ | Hugla and Thome 1999 |
| PCB-ORN | Zebrafish | Danio rerio | Warm | Fresh | 91 | di | 1.1 | M | 30 | Q2 | ↓ Eggs/♀ | Orn et al. 1998 |
| PCB-BLA | Mummichog | Fundulus heteroclitus | Warm | Salt | 40 | inj | 2.85 | Ip | 60 | Q3 | ↓ Eggs/♀ | Black et al. 1998a |
| Clo A50 | Eurasian minnow | Phoxinus phoxinus | Warm | Fresh | 300 | di | 15 | M | 40 | Q3 | ↓ # Spawns | Bengtsson 1980 |
| A1242 | Fathead minnow | Pimephales promelas | Warm | Fresh | 255 | aq | 107 | M | 65 | Q4 | ↓ Eggs/♀ | Nebeker et al. 1974 |
| Clo A50 | Three-spined stickleback | Gasterosteus aculeatus | Cold | Fresh | 105 | di | 289 | M | 69 | Q5 | ↓ # Spawns | Holm et al. 1993 |
| A1254 | Fathead minnow | Pimephales promelas | Warm | Fresh | 255 | aq | 429 | M | 58 | Q5 | ↓ eggs/♀ | Nebeker et al. 1974 |
- PCB = mixture of polychlorinated biphenyls (see SI Table PCBs in the Supplemental Data for description); Temp. = testing temperature range; Saline = salinity conditions used in testing; Exp (d) = duration of testing in days; Route = route of PCB exposure (di = dietary; inj = injection; aq = aqueous). Concn. = PCB tissue concentrations in μg/g in total PCB whole-body wet weight; Concn. code = code defining method of determining PCB tissue concentrations (m = direct measure; d = measured dry weight converted; fd = dietary exposure converted; ip = injected dose converted (see Materials and Methods and Supplemental Data, Table S3 for further description); Quint = identifies 20th percentile quintile for each data (Q1 = 0–20; Q2 = 20–40, Q3 = 40–60; Q4 = 60–80; Q5 = 80–100); Endpoint = method used to assess adverse reproductive outcomes.
| Mortality | Growth | Reproduction | ||
|---|---|---|---|---|
| Data inclusion | Studies | 22 | 15 | 7 |
| Data points | 29 | 17 | 8 | |
| PCB mixes | 10 | 10 | 7 | |
| Fish species | 16 | 11 | 6 | |
| Life history parameters | Warm/cold water | 22/7 | 11/6 | 7/1 |
| Fresh/salt water | 16/13 | 12/5 | 7/1 | |
| Adult/early life stage | 9/20 | 5/12 | 8/0 | |
| Tissue concentration information (μg/g) | Mean | 271 | 58.4 | 105.6 |
| SE | ±107 | ±16.9 | ±58.3 | |
| Median | 106 | 43.0 | 8.9 | |
| GeoMean | 49 | 19.3 | 9.8 | |
| GeoMean SE | ±8.8 | ±4.2 | ±4.7 | |
| Minimum | 0.8 | 0.14 | 0.26 | |
| Maximum | 2950 | 260 | 429 | |
| Normalitya | Fail (p < 0.001) | Pass (p = 0.063) | Fail (p = 0.009) | |
| Log-normalityb | Pass (p = 0.077) | Pass (p > 0.20) | Pass (p > 0.20) | |
| 20th Percentile | 5.6 | 3.0 | 0.8 | |
| 40th Percentile | 23.0 | 23.0 | 2.5 | |
| 60th Percentile | 124 | 50.0 | 33.4 | |
| 80th Percentile | 276 | 110 | 216 | |
| Percentage effect information | Mean | 54.7% | 26.5% | 50.0% |
| SE | ±4.4% | ±3.8% | ±5.7% | |
| Median | 50.0% | 25.0% | 55.0% | |
| Minimum | 5.8% | 4.1% | 26.0% | |
| Maximum | 100.0% | 66.0% | 69.0% | |
| Normalitya | Pass (p > 0.20) | Pass (p > 0.20) | Pass (p > 0.20) |
- a Normality determined by Kolmogorov–Smirnov with Lilliefors correction.
- b Tissue concentration data were log-transformed and then retested for normality.
- PCB = polychlorinated biphenyl; SE = standard error.
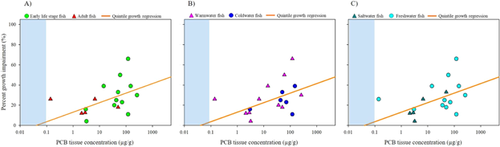
Probabilistic sensitivity distributions for MGR
Probabilistic concentration-percentage rank regressions were calculated for the MGR datasets (Figure 3), Developed independently for each dataset, these highly correlated regressions (Supplemental Data, Table S5) were used to generate likelihood of significant effect and concentration predictions (Tables 5 and 6). For example, at a given tissue concentration of 1 μg/g PCB, the regressions predict that 7% of fish species would experience significant mortality, 11% of species would experience adverse growth effects, and 27% of species would experience reproductive impairment (Table 5). As an inverse example, significant MGR in 25% of fish species would occur at PCB tissue concentrations of 7.5, 3.9, and 0.8 μg/g, respectively (Table 6). It would be likely that some, if not all, of these effects would be occurring simultaneously among the most sensitive species (those impacted at the lowest PCB concentrations).
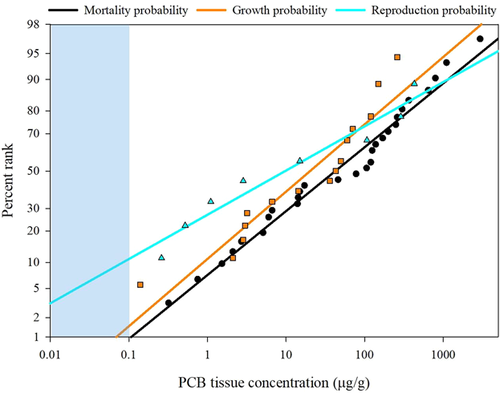
| Predicted % fish species associated with specified PCB tissue concentration (μg/g) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dataset | 0.001 | 0.01 | 0.05 | 0.1 | 0.5 | 1 | 5 | 10 | 50 | 100 | 500 | 1000 | 5000 |
| Mortality (%) | 0.00 | 0.06 | 0.44 | 0.9 | 4.2 | 7.3 | 20.3 | 28.7 | 52.5 | 63.0 | 83.1 | 89.0 | 96.8 |
| Growth (%) | 0.00 | 0.09 | 0.70 | 1.5 | 6.5 | 10.9 | 28.3 | 38.6 | 64.4 | 74.3 | 90.5 | 94.5 | 98.8 |
| Reproduction (%) | 0.7 | 3.2 | 7.8 | 10.9 | 21.2 | 27.0 | 42.8 | 50.2 | 67.0 | 73.4 | 85.5 | 89.4 | 95.3 |
- a Regression slope and intercept information are found in the Supplemental Data, Table S5. Data do not indicate the degree of effect (percentage effect) but rather the likelihood that a significant effect will occur at a given PCB concentration. Shaded areas are outside the predictive range of the dataset.
