Application of sediment toxicity identification evaluation techniques to a site with multiple contaminants
Abstract
Sediment toxicity identification evaluations (TIEs) are conducted to determine causes of adverse effects observed in whole-sediment toxicity tests. However, in multiple contaminant scenarios, it is problematic to partition contributions of individual contaminants to overall toxicity. Using data from a site with multiple inputs and contaminants of concern, the authors describe a quantitative approach for the TIE process by tracking toxicity units to determine whether all toxicity is accounted for. The initial step established the level of toxicity associated with the whole sediment and then partitioned sources of toxicity into general contaminant classes (e.g., ammonia, metals, nonpolar organic compounds). In this case, toxicity was largely the result of nonpolar organics, so the sediments were extracted and the extracts added back into dilution water and tested to confirm recovery of toxicity. Individual fractions were then generated using a solvent gradient and tested for toxicity. Fractions of interest were evaluated with gas chromatography/mass spectrometry to identify specific constituents associated with toxicity. Toxicity units associated with these constituents were then evaluated to determine probable associations with cause and whether all toxicity was accounted for. The data indicated that toxicity was associated with 2 contaminant classes, representing legacy compounds and contaminants of emerging concern, with the contribution of each varying across the site. Environ Toxicol Chem 2016;35:2456–2465. © 2016 SETAC
INTRODUCTION
The process of identifying causes of toxicity in environmental samples is generally referred to as a toxicity identification evaluation (TIE). Procedures for conducting TIEs on water samples are well documented and widely applied; see, for example, the now classic US Environmental Protection Agency (USEPA) guidance documents 1-3 as well as contributions by many others 4-8. Conversely, while comprehensive guidance documents for performing TIEs on sediments have been published 9, 10, the techniques have not been as widely applied or refined as with water samples, at least in part because of the intrinsic difficulties associated with working with sediments. For example, a number of investigators have noted the relative complexity of determining the causes of toxicity in sediment samples 11, 12. These include the presence of naturally occurring nonpersistent toxicants (e.g., ammonia, sulfide), different matrix effects (e.g., varying levels of organic matter, acid-volatile sulfide), a wide range of potential contaminants (e.g., metals, organics), and high potential for multiple toxicants. These issues are further complicated by the fact that toxicants may be associated with the solid or aqueous phase or both, which presents additional difficulties in terms of quantifying toxicity and analytical validation.
In addition to toxicant identification, practical applications of sediment TIEs include separation of naturally occurring toxicants (e.g., ammonia, hydrogen sulfide) from anthropogenic contaminants, development of appropriate remediation strategies and targets for specific contaminants, and identifying toxicant sources and responsible parties. However, in multiple contaminant scenarios, it is often problematic to partition contributions of individual contaminants to overall toxicity 13-15. Moreover, studies that are intended to support regulatory action demand a high level of rigor to ensure that the conclusions are based on appropriate data, and developing procedures for quantitative identification of causes of toxicity in sediments has been identified as a high priority by water quality managers 16.
To address this issue, investigators have developed a number of techniques aimed at further improving the usefulness of sediment TIEs. Extraction of sediments with different resins, supercritical fluids, and various membrane devices has been used in an effort to recover biologically available toxicants 10, 13, 17-20. Biochemical reagents (i.e., piperonyl butoxide, carboxyl esterase) have also been used to identify toxicity related to specific pesticides in sediments 21, 22. Genomics may also have promise for detecting responses associated with specific toxicants 23; but the science is still in its infancy, and genomics may have more relevance as a marker than a quantitative measure of toxicity. Despite these advances, as recently as 2013, Burgess et al. 24 compared the pursuit of environmental toxicants to the legendary exploits of the Victorian detective Sherlock Holmes but also noted that additional refinements to phase II identification methods were needed, particularly with respect to the recovery or extraction of toxicants from sediments consistent with their actual bioavailability, as well as separation of toxicants that are present in a manner consistent with identifying their individual contributions to toxicity.
In the present study, sediment samples were collected from a site characterized by significant historical contamination, as well as current discharges from stormwater and combined storm sewer overflows. Samples were collected from different locations across the site and tested for toxicity with the amphipod Leptocheirus plumulosus to identify the spatial distribution of toxicity; samples that exhibited toxicity were further investigated to identify the cause(s). The primary objective was to obtain quantifiable results suitable for delineating different causes of toxicity, rather than simply characterizing general contaminant classes associated with toxicity.
MATERIALS AND METHODS
Sample collection, transport, and storage
Samples were collected with a stainless steel Van Veen grab sampler. Following each grab, the top 2.5-cm layer of sediment was carefully removed and transferred into a food-grade polyethylene bag contained within a plastic bucket. Once sufficient volume was obtained, any remaining headspace was removed, the bag was sealed, and the bucket was placed in an insulated cooler, covered with ice, and shipped overnight to Nautilus Environmental for testing. On arrival at the laboratory, buckets were transferred to a 4 °C sample storage room, where there were held in the dark until testing.
Toxicity testing
Test organisms
Leptocheirus plumulosus was selected as the test species based on its comparatively high tolerance for ammonia and a wide range of sediment grain sizes 25-28; this minimized the need for purging samples prior to testing and facilitated focus on other sources of toxicity. Test organisms (2–4 mm) were obtained from Chesapeake Cultures and acclimated to laboratory conditions for a minimum of 3 d prior to testing. During this period, they were held at 25 °C in clean sediment obtained from the Sail Bay reference site (Mission Bay, San Diego, CA, USA), which is comprised of 85.7% fines. During acclimation organisms were fed a Tetramin slurry daily and held at a 16:8-h light: dark photoperiod.
Baseline toxicity tests
All sediment samples were initially screened for toxicity using the 10-d amphipod survival test, according to procedures described by the USEPA 25 and the Puget Sound Estuary Program 29. The tests were initiated within 22 d of sample collection. Seawater used in the tests was obtained from Scripps Institution of Oceanography and filtered (20 μm) and diluted to 20 ppt salinity with Nanopure deionized water prior to use. The control sediment (Sail Bay) was sieved (0.5 mm Nitex) and rinsed with seawater prior to use.
All test sediments were homogenized but not sieved prior to testing. Porewater was extracted by centrifugation and analyzed for ammonia, sulfide, pH, and salinity. Five replicates, each containing 20 organisms, were used for evaluation of each sample and the control. Tests were conducted in 1-L glass jars using 175 mL of sediment and approximately 750 mL of overlying seawater. Sediment and seawater were added to the test containers and allowed to equilibrate for 24 h prior to adding the test organisms. Tests were conducted at 25 ± 1 °C under continuous lighting at a salinity of 20 ppt. The overlying water was not changed and the organisms were not fed during the exposure. Gentle aeration of the overlying water was provided throughout the exposure period, and water quality parameters (dissolved oxygen [DO], pH, salinity, and temperature) were measured daily. Ammonia and sulfide were also measured in the overlying water at test initiation and termination; note that ammonia and sulfide were measured according to USEPA Methods 350.2 and 376.2, respectively, using Hach reagents.
After 10 d of exposure, the contents of each replicate were gently sieved, and the number of live organisms was determined and recorded. Numbers of surviving amphipods were then compared statistically using Biostat (Army Corp of Engineers 2006), to identify samples that exhibited a significant decrease in survival relative to the control (t test, p ≤ 0.05). The criterion for acceptable test performance was ≥90% survival of the control organisms. Reference toxicant tests were also conducted with each batch of L. plumulosus, using cadmium chloride as the toxicant, and the results compared with previous test data for this species to determine whether they were similar to historical values. All controls met the test acceptability requirements, and reference toxicant 50% lethal concentrations (LC50s) were within 2 standard deviations of the historical mean, suggesting that the test organisms were of similar sensitivity 25.
Toxicity identification evaluation
Sediment-dilution tests
To evaluate the degree of removal and recovery of toxicity associated with different TIE treatments, it was necessary to quantify the level of baseline toxicity associated with each of the samples. These tests were conducted in 300-mL glass beakers containing 200 mL overlying water and 50 mL of sediment. Each sample was tested at 25%, 50%, and 100%, with dilutions prepared by adding the appropriate amount of control sediment. Exposures were conducted at 25 ± 1 °C under constant illumination, and aeration was provided via microtubing at 100 ± 10 bubbles/min. Three replicates were used per treatment, each containing 10 organisms. Two replicates of 100% sample were terminated at 96 h to determine if the results were comparable to the original 10-d test. If so, all of the remaining replicates and dilutions were terminated. If not, the remaining replicates and treatments were continued for an additional 48 h to produce an unambiguous response. Evaporative losses in the test containers were replaced with deionized water. Control acceptability was ≥90% survival, and organisms were not fed during the test. Following exposure, sediments were sieved to recover the test organisms and LC50 values estimated by interpolation based on the pattern of survival across dilutions. Toxicity units were calculated based on full-strength sediment (i.e., 100%) divided by the LC50 (percentage).
Characterization of toxicity in the solid phase (phase I)
Phase I treatments were conducted concurrently with the untreated baseline sediment-dilution tests and consisted of 2 solid-phase sorbents (i.e., Amberlite XAD4 and SIR-300). Amberlite selectively binds nonpolar organic materials, and SIR-300 binds metals 10, 13; therefore, reduction of toxicity by either of these materials provides an indication of the presence of a specific class of contaminant that contributes to toxicity. Each of these reagents was activated prior to use according to USEPA 10 and manufacturer guidance and mixed with the sediment at 40% by volume (i.e., 20 mL of sorbent added to 50 mL sediment). Each of the solid-phase treatments was added independently to dilutions of the test sediment (i.e., 100%, 50%, and 25%) prepared as described previously. Note that the use of sediment dilutions and relatively high volumes of solid-phase sorbents was necessary because of the relatively high level of toxicity associated with some of the samples. All sediment dilutions and treatments were prepared the day prior to addition of the test organisms and allowed to equilibrate overnight. The phase I treatments were terminated after 96 h or 144 h, depending on the mortality observed in 100% untreated sample at 96 h in the sediment-dilution tests. Following test termination, LC50 values for the treated samples were estimated based on the pattern of survival across replicates and toxicity units calculated and compared with toxicity units for the untreated sample to evaluate effectiveness of the treatments.
Potential toxicity associated with ammonia and/or sulfides was identified by direct analysis and further investigated by purging the sample prior to initiating exposure. Purging was accomplished by exchanging the overlying water with fresh 20 ppt seawater twice daily until ammonia was within the desired range. This treatment was performed only on the sample that exhibited the highest concentration of ammonia and did not affect overall toxicity.
Water quality (DO, pH, salinity) in the sediment dilutions and TIE treatments was measured in 1 replicate at 0 h and 96 h and again at 144 h if the test was extended. Temperature was measured daily and aeration checked twice daily.
Toxicant identification (phase II)
Because the characterization phase largely indicated that metals and ammonia were not significant contributors to toxicity across the sites, this phase focused on identifying the organic constituents that contributed to toxicity using direct methanol extraction of the sediments to recover organic constituents. Note that the use of water-accommodated fractions and porewater was also evaluated but not found to be as consistent in terms of quantitative recovery of toxicity. The methanol fraction was dissolved back into water and organic constituents captured on C8 solid-phase extraction (SPE) columns. Extracts from the columns were then added back into water and tested for toxicity over a series of dilutions, and the level of toxicity associated with the add-backs was compared with the level of toxicity exhibited in the sediment-dilution tests to confirm the contribution of the organic fraction to the level of toxicity observed in the whole sediment. Additional details of the underlying methodology are presented in the following paragraphs.
For methanol extraction of the sediment, a subsample of 600 mL of sediment from each site was thoroughly homogenized and excess water removed by centrifugation at 4000 rpm for 30 min. The sediment was then mixed overnight on a shaker table at 200 rpm with 240 mL of methanol in a 1-L glass jar capped with a Teflon-lined lid. The following morning, sediments were again centrifuged (3000 rpm for 15 min), the resulting supernatant was siphoned off and placed in a 400-mL glass beaker at 25 °C, and the methanol volume was reduced to 50 mL by evaporation using pressurized air flow directed at the supernatant surface. The evaporation process was generally completed within 72 h.