| Predicted PCB tissue concentrations (μg/g) associated with specified % fish species with significant effects | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dataset | 5% | 10% | 20% | 25% | 30% | 40% | 50% | 60% | 70% | 75% | 80% | 90% | 95% |
| Mortality | 0.6 | 1.6 | 4.9 | 7.5 | 11.0 | 22.1 | 42.5 | 81.5 | 164 | 241 | 370 | 1148 | 2922 |
| Growth | 0.4 | 0.9 | 2.6 | 3.9 | 5.6 | 10.9 | 20.3 | 37.6 | 72.9 | 105 | 158 | 463 | 1125 |
| Reproduction | 0.02 | 0.08 | 0.43 | 0.80 | 1.4 | 3.8 | 9.8 | 25.1 | 68.7 | 120 | 223 | 1143 | 4409 |
- a The regression slope and intercept information are found in the Supplemental Data, Table S5. Data do not indicate the degree of effect (percentage effect) but rather the likelihood that a significant effect will occur at a given PCB concentration. Shaded areas are outside the predictive range of the dataset.
PCB-induced fish mortality dataset meta-analysis
PCB concentration–mortality effects regression
The linear regressions of percentage mortality and PCB tissue concentration had nearly identical slopes for the full dataset and for the quintiles condensed datasets (Supplemental Data, Table S8 and Figure 4). Calculated quintile average datapoints (PCB tissue concentration and percentage mortality pairs; Table 7) were used to generate the quintile regression. For example, the second quintile, Q2 (20–40th percentiles), fell between 5.6 and 23.0 μg/g and contained 6 LOAER values (Table 1, Q2: 6.0, 6.7, 14.0, 14.3, 15.0, and 17.2 μg/g), resulting in a geometric mean PCB tissue concentration of 11.3 μg/g (± 1.0 μg/g SE); the corresponding percentage mortalities (Table 1, Q2: 5.6, 13, 66, 54, 28, and 50%, respectively) resulted in a Q2 average percentage mortality of 36.1 ± 9.9%. The log PCB concentration values were used in both regressions because concentration was log-normally distributed. Both regressions were significant (p ≤ 0.05) and passed the normality (p > 0.05) and homogeneous variance (p > 0.05) tests. The mortality quintile regression improved the dataset correlation (full: r2 = 0.46 vs quintile: r2 = 0.79; Supplemental Data, Table S8). The expected percentage mortality was calculated across a range of PCB exposure tissue concentrations (Table 8). The quintile regression indicated that mortality would not be expected at or below PCB tissue concentrations of 0.1 μg/g. However, at a concentration of 0.5 μg/g, the quintile regression predicted a mortality of 10%. The quintile regression predicted that mortality would increase by approximately 20% with every order of magnitude increase in exposure concentration. Furthermore, expected PCB exposure concentrations were predicted at a set of given mortality rates (%; Table 9). This provided a way to identify the concentrations associated with expected magnitudes of effect, for example, 10% mortality would be expected at 0.4 μg/g and 25% mortality would be expected at 2 μg/g.
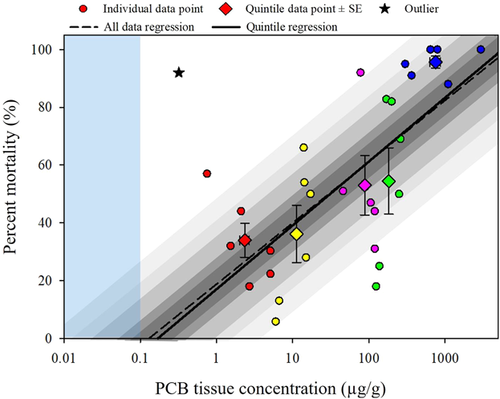
| Mortality effects | Growth effects | Reproduction effects | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Percentile range | Quintiles | Quintile range (µg/g) | Quintile tissue concn. (µg/g ±SE) | Quintile % effect (% ±SE) | Quintile range (µg/g) | Quintile tissue concn. (µg/g ±SE) | Quintile % effect (% ±SE) | Quintile range (µg/g) | Quintile tissue concn. (µg/g ±SE) | Quintile % effect (% ±SE) |
| 0–20% | Q1 | 0.75–5.6 | 2.4 ± 0.3 | 33.9 ± 5.9 | 0.14–3.0 | 1.3 ± 0.5 | 16.6 ± 3 | 0.26–0.75 | 0.37 ± 0.08 | 39 ± 13 |
| 20–40% | Q2 | 5.6–23 | 11.3 ± 1.0 | 36.1 ± 9.9 | 3.0–23 | 6.7 ± 1.5 | 23.0 ± 10 | 0.75–2.5 | 1.1 | 30 |
| 40–60% | Q3 | 23–124 | 88.6 ± 7.8 | 53.0 ± 10.2 | 23.0–50.0 | 44.4 ± 1.7 | 24 ± 3.3 | 2.5–33.4 | 6.5 ± 3.3 | 50 ± 10 |
| 60–80% | Q4 | 124–276 | 183 ± 10.8 | 54.5 ± 10.9 | 50.0–110 | 65.3 ± 3.4 | 36.5 ± 13 | 33.4–216 | 107 | 65 |
| 80–100% | Q5 | 276–2950 | 753 ± 120 | 95.7 ± 2.1 | 110–260 | 160 ± 14.1 | 36.5 ± 11 | 216–429 | 352 ± 43 | 63.5 ± 5.5 |
| Normality | p | >0.20b | 0.198 | >0.20b | >0.20 | >0.20b | >0.20 | |||
- a Including: the range of polychlorinated biphenyl (PCB) tissue concentration for each quintile, average quintile tissue concentration ± standard error (SE), average quintile percentage effect (±SE), and normality evaluations for quintile datasets.
- b Normality for quintile tissue concentrations was evaluated using log-transformed values.
| Predicted % effect associated with specified PCB tissue concentration (μg/g) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dataset | 0.001 | 0.01 | 0.05 | 0.1 | 0.5 | 1 | 5 | 10 | 50 | 100 | 500 | 1000 | 5000 |
| Mortality (%) | —b | —b | —b | —b | 12.6 | 18.9 | 33.7 | 40.1 | 54.9 | 61.2 | 76.0 | 82.4 | 97.2 |
| Quintile mortality (%) | —b | —b | —b | —b | 10.4 | 17.0 | 32.5 | 39.1 | 54.6 | 61.2 | 76.7 | 83.3 | 98.8 |
| Growth (%) | —b | 1.6 | 6.9 | 9.2 | 14.5 | 16.8 | 22.1 | 24.3 | 29.6 | 31.9 | 37.2 | 39.5 | 44.8 |
| Quintile growth (%) | —b | —b | 2.8 | 5.6 | 12.2 | 15.0 | 21.6 | 24.4 | 30.9 | 33.8 | 40.3 | 43.1 | 49.7 |
| Reproduction (%) | 13.3 | 22.5 | 28.9 | 31.7 | 38.1 | 40.9 | 47.3 | 50.1 | 56.5 | 59.3 | 65.7 | 68.5 | 74.9 |
| Quintile reproduction (%) | 5.4 | 16.4 | 24.1 | 27.4 | 35.2 | 38.5 | 46.2 | 49.5 | 57.2 | 60.5 | 68.2 | 71.6 | 79.3 |
- a For each dataset, slope and intercept data (Supplemental Data, Table S8) were used to predict percentage effect at given PCB tissue concentrations. Values above and below the range of PCB tissue concentrations within the dataset are shown in gray.