The 50 mL of concentrated methanol supernatant was then injected into 2.4 L of 20 ppt natural seawater using a Teflon-lined glass syringe and the solution mixed on a magnetic stir plate for 15 min. Four separate 600-mL aliquots of this solution were pumped through separate C8 solid-phase extraction columns at 3 mL/min. Prior to passing samples through the columns, each of the columns was rinsed with 200 mL of 20 ppt seawater; the rinse volume from 1 column/d of sample processing was saved for later testing as a column control. Following the extractions, loaded columns were labeled and frozen for storage. Given that each column nominally contained the nonpolar organics associated with 150 mL of sediment dissolved in 600 mL of seawater, each of the 4 columns contained the nominal equivalent of aqueous equilibrium concentrations in the overlying water in the original 10-d whole-sediment baseline toxicity tests.
To confirm the recovery of toxicity from C8 columns, a 2-mL aliquot of methanol was pumped through 1 of the 4 columns from each sample in discrete 1-mL volumes at the rate of 2 mL/min. The total 2 mL of methanol was then injected into 200 mL of 20 ppt natural seawater and mixed for 15 min to create a 3× solution (compared with the baseline sediment volume: 600 mL on column [nominally equivalent to 150 mL sediment] divided by 200 mL test volume = 3×). Serial dilutions were then prepared to achieve test concentrations of 3×, 1.5×, 0.75×, 0.38×, and 0.19×, using 20 ppt seawater as the dilution water. Each dilution was tested in 2 50-mL replicate volumes in 100-mL beakers with 5 test organisms (L. plumulosus) per replicate; recovery of toxicity in the add-backs indicated that organic toxicants were present. Water quality measurements (i.e., DO, pH, temperature, salinity) were taken on the dilution water at the beginning of the tests and in the test solutions at the end of the exposures (96 h) or when all of the test organisms died. Seawater and methanol controls (1%) were also tested concurrently.
If toxicity was observed in the add-backs, the toxic component(s) was further isolated using a solvent gradient. Eight dilutions of methanol (i.e., 50%, 70%, 75%, 80%, 85%, 90%, 95%, and 100%) were prepared by mixing deionized water with methanol. Aliquots of each of these solutions (2 mL) were then sequentially passed through 2 of the 3 remaining C8 columns archived from each sample at 2 mL/min; to confirm that all potential contaminants were recovered from the column, 2 additional 100% methanol aliquots were also passed through each column. The corresponding fractions from the 2 columns were then pooled for a total volume of 4 mL/fraction. Two milliliters of each fraction was then mixed into 200 mL of 20 ppt natural seawater to again create nominal 3× solutions (compared with the initial 600-mL volume placed on column); the remaining 2 mL of each fraction was placed in a Teflon-capped amber vial and retained for analysis of unknowns.
Each fraction was tested in 2 100-mL replicates with 5 amphipods per replicate. Otherwise, test procedures (e.g., water quality measurements) were the same as described above for the add-backs tests.
Identification and confirmation of unknowns (phase III)
Results from the toxicity tests conducted on the methanol:water fractions identified specific fractions that were associated with toxicity. To further identify constituents that contributed to toxicity, fractions that exhibited toxicity as well as adjacent nontoxic fractions were submitted to Physis Environmental Laboratories for analysis of unknowns. Each fraction was blown to near dryness with a gentle stream of nitrogen, and then approximately 50 μL of dichloromethane was added to the vial. This extract was transferred to a 100-μL glass insert, and the vial was rinsed 2 more times with dichloromethane, with each of the rinsates also added to the insert. An internal standard solution containing d10-anthracene, dibromobiphenyl, and tetrabromobiphenyl was then added to the insert; and the sample was analyzed using gas chromatography/mass spectrometry (GC/MS). The GC/MS system was equipped with a 60 m, 0.25 mm inner diameter, 0.25 μm film thickness DB-5 capillary column and was temperature-programmed from 125 °C to 285 °C at 2.5 °C per minute. The MS was operated in the full-scan mode from 45 atomic mass units to 550 atomic mass units at 1.67 scans/s in the electron ionization mode. Quantitation for polycyclic aromatic hydrocarbons (PAHs) and congener polychlorinated biphenyls (PCBs) was based on a 5-point calibration curve using a commercial solution (Accustandard) traceable to National Institute of Standards Technology by the internal standard method. Quantitation of the PAH alkyl homologs was based on response factors for the parent PAH compounds, and quantitation for alkanes was based on a commercial n-alkane standard ranging from C10 to C36 (Accustandard).
Compounds of interest were identified by setting a minimum peak size and then comparing the spectrum for each peak with the NIST08 spectral library, which contains approximately 120 000 spectra. This resulted in a list of several hundred compounds for each fraction that was further reduced based on 1) presence of a previously identified compound or an internal standard that was added to the extract (e.g., d10-anthracene); 2) purity of the match, with 100 being a perfect match (a 50% match was generally the cutoff, but the results for poorer matches were retained for large peaks because they might provide an indication of the types of compounds they could be); and 3) peak intensity—thus, very small peaks with low match quality were deleted. General associations with toxicity were identified by comparing relative mass of potential toxicants (derived from the area under the curve of the chromatogram relative to the internal standard anthracene which was added at a known mass) in toxic and nontoxic fractions within and across samples. Candidate sources of toxicity were identified on the basis of an increase in toxicity associated with an increase in mass; conversely, constituents whose concentrations did not vary consistently with survival were eliminated from consideration.
Recovery of toxicity
Toxicity units were tracked to ensure that toxicity was not added or lost during the process as an artifact of the manipulations and that contributions of all sources of toxicity were identified. This procedure began with the solid-phase testing on diluted sediments wherein an LC50 (percentage of sample) could be determined and toxicity units for the whole sediment calculated as 100 ÷ LC50. Thus, an LC50 of 50% would be equivalent to 2 toxicity units. In cases where <50% mortality occurred in the full-strength sample but there was still an apparent effect on survival, it was assumed that 0.5 toxicity units were present.
Proceeding through the TIE process, the proportion of toxicity recovered in the organic fraction (i.e., the methanol add-back) was determined by calculating the LC50 for the add-back and comparing the associated toxicity units with the toxicity units determined for the whole-sediment dilutions (thus, assuming minimal losses, 1 toxicity unit would be equivalent to 50% mortality at a 1× add-back and an LC50 of 100% in whole sediment). Contributions of individual contaminants to toxicity were determined by calculating mass as a function of relative areas under the curves from the chromatograms for toxic and nontoxic fractions. The LC50s were then calculated based on the relationship between mass and mortality using linear regression (CETIS, V.1.8.7.16; Tidepool Scientific Software). Finally, based on different patterns of toxicity associated with methanol:water fractions from different samples, it was clear that multiple toxicants were present. Consequently, a regression “model” for toxicity based on additivity and toxicity units associated with different contaminants was developed and compared with total toxicity units present in the methanol extracts of the original sediment samples to evaluate how successfully the TIE process characterized the toxicity present (JMP, Ver 10; SAS Institute).
RESULTS
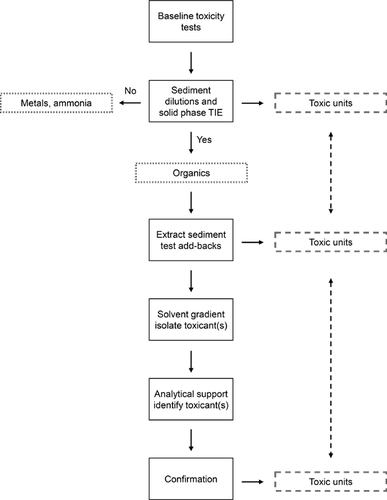
To aid the reader in following the progression of results, a general schematic of the TIE process is shown in Figure 1.
Baseline toxicity
Survival in the test sediments averaged between 0% and 90%, compared with an average control survival of ≥95%; survival was significantly reduced (p ≤ 0.05) in all 36 samples compared with their respective controls. One sample (3C01) exhibited DO concentrations of ≤0.1 mg/L in the overlying water, despite aeration during the test. To remove the effects of low DO on survival, this sample was also tested following multiple exchanges (2 times/d for 2 wk) of overlying water until DO reached 4 mg/L; average survival in the purged sample was 79% compared with 0% in the unpurged sample. With the exception of the 1 sample with extremely low DO, general water quality conditions were within ranges suitable for survival of the test organisms, specifically temperature (24.3–26.3 °C), DO (4.0–7.1 mg/L), pH (7.26–8.82), and salinity (19.0–22.1 ppt).
Total ammonia concentrations in overlying water ranged from <0.5 mg/L to 29.9 mg/L, corresponding to a range of 0.005 mg/L to 2.75 mg/L un-ionized ammonia, calculated on the basis of pH, temperature, and salinity the day the ammonia sample was collected 30. Concentrations of total ammonia in porewater were substantially greater than observed in the overlying water (i.e., 4 samples exceeded 50 mg/L). Sulfide concentrations in overlying water reached as high as 1400 µg/L at day 0 (mean day 0 sulfide for all samples was 97 µg/L) but typically declined to <5 µg/L by day 10. As observed with ammonia, concentrations of sulfide in porewater were higher than those found in the overlying water, with concentrations in 7 samples exceeding 1000 µg/L. In general, measured concentrations of total ammonia and sulfides did not exceed levels of concern identified in the literature 27, 28, and based on inspection of the data, levels of sulfides and total and un-ionized ammonia were generally not related to the levels of mortality observed in the samples.
Sediment dilutions and effect of solid-phase resin treatments
Because all of the samples exhibited statistically significant reductions in survival compared with the controls and the level of effort required to conduct extensive TIE manipulations on each sample would be prohibitive, a subset of 10 samples representing a range of responses and different locations across the site were selected for the initial follow-up investigation. Survival in sediment dilutions and solid-phase TIE treatments conducted on the 10 selected samples (batch 1) is shown in Table 1. Survival in the controls averaged 90%, whereas the samples exhibited varying levels of toxicity that ranged from only a small effect in undiluted sediment to 85% mortality in 25% sample. Notably, in all cases, the Amberlite XAD4 resin either eliminated or significantly reduced toxicity, implying that mortalities were largely caused by nonpolar organic toxicants and further suggesting that ammonia and/or sulfide were not major contributors to toxicity.
| Survival (%) | ||||
|---|---|---|---|---|
| Sample | Sediment concentration (%) | Untreated | Amberlite | SIR-300 |
| 3C01 | 100 | 0 | 95b | 20 |
| 50 | 75 | 90b | 90 | |
| 25 | 95 | 100 | 95 | |
| 3C10 | 100 | 35 | 90b | 30 |
| 50 | 65 | 100b | 60 | |
| 25 | 85 | 100 | 75 | |
| 3C11 | 100 | 0 | 25b | 0 |
| 50 | 25 | 70b | 30 | |
| 25 | 50 | 90b | 45 | |
| 3C12 | 100 | 50 | 100b | 40 |
| 50 | 75 | 95b | 80 | |
| 25 | 95 | 100 | 90 | |
| 3C13 | 100 | 5 | 95b | 95b |
| 50 | 90 | 100 | 95 | |
| 25 | 95 | 100 | 85 | |
| 3C15 | 100 | 0 | 5 | 0 |
| 50 | 5 | 75b | 0 | |
| 25 | 15 | 90b | 30 | |
| 3C16 | 100 | 80 | 95b | 80 |
| 50 | 90 | 100 | 85 | |
| 25 | 100 | 100 | 90 | |
| 3C25 | 100 | 15 | 80b | 60b |
| 50 | 60 | 95b | 75 | |
| 25 | 75 | 100b | 90 | |
| 3D01 | 100 | 45 | 95b | 55 |
| 50 | 80 | 95b | 90 | |
| 25 | 100 | 100 | 95 | |
| 3D05 | 100 | 66 | 100 | 75 |
| 50 | 90 | 100 | 85 | |
| 25 | 100 | 100 | 100 | |
- a Survival is the mean of 2 or 3 replicates.
- b Denotes treatments that reduced toxicity.