- b Predicted percentage effect <0.
| Predicted PCB tissue concentrations (μg/g) associated with specified % effects | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dataset | 5% | 10% | 20% | 25% | 30% | 40% | 50% | 60% | 70% | 75% | 80% | 90% | 95% |
| Mortality | 0.22 | 0.38 | 1.12 | 1.93 | 3.33 | 9.90 | 29.4 | 87.4 | 260 | 447 | 771 | 2290 | 3947 |
| Quintile mortality | 0.29 | 0.49 | 1.43 | 2.43 | 4.13 | 11.9 | 34.5 | 99.9 | 289 | 492 | 836 | 2420 | 4116 |
| Growth | 0.03 | 0.13 | 2.68 | 12.2 | 55.7 | 1161 | —b | —b | —b | —b | —b | —b | —b |
| Quintile growth | 0.09 | 0.29 | 3.42 | 11.7 | 39.8 | 464 | 5406 | —b | —b | —b | —b | —b | —b |
| Reproduction | 0.0001 | 0.0004 | 0.01 | 0.02 | 0.07 | 0.8 | 9.8 | 119 | 1458 | 5094 | —b | —b | —b |
| Quintile reproduction | 0.001 | 0.003 | 0.02 | 0.06 | 0.17 | 1.4 | 11.1 | 89.5 | 722 | 2051 | 5825 | —b | —b |
- a For each dataset, slope and intercept data (Supplemental Data Table S8) were used to predict PCB tissue concentration given specified percentage effects. Values above and below the range of PCB tissue concentrations within the dataset shown in gray.
- b Beyond the scope of theoretical bioaccumulation (>10 000 μg/g).
The uncertainty regressions representing a range of effect probabilities were generated (Figure 4) and allowed probability ranges to be calculated for any PCB tissue concentration (Supplemental Data, Uncertainty). For example, at a 5-μg/g PCB exposure concentration, the predicted 32.5% mortality has a 50% confidence interval (CI) ranging from 26.5 to 38.5% and a 95% CI range from 10.3 to 54.5% mortality.
During the initial regression diagnostics process, one datapoint (0.32 μg/g and 92% mortality; Hugla and Thome 1999) was identified as an outlier (studentized deleted residual value > 3). A preliminary regression including this datapoint predicted substantial mortality at PCB tissue concentrations not supported by any other studies within the dataset. For those reasons, this datapoint was excluded from further analysis and all regressions excluded the outlier.
Evaluation of ancillary variables within PCB mortality dataset
Influence of specific PCB mixtures on mortality
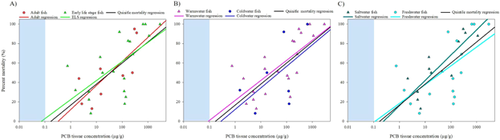
The average LOAER values for PCB mixtures followed the same concentration–response trend as the quintile data (Figure 5A and Supplemental Data, Table Average PCB Mixtures). Regression analysis showed a significant, positive log-linear relationship among PCB mixture concentration–mortality pairs (regression p = 0.001; r2 = 0.74), and none of the average PCB mixture values were observed to be an outlier or to be exerting undue influence on the regression. This trend suggested dose–response as an important factor among average PCB mixture datapoints (Figure 5A). However, differences in potency certainly exist among technical mixtures and individual PCB congeners, as established in the literature. Furthermore, due to a paucity of available, appropriate data, mixture-specific LOAER values would be based on 1 or 2 datapoints for most PCB mixtures. For those mixtures with multiple datapoints, individual mortality-based LOAER regressions for both A1254 (n = 13) and A1016 (n = 4) had positive dose–response relationships (Figure 5B). For A1254 there was a significant log-linear regression (p = 0.01; r2 = 0.46), whereas A1016 LOAERs in fish increased with concentration; however, the relationship was not significant (p = 0.2 r2 = 0.58), likely due to the small number of studies in the dataset (n = 4). The percentage effects associated with the different PCB mixtures were significant. However, no individual pairs of LOAER values for PCB mixtures tested as significantly different (Tukey's test, p > 0.05). This may be due to the wide range of mortality responses and the overall influence of PCB concentration on the percentage effect. For example, the A1242 LOAER caused 100% mortality (Nebeker et al. 1974), whereas a study with a mixture of Aroclors caused an 8.2% increase in mortality (Fisher et al. 1994). The strong trend of percentage effect increasing with concentration, observed across the mortality dataset generally, supported our inclusion of all the vetted data, which, in turn, provided better resolution across the spectrum of PCB exposure-related mortalities.
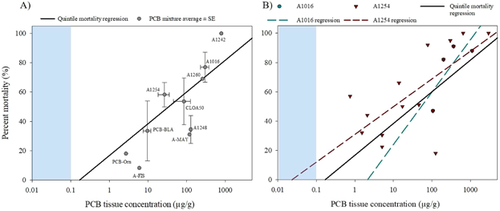
Influence of species tested on mortality
Whereas species average LOAERs varied in both PCB concentration and percentage mortality, the relationship between geomean PCB LOAER and average percentage mortality for individual species generally followed the same concentration–response trend as the quintile regression (Figure 6A) and was significant (p = 0.01, r2 = 0.39). Residual analysis of that regression found no outliers. The geometric mean mortality-based LOAER values in fish spanned 4 orders of magnitude, from 0.75 μg/g for tilapia to 399 μg/g for fathead minnow (Supplemental Data, Table Average Fish Species). Many species occurred singly within the dataset. Species with multiple mortality studies generally had large SEs. The geomean mortality LOAER values for pinfish (38 ± 24 μg/g; n = 2), mummichog (9.7 ± 2.3 μg/g; n = 2), sheepshead minnow (93 ± 42 μg/g; n = 6), and fathead minnow (399 ± 116 μg/g; n = 5) had SEs ranging from 20 to 60% of the geomean mortality LOAER value. Species-specific data for both PCB LOAER values and percentage effect failed the normality test and were therefore assessed with an ANOVA of ranked data. No significant differences were observed in either geomean mortality-based PCB tissue LOAER values (p = 0.24) or average percentage mortality (p = 0.78) among the species evaluated in this dataset.