- SIR-300 = solid-phase sorbent.
The general lack of effectiveness of SIR-300 at removing toxicity indicated that metals were not responsible for a significant portion of toxicity across sites. Two exceptions were observed: the Amberlite and SIR-300 treatments appeared to be similarly effective at reducing toxicity for 3C13, possibly suggesting that both metals and organic constituents were present at toxic concentrations or that the effect was simply diluted by addition of the resins (the latter hypothesis is supported by the absence of toxicity in the 50% dilution of this sample). The SIR-300 treatment also appeared to remove a component of toxicity in sample 3C25, potentially suggesting that metals were contributing to toxicity; however, the Amberlite treatment removed all toxicity in this sample.
When originally evaluated for baseline toxicity, sample 3C01 required extended exchanges of overlying water to stabilize the dissolved oxygen. In this phase, care was taken to not include toilet paper and fecal material in the test sediment, and DO was maintained above 4 mg/L. Thus, toxicity was deemed representative of underlying sediment quality at this site, and removal of toxicity by Amberlite suggested that nonpolar organics were the primary cause of the observed mortalities.
Contribution of the nonpolar organic fraction to toxicity
Results for the methanol add-backs for the samples that were also tested with sediment dilutions (i.e., batch 1) are summarized in Table 2; measurable toxicity was recovered from all of the samples. To determine the extent to which the methanol extractions of the sediment could account for whole-sediment toxicity, the level of toxicity exhibited in the sediment-dilution tests (Table 1) is also shown in Table 2 to compare with the level of toxicity recovered in the methanol add-backs.
| Add-back concentration | Definitive sediment test | ||||||
|---|---|---|---|---|---|---|---|
| Sample | 0.2X | 0.4X | 0.75X | 1.5X | 3X | Toxicity units | Toxicity units |
| 3C01 | 90 | 30 | 0 | 0 | 0 | 3.5 | 1.4 |
| 3C10 | 100 | 90 | 60 | 0 | 0 | 1.3 | 1.8 |
| 3C11 | 50 | 20 | 0 | 0 | 0 | 5.0 | 4.0 |
| 3C12 | 100 | 70 | 10 | 0 | 0 | 1.8 | 1.0 |
| 3C13 | 30 | 0 | 0 | 0 | 0 | >5 | 1.4 |
| 3C15 | 100 | 90 | 20 | 0 | 0 | 1.8 | >4.0 |
| 3C16 | 100 | 100 | 100 | 40 | 0 | 0.7 | 0.5 |
| 3C25 | 100 | 50 | 0 | 0 | 0 | 2.5 | 2.0 |
| 3D01 | 100 | 100 | 30 | 0 | 0 | 1.8 | 1.0 |
| 3D05 | 90 | 70 | 100 | 70 | 50 | 0.3 | 0.5 |
- a Data show the mean of 2 replicates; control survival was ≥90%.
In general, agreement between the results from the sediment-dilution tests and the methanol add-backs was reasonably good; that is, toxicity units for most of the 10 samples were within a factor of 2, suggesting that toxicity was the result of organics and was recovered at least semiquantitatively by extracting the sediments with methanol. Notably, there was only 1 sample for which the toxicity units differed by at least a factor of 3 (3C13). This sample exhibited substantially more toxicity in the methanol add-backs compared with the whole-sediment test, implying that there was more potential toxicity present than “bioavailable” in the solid phase. Closer inspection of this sample revealed that there was a layer of either tar or viscous material associated with the larger substrate particles, for example, gravels, which would be extractable with a solvent but not in direct association with the test organisms, which were typically associated with the finer particulates. Equilibrium could also play a role with respect to bioavailability if excess material was present. Regardless, these results suggested that material was available to the extraction process but not to the organisms under the actual exposure conditions.
Interestingly, 3C15 exhibited appreciably less toxicity in the methanol add-backs than observed in the whole-sediment exposures. However, the fact that Amberlite removed >75% of the toxicity indicated that toxicity was largely caused by nonpolar organics. Thus, low recovery in the methanol add-back suggests poor recovery from the C8 column and/or inefficient resolubilization of the extract back into water.
Regardless of these discrepancies, it is noteworthy that the samples exhibited evidence of toxicity primarily caused by organic contaminants when tested as whole sediments and that toxicity was recovered in methanol extracts from all of the sediments. Thus, this approach facilitated additional steps to further identify the cause(s) of toxicity by focusing on methanol fractions. Another notable observation was the potential for recovering toxicity in cases where toxicity had dissipated in the solid phase during storage. For example, sample 3C16 exhibited toxicity when initially tested, but toxicity was largely absent when subsequently tested with whole-sediment dilutions. However, we were able to recover toxicity with the methanol add-back at a level of 0.7 toxicity units, which is consistent with the absence of toxicity in the definitive whole sediment–dilution test.
Based on these results, add-backs were also conducted with methanol extracts from an additional 12 sediment samples (batch 2) that exhibited 0% to 66% survival in the original baseline tests (data not shown). Toxicity units recovered in the extracts ranged between 0.9 and 5, with a median of 2.4, which is similar to the distribution of toxicity units for the add-backs presented in Table 2.
Toxicity of solvent gradient elutions
Results of 96-h exposures to the gradient of methanol:water fractions are summarized in Table 3 for all 22 samples tested in batches 1 and 2. At 96 h, toxicity tended to be primarily associated with the 75% to 85% fractions, whereas the 24-h results (data not shown) exhibited toxicity primarily in the 80% and 85% fractions, suggesting that toxicity tended to be more concentrated in these fractions. Notably, some samples exhibited different patterns of toxic fractions, potentially indicating different causes of toxicity.