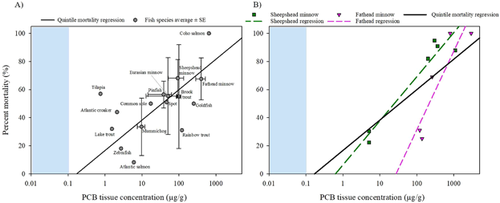
Individual regressions for fathead minnow (n = 5) and sheepshead minnow (n = 6) showed significantly positive concentration–response data (p = 0.04, r2 = 0.81; and p = 0.002; r2 = 0.92, respectively; Figure 6B). Slopes of the linear regressions for both were steeper and shifted to the right, relative to the quintile mortality regression, suggesting a more homogeneous response among the populations of fish tested (steeper slope) and lesser sensitivity (shifted right), compared with the all-species regression. However, these datasets were small and confounded by datapoints generated from multiple PCB mixtures (4 for fathead minnow, 2 for sheepshead minnow). Overall, the lack of significant difference suggests that each species represents an overlapping continuum of their individual sensitivities. Again, these analyses suggest the usefulness of an overall approach that is more inclusive of all available data and species.
Influence of life history variable on mortality
No significant differences were observed among averages of paired test condition variables (warm/cold water, fresh/salt water, adult/ELS). There was a great degree of overlap among PCB LOAER and percentage mortality averages for these data. Enough data were available that concentration–response regressions could be generated for each life history variable set: life stage (adult/ELS; Figure 7A), temperature (warm/cold; Figure 7B), and salinity (fresh/salt water; Figure 7C). Each of these life history variable regressions of the LOAER mortality data was significant (p < 0.05). However, the regression slopes and intercepts, and the degree of overlap, suggested that these differences in fish life history did not appear to influence the PCB tissue concentration–mortality relationship. Life history–based paired variable regressions of LOAER values for mortality did not vary substantially from the quintile regression that represented the same data from the entire dataset. Overall, this analysis suggested that when one is deriving mortality-based effects thresholds in fish, there is little support for excluding or separating data based on these life history parameters.
PCB concentration–growth effects regression
PCB concentration: Growth effects regression
There was minimal difference in the linear regressions between the full growth dataset and the quintile dataset (Figure 8 and Supplemental Data, Table S8). Both regressions passed the normality test (p ≥ 0.05) and the homogeneous variance test (p > 0.05). The full dataset regression approached significance (p = 0.09) but was poorly correlated (r2 = 0.18), whereas the quintile dataset regression was significant (p = 0.04), with an improved correlation coefficient (r2 = 0.80). The expected percentage growth was calculated across a range of PCB tissue concentrations (Table 8). As PCB tissue exposure concentrations increased, the predicted growth inhibition did not exceed 50% based on the regression. The PCB-induced growth inhibition had an upper limit, that is, only a certain degree of growth inhibition was observed before alternative pathways of toxicity were manifested in the exposed organisms, specifically mortality. This was observed in half of the studies in which both mortality and growth inhibition were monitored (DeFoe et al. 1978; Mayer et al. 1985; Thomas and Wofford 1993; Gutjahr-Gobell et al. 1999). In these studies, once mortality began to occur, individuals were removed from the population being evaluated for effects on growth. Thus the range of percentage growth reductions within individual studies of the present analysis achieved a maximum of 66% (DeFoe et al. 1978; Table 2), and the average percentage growth inhibition in the fifth quintile (Q5) was 37 % (±11%; Table 7). Furthermore, at a certain PCB exposure concentration, the percentage growth inhibition could be predicted. For example, a 25% inhibition of growth was predicted at 12 μg/g (Table 9).
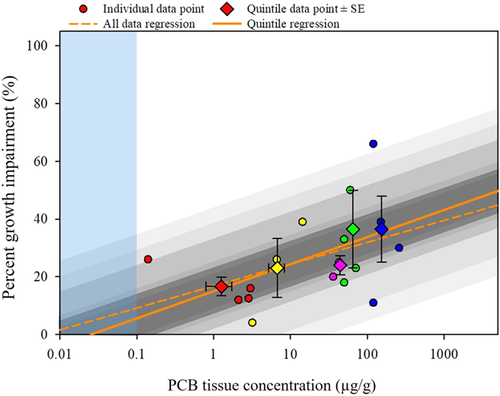
The uncertainty regressions were generated for the growth dataset, representing a range of probabilities (Figure 8) and allowed probability ranges to be calculated for any PCB tissue concentration (Supplemental Data, Uncertainty). For example, at 10 μg/g, the quintile growth regression predicted 24.4% growth inhibition with a 50% CI ranging from 18.2 to 30.5%, and a 95% CI range from 1.2 to 47.5% from the uncertainty regressions.
Evaluation of ancillary variables on the growth dataset
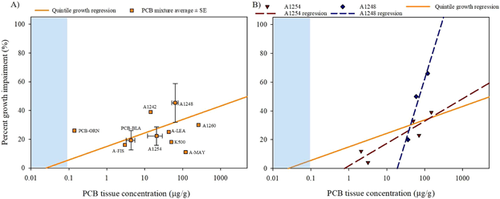
Influence of PCB mixture on growth
A concentration–percentage effect regression of the 10 PCB mixture average datapoints (LOAER concentration–growth effect pairings) was not significant (Figure 9A; p = 0.78; nonsignificant regression line not shown). The plotted average PCB mixture datapoints relative to the quintile growth regression (Figure 9A) suggested differences. They did not follow the same relative exposure concentration–effect patterns observed in the overall growth regression, with several individual mixtures shifted away from the quintile regression line. No significant differences among mixture average LOAER concentrations (p = 0.36) or effects (p = 0.63) were observed (Kruskal–Wallis, p > 0.05). The majority of PCB mixtures were only tested once within the growth dataset, and those that were tested in multiple studies had highly variable results. The 3 PCB mixtures with multiple study datapoints (A1254, A1248, PCB-BLA) had large SEs for the LOAER concentration (Supplemental Data, Table Average PCB Mixtures). Strong concentration–response trends were observed for A1248 (with 3 datapoints, the geomean concentration was 62 ±12 μg/g) and A1254 (with 5 datapoints, the geomean was 20 ± 8.5 μg/g; Figure 9B). The regression of A1254 datapoints was similar to the quintile regression. The slope of the A1248 regression was much steeper, but all datapoints in that regression came from one species (fathead minnow), suggesting that these differences did not wholly result from differences in PCB mixtures.
Influence of species on growth dataset
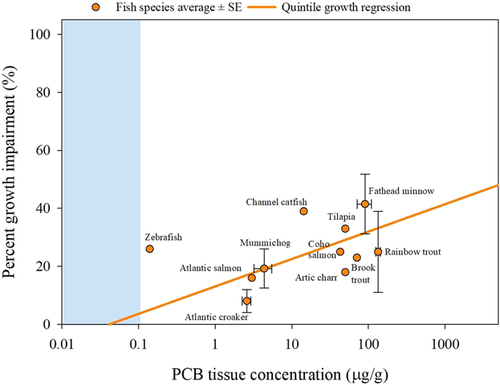
Differences in species average PCB concentration LOAER values for growth inhibition across the 11 different species of fish were substantial, varying by 3 orders of magnitude, but were not statistically significant (Supplemental Data, Table Average Fish Species). Percentage growth effect also varied by species, but variation was not significant. Most species average growth concentration–response datapoints fell near the quintile regression line (Figure 10). The species that were not near the quintile regression (e.g., catfish and zebrafish) represented a single study, but were still within the 70% CI (Figure 8).
Influence of life history variable on growth dataset
No significant differences in average LOAER or percentage growth inhibition were observed for salinity (salt vs fresh) or temperature (warm vs cold). For life stage, a significant difference was observed between the LOAER growth values of ELS fish (78 ± 22 μg/g) and adult fish (12 ± 9.5 μg/g). However, the distribution of the datapoints for growth LOAERs in adult fish overlapped the range of the ELS LOAER values with the exception of one value (Figure 11A). This finding suggests that the significant differences of the average responses may be an artifact of testing approaches (e.g., species tested, PCB mixture, concentrations tested), rather than a true difference. The pattern holds for salinity and temperature variables, for which the individual LOAER values were distributed across the entire range of responses (Figures 11B and C). An analysis comparing individual variable regressions with that of the quintile regression was not conducted, because individual variable regressions were not significant.
Growth effects in context with other effects
Growth inhibition LOAER values for PCB were influenced by mortality, particularly at higher exposure concentrations. Indeed, no studies had reductions in growth >66% of the control treatments (Table 2). It was likely that PCB-induced growth impacts in fish were limited due to the co-occurrence of mortality at the same exposure concentrations. Because mortality occurred at a given exposure concentration within a study, those individuals that died were not available for assessment of other adverse outcomes, including impairment of growth. Significant growth impacts occurred in several studies at the same exposure concentrations at which significant mortality was also occurring (DeFoe et al. 1978; Mayer et al. 1985; Thomas and Wofford 1993; Gutjahr-Gobell et al. 1999). In some cases, PCB-induced reductions in growth occurred at the next lower exposure concentration, where mortality occurred but was not statistically significant (Nebeker et al. 1974; Mauck et al. 1978; Fisher et al. 1994; Black et al. 1998b; Orn et al. 1998). Growth effects caused by PCBs in fish may also be less prominent due to size-selective mortality. If, as has been suggested, smaller fish are more susceptible to PCBs and die first (Seelye and Mac 1981; Garrido et al. 2015), then the growth (or size) differences between average control and PCB-exposed fish would appear to be not as great, whereas the impact on individual fish (not measured) may be greater. Conversely, when expected mortality was low, there was a greater degree of impact on growth (Tables 1 and 2). This was the case with fathead minnows, a species observed to be among the least sensitive to PCB-induced mortality. Fathead minnows had the greatest degree of growth impairment, 50% and 66% reduction in growth compared to controls (Nebeker et al. 74; Defoe 1974; DeFoe et al. 1978).
The majority of studies reporting effects on growth also monitored and reported other effects occurring concurrently. In the evaluation of A1248 and A1260 (DeFoe et al. 1978), MGR effects were reported. A number of studies measured sublethal effects along with mortality, including physiological effects (Hansen et al. 1976; Mauck et al. 1978; Cleland et al. 1998; Shiau and Chen 1992; Thomas and Wofford 1993), reproductive and physiological effects (Black et al. 1998a; Orn et al. 1998; Gutjahr-Gobell et al. 1999), physiological and immunological responses (Leatherland and Sonstegard 1978; Mayer et al. 1985; Jorgensen et al. 2004), and behavioral responses (Fisher et al. 1994; McCarthy et al. 2003). The PCBs disrupt fish and other organisms through several different pathways and mechanisms of action (Monosson, 2000; Meador et al. 2002). Thus, it was not surprising that most studies reported simultaneous adverse effects occurring at single exposure concentrations of PCBs, including growth inhibition and mortality.
PCB-induced fish reproductive effects dataset meta-analysis
PCB concentration: Reproductive effects regression. The reproduction full and quintile dataset regressions were similar in slope and intercept (Figure 12 and Supplemental Data, Table S8). Both regressions were significant (full p = 0.04; quintile p = 0.03) and passed normality and variance testing. The reproduction quintile dataset regression showed a stronger correlation versus the full dataset (full r2 = 0.52; quintile r2 = 0.85). The reproduction regressions were used to predict reproductive effects across all applicable PCB tissue concentrations (Table 8). For example, 39% of fish would have predicted reproductive effects at PCB tissue concentrations of 1 μg/g. Regressions were also used to identify tissue concentrations associated with specific percentage reproductive impairment (Table 9). The reproduction regression predicts effects at a concentration below the level of data applicability of 0.1 μg/g (e.g., 20% loss of reproduction predicted at 0.02 μg/g). However, additional research is needed to support predictions in this range of exposure.
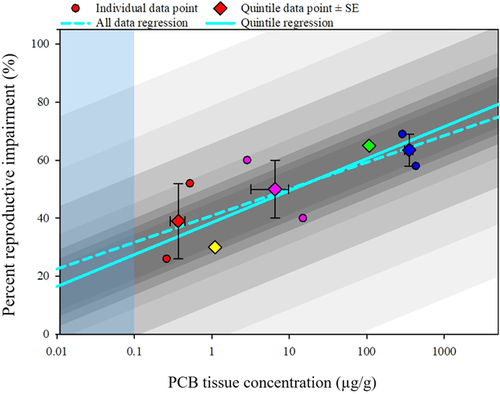
A critical caveat in generating uncertainty regressions for the reproductive dataset (Figure 12) was the small number of reproductive studies (n = 8). Only 3 quintiles had sufficient data to calculate the SEs. The uncertainty factors were larger (Supplemental Data, Uncertainty), and the corresponding regressions were wider than the uncertainty estimates for mortality and growth. For example, at 0.5 μg/g, the quintile reproduction regression predicted 35.2% effect with a 50% CI ranging from 27.2 to 42.9%, and a 95% CI ranging from 0 to 76%, using the uncertainty regressions. This broad uncertainty range reflects the limited reproductive data.
Analysis of the influence of life history characteristics, PCB composition, or other test parameters on reproductive outcomes in fish was not attempted due to the limited number of studies in this dataset. Only 1 of 8 studies was conducted using cold water fish or salt water fish, and only 2 studies used the same PCB mixture. Only 2 species (zebrafish and fathead minnow) were used twice within the 8 included assays. These limitations made it impossible to develop meaningful trends among individual variables (life history or PCB mixture tested). Consequently, the same data-inclusive approach was used with the reproductive effects studies as the evaluation of mortality and growth endpoints.
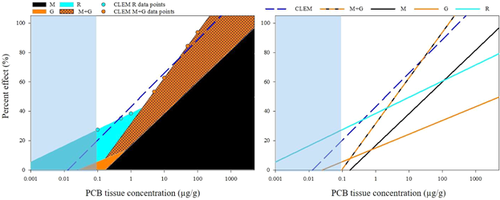
Evaluation of cumulative MGR effects of PCBs on fish
The CLEM dataset regression estimated cumulative effects across the wide range of PCB exposure concentrations in fish (Figure 13). The largest percentage effects (reproduction or mortality + growth) were selected for analysis (Figure 13 and Supplemental Data, Table CLEM). Reproductive data drove the regression at lower PCB concentrations (≤1 μg/g), switching to mortality + growth at greater PCB tissue concentrations. The CLEM-predicted effects were, as expected, greater than any single effect (mortality, growth, or reproduction; Figure 13B). For example, at 1 μg/g PCB concentration, the CLEM would predict 43% cumulative effect compared with 17, 15, and 39% for mortality, growth, and reproduction, respectively (Figure 13B and Supplemental Data, Table CLEM).