| % Methanol | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | 50 | 70 | 75 | 80 | 85 | 90 | 95 | 100 | 100 | 100 |
| 3C01 | 100 | 80 | 0 | 0 | 0 | 0 | 90 | 100 | 100 | 100 |
| 3C10 | 100 | 100 | 0 | 0 | 0 | 100 | 100 | 90 | 100 | 100 |
| 3C11 | 100 | 80 | 0 | 0 | 0 | 50 | 100 | 100 | 100 | 100 |
| 3C12 | 100 | 0 | 0 | 0 | 0 | 80 | 100 | 100 | 100 | 90 |
| 3C13 | 100 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 80 |
| 3C15 | 100 | 70 | 0 | 0 | 0 | 30 | 100 | 100 | 100 | 100 |
| 3C16 | 100 | 30 | 40 | 80 | 90 | 100 | 100 | 100 | 100 | 100 |
| 3C25 | 100 | 100 | 80 | 0 | 0 | 10 | 100 | 90 | 100 | 100 |
| 3D01 | 100 | 100 | 0 | 0 | 0 | 100 | 100 | 100 | 100 | 100 |
| 3D05 | 100 | 20 | 100 | 90 | 90 | 90 | 90 | 100 | 100 | 100 |
| 3C08B | 100 | 100 | 0 | 0 | 0 | 20 | 90 | 100 | 100 | 100 |
| 3C09 | 100 | 90 | 0 | 0 | 0 | 80 | 100 | 100 | 100 | 100 |
| 3C14 | 100 | 0 | 0 | 0 | 0 | 80 | 100 | 100 | 100 | 100 |
| 3C18 | 100 | 0 | 0 | 0 | 0 | 100 | 100 | 90 | 100 | 100 |
| 3D00 | 100 | 100 | 0 | 0 | 0 | 60 | 100 | 100 | 100 | 100 |
| 3D02 | 100 | 100 | 0 | 10 | 0 | 100 | 100 | 100 | 100 | 100 |
| 3D07 | 100 | 30 | 0 | 0 | 0 | 0 | 30 | 80 | 90 | 90 |
| 3D09 | 100 | 100 | 80 | 100 | 100 | 90 | 100 | 100 | 100 | 90 |
| 3C17 | 100 | 80 | 0 | 80 | 40 | 90 | 100 | 100 | 100 | 90 |
| 3C19 | 100 | 0 | 0 | 0 | 0 | 0 | 80 | 90 | 100 | 100 |
| 3D03 | 100 | 30 | 0 | 30 | 20 | 80 | 100 | 70 | 80 | 100 |
| 3D04 | 100 | 0 | 0 | 0 | 0 | 30 | 90 | 100 | 90 | 100 |
- a Data show means of 2 replicates; control survival was ≥90%.
Analysis of methanol fractions
Up to this point, the results suggested that the bulk of toxicity in the samples was caused by nonpolar organic contaminants that were recovered in methanol extracts of the sediments. In addition, toxicity partitioned into discrete methanol:water fractions, suggesting that constituents present in the toxic fractions could be used to identify the cause(s) of toxicity. An initial analysis of unknowns associated with the fractions revealed the following general contaminant groupings of interest. First, n-Alkanes, primarily representing the C30–C36 range, representative of a heavy oil (i.e., not gasoline, diesel, or motor oil), tended to elute in the lower fractions (i.e., 50% and 70% methanol) that did not exhibit toxicity. Second, health care and household products (e.g., triclosan and chlorophene) dominated the lower-eluting (70–75%) toxic methanol fractions. Chlorophene, a disinfectant, accounted for most of the mass recovered, with triclosan present at lower concentrations. Third, polycyclic aromatic hydrocarbons PAHs comprised most of the total mass recovered in the middle range of toxic fractions (e.g., 80–85% methanol). Thus, the primary contaminants of interest associated with the toxic fractions included disinfectants and PAHs, which tended to elute from the columns in that order. Chlorinated pesticides and PCBs were also present intermittently in some of the fractions and considered potentially relevant as “legacy” contaminants.
Contributions to toxicity
The investigation of cause focused on compounds present in toxic methanol:water fractions, with major contaminant classes of interest including disinfectants, PAHs, PCBs, and chlorinated pesticides. With the exception of the first few fractions, PAHs tended to dominate the mass of material recovered in the methanol:water fractions. Indeed, when toxicity was noted in some of the higher fractions (e.g., 95–100% methanol), it was thought that it might be a result of the presence of a different contaminant (e.g., PCBs, chlorinated pesticides). However, this pattern typically occurred in samples with higher toxicity (and more PAHs), and the analytical results showed that the PAHs tended to smear or elute across a wider range of fractions under these conditions.
The approach used to account for toxicity was based on the following assumptions: 1) the bulk of toxicity was associated with disinfectants (e.g., chlorophene, triclosan) and PAHs (notably, PAHs were predominantly present in the higher percentage methanol fractions, whereas the disinfectants were largely associated with the lower fractions) and 2) toxicity not accounted for by these 2 components would suggest that additional sources were present. The process was undertaken by deriving LC50 values that could be used to estimate toxicity units for each of the different contaminant classes. These were derived from a subset of samples (i.e., n = 18 samples) for which toxicity data were available for the same fractions that were analyzed for unknowns. For PAHs, this procedure focused on the higher percentage methanol fractions that contained minimal or no concentrations of the disinfectants (n = 76). Relationships between mass (nanograms) and mortality were calculated for low–molecular weight and high–molecular weight PAHs and total PAHs using linear regression. Inspection of the dose–response curves and associated confidences intervals suggested that high–molecular weight PAHs exhibited the tightest relationship with mortality. Moreover, concentrations of low–molecular weight and high–molecular weight PAHs were well correlated (R2 = 0.92), suggesting that high–molecular weight PAHs provided a good marker for PAH concentrations in general. Therefore, the LC50 (i.e., 4759 ng; confidence interval = 3842–5811; Figure 2) estimated for high–molecular weight PAHs was used to estimate toxicity units representing PAH toxicity.
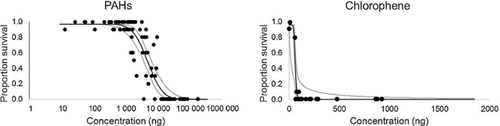
For the disinfectants (e.g., chlorophene and triclosan), this procedure focused on the lower percentage methanol fractions that contained these contaminants and minimal concentrations of PAHs (n = 19), and LC50s were determined similarly to the PAHs. Inspection of the dose–response curves and associated confidence intervals suggested that chlorophene represented a tighter relationship between mass and toxicity than did the combination of chlorophene + triclosan; therefore, the estimated LC50 (i.e., 57.4 ng; confidence interval = 31.6–64.7; Figure 2) for chlorophene was used to estimate toxicity units for disinfectant-related toxicity.