Use of field PCB studies to corroborate laboratory-derived PCB effects and regressions
Twenty-four PCB field studies met the inclusion criteria and contained an even distribution of mortality, growth, and reproduction endpoints (Table 10). Egg hatchability was a common endpoint in studies in which wild-exposed fish were brought into the laboratory to evaluate fertilization and hatching relative to maternal PCB exposure. This provided a direct comparison with egg mortality in laboratory studies. There was overlap in the average responses compared with equivalent laboratory results (Figure 14). Furthermore, similar amounts of variation in concentration and effect were observed among both field and laboratory LOAER data.
| Specific PCB or location | Common name | Scientific name | Life stage | Temp. | Saline | MGR | PCB tissue concn. μg/g | % Change | Endpoint | References |
|---|---|---|---|---|---|---|---|---|---|---|
| New Bedford, MA, USA: | Mummichog | Fundulus heteroclitus | Adult | Warm | Salt | M | 5.2 | 23 | ↑ Mortality | Black et al. 1998b |
| Measured PCBs: 118, 105, 167, 156, 157, 189, 77, 126, 169 | Adult | Warm | Salt | G | 2.37 | 15 | ↓ Growth | |||
| Larval | Warm | Salt | M/R | 7.3 | 30 | ↓ Larval survival | ||||
| Larval | Warm | Salt | M | 7.3 | 20 | ↑ Larval spine abnormalities | ||||
| New Bedford, MA, USA | Winter flounder | Pseudopleuronectes americanus | Larval | Warm | Salt | G | 9.9 | 18/6 | ↓ Weight/length at hatch | Black et al. 1988 |
| R | 3.9 | 18.9 | ↓ %Fertilization | |||||||
| Baltic Sea | Baltic flounder | ELS | Cold | Salt | M | 0.21 | 66 | ↓ Hatchability | Von Westernhagen et al. 1981 | |
| Baltic Sea: A1260b | Baltic herring | Clupea harengus | ELS | Cold | Salt | M | 0.19 | 38 | ↓ Hatchability | Hansen et al. 1985 |
| A1242 | Rainbow trout | Oncorhynchus mykiss | ELS | Cold | Fresh | M | 2.7 | 68 | ↓ Hatchability | Hogan and Brauhn 1975 |
| San Francisco Bay, CA, USA | Starry flounder | Platichthys stellatus | ELS | Cold | Salt | M | 2.8 | 38 | ↓ Hatchability | Spies and Rice 1988 |
| Newfoundland, CA, USA | Shorthorn sculpin | Myoxocephalus scorpius | Adult | Cold | Salt | G | * | 14 | ↑ Lesion | Khan 2011 |
| Adult | Cold | Salt | O | * | 100 | ↓ Growth | *no direct concentration available | |||
| Newfoundland, CA, USA | Winter flounder | Adult | Cold | Salt | G | 50 | 50 | ↓ Growth | Khan 1999 | |
| Housatonic River, MA, USA | Largemouth bass | Micropterus salmoides | ELS | Warm | Fresh | M | 33 | 41 | ↑ Mortality | Tillitt et al. 2001 |
| G | 36 | ↓ Growth | ||||||||
| Lake Geneva, FR | Artic charr | Salvelinus alpinus | ELS | Cold | Fresh | M | 0.32 | 42 | ↓ Hatchability | Monod 1985 |
| Adult | Cold | Fresh | R | 0.32 | 14 | ↓ Fertility | ||||
| Puget Sound, WA, USA | English sole | Parophrys vetulus | ELS | Cold | Salt | M | 2.56 | 28 | ↓ Larval survival | Casillas et al. 1991 |
| Adult | R | 3.47 | 21 | ↓ %Spawners | ||||||
| Puget Sound, WA, USA | English sole | Parophrys vetulus | Adult | Cold | Salt | R | 10 | 30 | ↑Fecundity, | Johnson et al. 1997 |
| ELS | R | 10 | 27 | ↓ Egg weight | ||||||
| Clear Creek, IN, USA | Creek chub | Semotilus atromaculatus | Adult | Warm | Fresh | G | 19.2 | 21 | ↓ Growth | Henshel et al. 2006 |
| Oak Ridge, TN, USA: PCB and mercury | Redbreast sunfish | Lepomis auritus | Adult | Warm | Fresh | R | 1 | 45 | ↓ Fecundity | Adams et al. 1989, 1990, 1992 |
| Adult | Warm | Fresh | G | 1 | 32 | ↓ Growth | ||||
| Mersey Estuary UK: ICES7 | European flounder | Platichthys flesus | Adult | Cold | Salt | G | 0.416 | 11 | ↓ Growth | Kleinkauf et al. 2004 |
| Great Lakes, MI, USA | Lake trout | Salvelinus namaycush | ELS | Cold | Fresh | M/ | 2.4 | 23 | ↓ Hatchability | Mac and Schwartz 1992 |
- a Specific information about each study and selection of values is presented in the Supplemental Data, Table S10 and the Table Field Info.
- b 43 CFR Part 11.62; US Department of the Interior 2009.
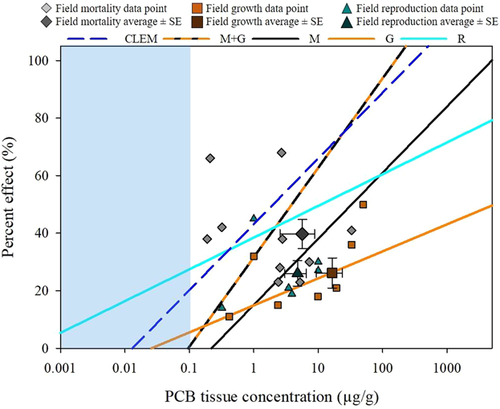
Overall, PCB-induced mortality in fish from field studies was in line with laboratory-generated mortality data. The average tissue concentration/percentage effect value for field responses fell very near the regression line generated from PCB laboratory mortality quintiles (Figure 14). The majority of field studies showed more severe effects than would be predicted by the laboratory-generated regression line. This finding suggested that the laboratory-generated regression line provided a conservative estimate of PCB-induced mortality (i.e., less than observed in field investigations). The one study with a response substantially below the regression line (Seelye and Mac 1981) was one of a number of lake trout studies (Mac and Schwartz 1992; Mac et al. 1993). When these field lake trout studies were taken together, the average datapoint for lake trout (6.35 μg/g and 25% mortality) fell directly on the regression line. It should also be noted that the range of tissue concentrations from field studies was much narrower and generally lower than those observations in laboratory-based studies with fish. This was likely a functional limitation of field studies, in which the organisms collected only represent survivors. In addition, the migratory nature of fish enhances uncertainty of site fidelity and hence decreases the certainty that a continuous exposure occurred.
Growth effects observed in field studies were very similar in magnitude to those observed in laboratory studies at similar measured PCB exposures (Figure 14). The field growth responses were at or near the quintile-derived PCB concentration–growth effect regression. The average value for all fish-PCB field growth studies (9.2 μg/g and 25% growth impairment) fell slightly above that regression line based on laboratory PCB exposure studies (Figures 8 and 14). As with mortality, this finding suggested that the laboratory-derived regression was a conservative approach to assessing growth effects in fish exposed to PCBs under environmental conditions. One caveat with studies from the field would be that growth, even more so than mortality, could be substantially influenced by abiotic and biotic environmental conditions that may not be readily assessed by the routine measurements made in the field ecotoxicological investigations. Thus, if confounding effects of contaminant-induced responses under field conditions were to occur, it would be highly unlikely they would be discovered through routine field investigations. Moreover, any confounding effects would generally tend to indicate increased sensitivity relative to the same exposures in the laboratory.
Unlike mortality and growth studies, the effects on reproduction in adult fish from the field were generally less severe than those observed in the laboratory, based on the 5 field studies evaluated (Table 10 and Figure 14). That is, the reproductive effects associated with PCB exposure measured in field studies were less than those predicted by laboratory-based studies with adult fish. Laboratory reproductive studies focus on fecundity via egg production. Of the 4 field studies below the regression line, 2 measured fertilization rates, 1 counted the number of spawning fish, and 1 evaluated egg weight. Environmental factors confound reproductive effects, influencing the accuracy of the measurements in the field. In addition, reproductive measures in fish can be challenging to assay in fish collected in the field, which may also have affected the measurements reported in Table 10.
Many of the PCB-induced physiological, molecular, and cellular responses observed in laboratory studies with fish were also observed in field-based investigations of PCBs. Induction of ethoxyresorufin-O-deethylase (EROD) activity, decreases in vitellogenin, and changes in liver or gonadal somatic indices were all observed in both laboratory and the field studies with fish exposed to PCBs. Although some of these effects were considered adverse, they can be initiated by many different contaminants, not just exposure to PCBs. In addition, the influence of environmental conditions on some of these suborganismal responses leads to highly variable responses of these endpoints. Field studies with the common barbel observed EROD/7-ethoxycoumarin-O-deethylase and cytochrome P450 induction and adverse changes in liver cell histology (Hugla et al. 1995). A follow-up laboratory study with the same species and PCB mixture was able to replicate these same responses, as well as adverse effects in reproduction at the same tissue concentration (Hugla and Thome 1999). The same approach was used for the mummichog, and field and laboratory studies showed similar responses to PCB exposures (Black et al. 1998a, 1998b; Gutjahr-Gobell et al. 1999). Even among these studies, the effects that occur at the physiological, cellular, and molecular level were difficult to scale to organismal level effects. Suborganismal biomarkers of PCB-induced responses in fish are important for development of lines of evidence for causation within risk assessment and injury determination paradigms, as part of an ecoepidemiological framework for determination of causality (Fox 1991) or adverse outcome pathway.
DISCUSSION
The available literature on PCB-induced injury in fish populations provided sufficient data for a successful meta-analysis using regressions of PCB-related LOAERs to deliver a unique, data-driven approach to injury assessment. The use of LOAER data, connected to definitive adverse effects, allowed quantitative estimates of PCB tissue concentrations related to MGR injury to fish populations.
The MGR sensitivity distributions (Figure 3 and Tables 5 and 6) provided a means to screen for injury within fish populations and communities. These sensitivity distributions can also provide an initial context to field-derived tissue concentrations. For example, a PCB tissue concentration of 1 μg/g was related to 7% of species within that community experiencing significant mortality, and another 11 and 27% could be expected to exhibit reductions in growth and reproduction, respectively. Alternatively, if an effects threshold was set for a fish population, for example 25%, then adverse MGR effects would be expected (according to this criteria) when PCB tissue concentrations were 7.5, 3.9, and 0.8 μg/g, respectively. The actual concentration or effects threshold that would lead to a management action or other regulatory activity would require more site-specific information, based on the needs of regulators and stakeholders, the presence of critical, sensitive, or endangered species, and site-specific exposure analysis.
Application of the individual concentration–effect responses provided the critical connection between tissue concentrations of PCBs and the degree of effect observed in fish. These relationships allow estimation of the specific degree of effect associated with a tissue concentration. As an example, the regressions predict that in a fish population with PCB tissue concentrations of 10 μg/g, 40% of the individuals are likely to have died, and within the remaining individuals, growth would be reduced by 24% and reproduction reduced by 50%. The regression approach also allows simple calculations of estimated injury in fish populations to be developed, based on specific needs of an assessment and site-specific information.
The concentration–effect regressions provided the ability to identify MGR effects across a range of exposures. However, in practical application, ecological risk assessments often develop thresholds based on site-specific criteria, such as the threshold of toxicological concern (TTC; Kroes et al. 2004; Belanger et al. 2015). A TTC is established using a risk value, such as the 5th percentile of the cumulative distribution (de Wolf et al. 2005; Gross et al. 2010; Williams et al. 2011; Mons et al. 2013; Hendriks et al. 2013; Gutsell et al. 2015). In this example, the 5th percentile cumulative distribution for these data would be the point where 5% of species are expected to have mortality, growth, or reproduction effects: at 0.6, 0.4, and 0.02 μg/g PCB tissue concentrations (respectively for mortality, growth, and reproduction; Tables 5 and 6). The corresponding levels of effects anticipated for mortality, growth, and reproduction are 11.8, 11.3, and 19.0%, respectively. Similarly, the 10th percentile TTC corresponds to tissue exposure concentrations of 1.6, 0.9, and 0.08 μg/g with anticipated effects of 21.1, 14.6, and 26.4% for mortality, growth, and reproduction, respectively. This type of approach can be used to estimate MGR effects or exposure based on risk management criteria for protection of fish populations.
The CLEM presented a general, conservative example of how to combine effects, although it was not suggested as a definitive approach to cumulative effects assessment. As with any toxicological model attempting to summarize multiple effects occurring simultaneously, this cumulative effects model approach should be used with caution. Reproductive responses drive the cumulative effects observed at lower concentrations in CLEM. Reproduction is the smallest, and generally the weakest, of the MGR dataset. It is populated with high-quality data, but the limited number of available studies necessarily makes the model less robust than the mortality dataset.
The approach to using these data and tools will depend on the objectives and requirements of the end user. We presented data in several different ways to address varying needs of injury assessment practitioners or other natural resource managers responsible for estimation of effects of PCB exposure to fish populations. The LOAER-based sensitivity distributions for MGR may be used individually. For example, reductions in growth could impact the overwintering success of salmon populations (Quinn and Peterson 1996). The PCB- LOAER growth sensitivity distribution could connect field-measured PCB concentrations in salmon to the likelihood of significant effect, and then to a detailed life history (e.g., overwintering success) to generate the associated injury. Uncertainty regressions can be used to determine the bounds of the estimates. The upper bound of the uncertainty regressions could be used to provide a measure of confidence for the protection of species of special concern, like a threatened or endangered species, a species suspected of being particularly sensitive, or an ecologically or economically important species.