The potential contribution of toxicity units related to disinfectants and PAHs was calculated for each sample by summing the total mass (nanograms) in all of the fractions to obtain an estimate of mass in the methanol fraction as a whole and then dividing by 3 to adjust for the fact that the amount on the column represented 3× the amount present in the original sample. The estimated mass in the original sample was then divided by the LC50 to determine how many toxicity units were present at 1×. This estimate of toxicity units was then compared with the toxicity units found in the original methanol add-backs to determine the extent to which disinfectants and PAHs could account for toxicity or if other nonpolar organic contaminants were potentially contributing to toxicity. These data are compared in Table 4 and suggest that 1) toxicity units estimated from the fractions were in general agreement with toxicity units found in the methanol add-backs (i.e., within a factor of 2 for most samples), and 2) toxicity for most of the sites could be explained largely on the basis of PAHs or disinfectants; however, in some cases, both classes of contaminants appeared to contribute to toxicity.
| Toxicity units | |||
|---|---|---|---|
| Sample | Chlorophene | PAHs | Methanol add-back |
| 3C01 | 1.1a | 0.2 | 3.5 |
| 3C08 | 2.0a | 0.5 | 3.5 |
| 3C09 | 3.2a | 0.6 | 3.5 |
| 3C10 | 1.1a | 0.4 | 1.3 |
| 3C11 | 3.2a | 0.8 | 5 |
| 3C12 | 1.3a | 0.3 | 1.8 |
| 3C13 | 0.6 | 32.4a | >5 |
| 3C14 | 0.5 | 3.8a | 1.8 |
| 3C15 | 2.1a | 1.2 | 1.8 |
| 3C16 | 0.3 | 0.2 | 0.7 |
| 3C17 | 0.7 | 0.8 | 1 |
| 3C18 | 0.2 | 3.8a | 1.8 |
| 3C19 | 1.5 | 5.9a | 3.5 |
| 3C25 | 0.5 | 3.9a | 2.5 |
| 3D00 | 10.1a | 1.0 | 3.5 |
| 3D01 | 1.6a | 0.2 | 1.8 |
| 3D02 | 0.7 | 0.8 | 1 |
| 3D03 | 1.0a | 0.6 | 0.9 |
| 3D04 | 1.9 | 2.8a | 3 |
| 3D05 | 0.2 | 0.0 | 0.3 |
| 3D07 | 6.3a | 7.4a | >5 |
| 3D09 | 0.4 | 0.2 | 0.9 |
- a Indicates dominant contribution(s) to toxicity.
- PAH = polycyclic aromatic hydrocarbon.
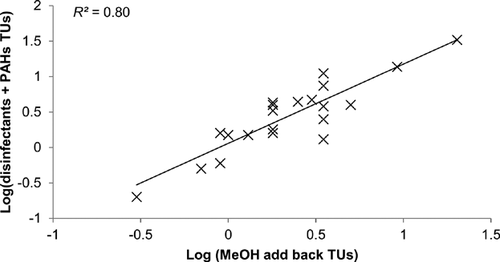
The relationship between toxicity units estimated from the fractions and toxicity units found in the methanol add-backs was further evaluated using regression analysis. Notably, toxicity units derived for disinfectants or PAHs explained similar proportions of the variance across samples, but including both variables in the equation significantly improved the fit (Table 5 and Figure 3). Collectively, these data suggest that these 2 contaminant classes largely accounted for toxicity across the site. Moreover, inspection of the residuals (Figure 3) suggests that no additional sources of toxicity were present across the site; thus, any unexplained variance was likely related to experimental variability. Note that mass patterns associated with PCBs and chlorinated pesticides were also evaluated with respect to patterns of mortality in the fractions, with no evidence of association.
| Contaminant class | F ratio | p | R2 |
|---|---|---|---|
| Disinfectants | 8.85 | 0.007 | 0.31 |
| PAHs | 13.7 | 0.002 | 0.42 |
| PAHs + disinfectants | 78.6 | <0.0001 | 0.80 |
- PAH = polycyclic aromatic hydrocarbon.
DISCUSSION
Toxicity identification evaluations conducted on sediments typically entail a general phase I characterization of toxicity (e.g., ammonia, sulfides, metals, nonpolar organics) to identify contaminant classes associated with toxicity. These results are then used to support more specific insights based on qualitative comparisons of analytical data and screening values for specific contaminants suggested by knowledge of the site that are consistent with the contaminant class(es) identified in the characterization phase. This approach is particularly effective if toxicity is driven by a single contaminant or class of contaminant that responds to a unique and targeted treatment (e.g., pyrethroid pesticides) 31 or otherwise readily distinguishable from other contaminants present 32. However, the potential for a clear line of evidence can quickly become obfuscated in the presence of matrix effects, multiple contaminants at potentially relevant concentrations, or an unknown toxicant not included in the original analytical screening process. Collectively, these factors may result in incorrect or partial identification of the cause of toxicity, which could further result in inappropriate remediation targets as well as improper assignment of source and responsibility. As a result, there is a need for improved identification techniques, particularly for sites with the potential for multiple nonpolar organic constituents to contribute to toxicity. Given that the present study site contained PAHs, PCBs, and chlorinated pesticides, as well as a range of potential unknowns associated with urban runoff and combined storm sewer overflows, the ability to separate individual contributions to overall toxicity was a primary consideration.