Although it was based on the best available data, the meta-analysis we conducted had inherent uncertainties built in to the calculations and LOAER estimates, based on limitations of the available data. Potency differences among the PCB mixtures were perhaps the greatest concern related to the uncertainty incorporated into the meta-analysis. Different PCB congeners and technical mixtures are without doubt more potent than others, particularly those with higher percentages of the dioxin-like PCBs (77, 81, 126, and 169; Safe 1994; Giesy and Kannan 1998; Van den Berg et al. 1998; Kannan et al. 2000; Burkhard and Lukasewycz 2008); nonetheless, the LOAER regressions using total PCB concentrations were robust enough across PCB mixtures to be useful with calculated degrees of uncertainty. The potential for various fish species to exhibit differential sensitivities also added uncertainty. Fish exhibit differing sensitivities to dioxins (Elonen et al. 1998; Tillitt et al. 2017), and differences among species in sensitivity to PCBs were evident in the data we compiled. Although the data in our analysis were derived from studies utilizing 21 species of fish, this represents only a small fraction of the diversity of fish species. There was also uncertainty surrounding the tests themselves, particularly the data from growth studies. due to the confounding effects of PCB-induced mortality that occurred simultaneously as growth effects. Another uncertainty comes from the changes in analytical detection methods and chemical quantification of PCBs that have occurred over the 5-decade time span represented within the dataset. During that time, significant improvements in analytical methods for the detection of PCBs occurred (Subedi and Usenko 2012). A more subtle difference among the data reported from different studies may have been in the degree or extent of statistical analysis, with more recent studies tending toward more statistically driven analysis. Lastly, the datasets used to generate the threshold response regressions were relatively small, particularly for growth (n = 17) and reproduction (n = 8). Small datasets are not ideal; however, the size of the dataset does not invalidate the analysis or the approach taken. Similarly, small datasets are considered valid when regulatory assessments are developed for Registration, Evaluation, Authorisation, and Restriction (REACH) in Europe (European Chemicals Agency 2008), water quality criteria in the United States (Stephan et al. 1985), and similar assessments in other countries (Belanger et al. 2017). Although it is critical to acknowledge these uncertainties, it is also important to understand that the data used to generate our meta-analysis were screened to meet select criteria, so as to include only the best available information.
A major advantage of this approach was the degree of transparency afforded in the data selection and analysis. Selection criteria provided a clear path to sorting through the fish PCB toxicity data appropriate to support fish injury assessments. The regressions were purposely kept as linear regressions, with defined equations (Equations 8 and 9) used to generate estimates of effect or exposure concentration. Exposure or effects thresholds can be quickly generated based on specific criteria for a fish injury assessment. The approach also allows for updating and is fully adaptable. As additional MGR data become available, they can easily be incorporated into the existing dataset.
The analyses based on the population-level MGR effects provide an anchoring point for relating tissue concentrations to percentage effects. Other effects endpoints, at the physiological, cellular, biochemical, and genetic levels of organization, can be connected and scaled to these MGR anchor points, following the adverse outcome pathway approach (Ankley et al. 2010; Hutchinson et al. 2013). As mechanistic models of toxicity of PCBs are further developed and key events become identified, the same approach, from data vetting to concentration–response regression, could also be applied. This same meta-analysis approach could also be adapted to other pollutants or pollutant classes (e.g., metals, oil, other legacy pollutants). A similar structural framework could be followed: gather data—develop criteria—vet the data—develop effect-specific regression—identify/analyze testing parameters—establish data-driven thresholds and uncertainty factors.
The approaches we describe focused on injury assessment in fish populations exposed to PCBs. Retrospective assessments, related to the discharge of PCBs into an environment, often require an evaluation of the literature on concentration—adverse effect relationships to help define an injury assessment. Quality data on lethal and sublethal effects, linked to concentrations, provide practitioners with the tools to assess the injuries that may have already occurred within fish populations. This was the purpose of selecting LOAER concentration–effect values from all the existing literature on PCB-induced responses in fish. At the LOAER exposure concentrations in each of the studies examined, a significant adverse effect occurred on mortality, growth, or reproduction. The purpose of the present meta-analysis was the development of an approach to facilitate injury assessments; however, the meta-analysis could also aid in the development of risk assessments. The inclusion criteria provided transparency and the scientific justification behind the data review and selection process. The use of concentration–response regressions provided a data-driven means to estimate PCB effects across the broadest range of concentrations. The approaches we have outlined create a framework for regulators and scientists to utilize the entire array of available data for the purposes of environmental injury assessments of PCB-induced effects on fish populations.
CONCLUSIONS
- This meta-analysis began with clear, transparent data selection criteria to assure use of the best available data.
- The analysis used LOAER to connect PCB exposure concentrations to definitive adverse effects on MGR.
- Sufficient high-quality data was available for this meta-analysis; 55 LOAER datapoints (M 30, G 17, R 8) across 31 studies that met criteria for inclusion.
- An applicability limit of 0.1 µg/g total PCB tissue concentration was established, below which MGR effects were not supported by the available data.
- Sensitivity distributions identified the probability of adverse effects in a population or community, for example, at 7.5 µg/g PCB in tissues, 25% of fish species would be impacted.
- Regressions connected PCB concentrations with the degree of adverse effect expected, for example 1 µg/g PCB in tissues would result in 17% increase in mortality, 15% inhibition of growth, and 39% inhibition of reproduction.
- LOAER regressions using the exposure metric of total PCB concentrations were sufficiently robust across different PCB mixtures to be useful for injury assessment of MGR in fish populations.
- This approach provides a framework to utilize the entire array of available data to assess PCB-induced environmental injury.
The present meta-analysis provides a tool that can be directly applied to injury assessment of fish populations exposed to PCBs. Application of the LOAER regression toxicity threshold values will be dependent on the specific objectives of different natural resource managers; however, the analysis and approach provide an important step forward for evaluation of PCB-related effects on fish populations across a range of environmental exposures.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.4335.
Acknowledgment
Funding for the present study came from the US Department of Interior, Office of Restoration and Damage Assessment (ORDA), and the Contaminants Biology Program, Environmental Health Mission Area, US Geological Survey. The authors thank J. Towns-Campbell for assistance with literature searches; S. Finger and J.E. Hinck for coordination and inspiration; and the ORDA office for patience during development and review of the document. Early drafts received thoughtful reviews and helpful comments from J. Meador, D. Nacci, A. Horner-Hanley, K. Skrabis, J. Isanhart, C. Schmitt, J. Gooch, T. Brosnan, K. Finkelstein, A. Merten, M. Greenberg, M. Sprenger, and L. Williams. We thank these reviewers and anonymous reviews from the journal for their time and expertise. The final manuscript greatly benefitted from their efforts.
Disclaimer
Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government.
Data Accessibility
Data, associated metadata, and calculation tools not in the Supplemental Data are available from the corresponding author ([email protected]).