The present approach built on TIE fundamentals that we were generally familiar with, as well as procedures described by others which had been shown to be effective in recoveries of contaminants of interest (PAHs, PCBs) associated with sediments. Thus, it appeared that methanol was a desirable solvent to work with in terms of recovery, and a solvent gradient (i.e., methanol:water) applied to a SPE column was sufficient to separate the contaminants of interest 33. Moreover, use of C8 instead of C18 SPE columns appeared appropriate to shift the contaminant distribution closer to the middle of the solvent gradient applied 5. Notwithstanding the demonstrated usefulness of porewater testing in sediment toxicity investigations 10, 11, 31-33, our initial efforts to work with elutriates and porewater did not provide the desired level of reproducibility relative to whole-sediment toxicity. Consequently, the main issue was how to consistently extract biologically available nonpolar organic constituents from the sediments. The approach used in most studies is to add different media to the sediments for varying periods of time to sorb the nonpolar organic constituents present, followed by extraction of the media to recover the sorbed nonpolar organic constituents. However, investigators implementing these procedures have reported variable levels of recovery and seemed in general agreement that they could be improved 13-15. Always in search of simplicity, we thought why not add solvent directly to the sediments and see what happens? By using a relatively unaggressive solvent (methanol) in conjunction with a relatively short extraction period, we hoped to recover the biologically available material present in the solid phase, without extracting the more tightly bound materials that might be removed by a more aggressive solvent (e.g., hexane or dichloromethane) or with a longer equilibration period 34. Furthermore, direct addition of solvent would eliminate 1 of the steps in a resin addition/solvent-extraction approach, which would hopefully improve recovery and reproducibility. By happy coincidence or luck, this approach turned out to be largely successful based on comparisons of toxicity units in the methanol add-backs with the toxicity units found in the whole sediments.
Overall, the results of the present study clearly implicated nonpolar organic constituents as the primary cause(s) of toxicity across the site and further demonstrated that ammonia, sulfide, and metals were not significant contributors to toxicity. Follow-up work showed that toxicity was largely a function of disinfectants that tended to elute in the lower methanol:water fractions and PAHs that tended to elute in the higher methanol:water fractions. Based on the relative toxicity units associated with each toxicant class, it would also be possible to infer sources of toxicity; for example, sites that exhibited toxicity dominated by disinfectants would suggest a relationship with sewage discharges. Notably, disinfectants were not part of the original suite of contaminants selected for analytical screening and would have been missed entirely in the absence of analyses conducted on the toxic fractions.
The presence of toxicity related to disinfectants was unexpected but significant; for example, chlorophene accounted for approximately 70% of the total peak area in the chromatogram for 1 of the toxic fractions tested. Notably, triclosan and chlorophene are emerging contaminants of concern and have been designated as high-priority chemicals for monitoring programs by the US Geological Survey 35. Both chemicals have been associated with endocrine-disrupting effects 36, 37, and Ho et al. reported adverse effects on meiobenthic and microbenthic faunal communities exposed to sediment spiked with triclosan 38. Although toxicity data on chlorophene are more limited than those on triclosan, the available values suggest that it is at least comparable 39.
The presence of PCBs and chlorinated pesticides in the samples also presented a potential confounding factor that would have been difficult to resolve without the TIE results, specifically the toxicological and analytical results for the methanol:water fractions that indicated no relationship between these contaminants and toxicity. Thus, having toxicity and analytical data for specific fractions provides greater resolution in terms of associating concentrations with actual effects than simply 1 value for the whole-sediment sample that might exceed sediment quality guidelines but not actually contribute to toxicity. By having multiple fractions across which to compare concentration and response data, it is readily apparent whether or not there is a relationship between the 2 variables. Notably, Greenstein et al. also commented on the importance of actual TIE and toxicity data to establish links between sediment toxicity and contaminants present, rather than relying on sediment quality guidelines 31.
One observation that came up was the presence of nondissolved hydrocarbon (e.g., tar, oily liquid) in some of the samples. Based on results from the whole-sediment tests, this material was largely not bioavailable (perhaps as a function of avoidance or close association with surfaces of larger sediment particles), but it did contribute to elevated toxicity in solvent extractions of the sediments. This underscored the importance of tracking toxicity units between manipulations because of its effect on toxicity in the methanol extractions. Likewise, the presence of nondissolved material in whole sediment would have affected correlations between measured contaminant concentrations and toxicity because the measured concentrations in these particular samples would have been high relative to actual toxicity. Thus, conducting the TIE manipulations provided additional relevance to the interpretation process.
In the present study, the occasional recovery of more toxicity units in the methanol add-backs than present in the whole sediment was related to the presence of nondissolved hydrocarbons in the sample. Conversely, Anderson et al. 15 reported that toxicity units in solvent extractions of carbonaceous resins mixed into the sample were typically higher relative to whole sediment, potentially because of sorption efficiencies of the resin in conjunction with an extended extraction period. In contrast, the approach used in the present study showed that toxicity units captured in the methanol add-backs were generally in good agreement with toxicity units associated with the whole sediment (i.e., the bioavailable fraction). The fact that toxicity units were also conserved between the methanol add-backs and solvent-gradient elutions further suggests that the combination of solvent and SPE column was appropriate in terms of optimizing efficiencies associated with extraction and elution. Notably, “off ramps” (i.e., points in the investigation where the toxicity units did not match) were part of the process and allowed for additional scrutiny to identify causes of the discrepancies.
The present study demonstrated a rigorous approach for identifying the cause(s) of toxicity in sediments from a site with multiple sources of toxicity. Specifically, the level of toxicity associated with each sample was determined and then used as a basis for evaluating the effectiveness of solid-phase TIE treatments to characterize the general cause(s) of toxicity. Given that the phase I results largely implicated nonpolar organic constituents as the primary cause of toxicity, methanol extracts of the sediments were used to recover the organic fraction, which was then further separated into toxic and nontoxic fractions based on polarity. The GC/MS analysis of the fractions identified toxicants that were of interest, as well as contaminants that were present, but did not contribute significantly to toxicity. In general, toxicity was quantitatively recovered throughout the TIE process, suggesting that all significant causes of toxicity were accounted for. Toxicity varied at different points across the site but appeared to be largely a function of disinfectants and PAHs, consistent with inputs from combined storm sewer overflows and historical uses of the site, respectively. Moreover, the procedures appeared to be robust, with the fractionation process capturing a relatively wide range of contaminants of interest. Given that these results are consistent with the goals of environmentally relevant extractions in conjunction with toxicant-specific TIEs identified by Burgess et al. 24, even Holmes himself might be sufficiently moved to say, “Well done, Watson.”
Data availability
Data are available on request from the corresponding author ([email protected]).




