Splenic immunotoxicity in developing cane toads (Rhinella marina) from Bermuda
Abstract
The impacts of contaminated sediment from 2 ponds in Bermuda on immune function in newly metamorphosed cane toads were examined. In the present study, a partial life-cycle experiment exposing Gosner stage 20 cane toad tadpoles to pond sediment and laboratory culture water through metamorphosis and into a juvenile state was performed. A basic immunology battery, including general necropsy, spleen somatic index, spleen white pulp content, splenocyte tissue density, and splenocyte viability, was conducted in newly metamorphosed Rhinella marina exposed to Bermuda freshwater sediment and baseline specimens collected from 2 separate populations in south Texas and south Florida, USA. Immune function was evaluated using a lymphocyte proliferation assay with subset specimens infected with Mycobacterium chelonae. In the Bermuda population exposed to pond sediment, splenocyte tissue density was markedly lower and lymphocyte proliferation substantially less relative to cohorts exposed to control sediment and to the North American populations. Considerable increases in spleen weight and liver and spleen lesions related to M. chelonae infection were recorded in challenged Bermuda R. marina compared with unchallenged specimens. Overall, immune function in Bermuda R. marina was compromised compared with North American mainland R. marina regardless of treatment but more dramatically in specimens exposed to Bermuda pond sediments. Environ Toxicol Chem 2016;35:2604–2612. © 2016 SETAC
INTRODUCTION
The Bermuda Amphibian Project was initiated in response to local concerns that the populations of Bermuda's amphibians were declining. Overall, the Bermuda Amphibian Project includes an amphibian field-monitoring program, amphibian-based ecological hazard assessment, assessment of potential impacts on other wildlife, epidemiology, and public education. Components of the ecological hazard assessment include exposure assessment, causality analysis, and population impact modeling. Prior to 1994, 3 species of amphibians, the cane toad (Rhinella marina) and 2 whistling frogs (Eleutherodactylus johnstonei and Eleutherodactylus gossei), introduced in the late 1880s were known to inhabit Bermuda. Presently, however, it appears that the whistling frog E. gossei has become locally extinct, and there is evidence of a decline in the cane toad (R. marina) populations, which is the only species of toad inhabiting Bermuda.
Previous studies discovered a high incidence of deformities in Bermuda's mature toad and newly metamorphosed toad populations 1 and suggested that adult R. marina from a contaminated site in Bermuda showed signs of immunosuppression 2. Gonadal abnormalities observed in adult toads from several sites suggested that endocrine-disrupting chemicals may have affected the process of gonad differentiation in these animals 3. Furthermore, sediments from Cloverdale and Warwick Ponds were shown to impair reproduction and affect steroidogenesis in fathead minnows 4. Steroidogenesis in larval toads inhabiting the same ponds was similarly affected (D.J. Fort and J.P. Bacon, unpublished data). Therefore, to further assess the effects of pond sediments on Bermuda's toads, R. marina tadpoles were exposed to contaminated site sediments from Cloverdale and Pitman's Ponds through metamorphosis in flow-through laboratory microcosms, and the effects on immune function were evaluated postmetamorphosis. These 2 ponds have a history of a high incidence of deformed metamorphic toads 1, 5. Immune function in R. marina populations in south Texas, USA and in south Florida, USA was also evaluated to establish a mainland baseline. No deformities have been reported for either North American R. marina population. A basic splenocyte immunology battery including general necropsy and evaluation of spleen white pulp content, splenocyte tissue density and viability, lymphocyte proliferation, and phagocytosis functionality was conducted. Response to infection was evaluated by exposing a subset of developing R. marina to the ulcerating bacterium Mycobacterium chelonae.
MATERIALS AND METHODS
Pond and control sediment
Pond sediment was collected from 2 freshwater ponds, Pitman's Pond and Cloverdale Pond, in Bermuda. Both ponds are zoned as nature reserves. Pitman's Pond is a 0.26-ha pond excavated on a portion of a former landfill site. This slightly brackish pond is a habitat for a variety of birds as well as for cane toads and red-eared sliders. Cloverdale Pond is a 0.16-ha excavated pond located on the grounds of an apartment complex and situated 50 m from a heavily traveled road, gasoline station, and apothecary 5. It is also a habitat for various birds, cane toads, and red-eared slider turtles (Trachemys scripta elegans).
Each sediment sample was comprised of 4 grab samples and composited in buckets in the field. Sediment was shipped via overnight courier to the laboratory and stored at 4 °C. Because no ponds within Bermuda could be classified as true controls, control laboratory sediment was prepared by mixing inert reef-grade sand (AES) with pesticide-free commercial organic soil (BWI) to comparable organic carbon levels, 5.6 mg/L and 8.0 mg/L for freshwater sites Pitman's Pond and Cloverdale Pond, respectively.
Culture (dilution) water and laboratory control
Dechlorinated (charcoal-filtered) tap water was used as culture or dilution water for the freshwater studies. Dechlorinated laboratory water was prepared by passing tap water through a 4-filter system; a multimedia filter to remove suspended solids in the feed water; a 10-inch pretreatment filter (5 μm) to remove any additional solids; a 3.6-cf activated virgin carbon treatment filter to remove chlorine, ammonia, and higher–molecular weight organics; and a 5-μm polishing filter to remove any carbon particles from the carbon treatment phase.
Other culture water quality characteristics measured weekly during the 21-d experiment were conductivity (salinity), hardness, alkalinity, ammonia-nitrogen, and residual oxidants. Trace contaminants, including organics (volatile and semivolatile) and inorganics (metals, nitrates/nitrites, fluoride, and iodide), were analyzed at the present study's initiation and were not detected.
Test animals and exposure
The exposure phase was initiated with Gosner stage 20 R. marina larvae collected from each respective pond in Bermuda. A control for each pond (site organisms in dechlorinated tap water and control sediment) and a treatment for each pond (site organisms exposed to site sediment/dechlorinated tap water) were used. Four replicates of 20 larvae per treatment (total 80 larvae/treatment) were initially exposed to the corresponding sediments for each control, unchallenged and later, as described below, ulcerating M. chelonae–challenged treatments. Forty of the 80 larvae were eventually selected for the challenge treatment at Gosner stage 40 and placed in a separate microcosm, consistent with previous sediment exposure, ultimately resulting in 40 larvae/treatment or control for the challenged and unchallenged treatments. Because mainland toads were only used to assess baseline immune function, only test organisms from Bermuda designated as the challenge treatment were injected with ulcerating M. chelonae intraperitoneally at Gosner stage 40 (∼culture day 45). Exposure was conducted for 75 d or until the toads reached metamorphic completion. All control and treatment test chambers consisted of 60-L plastic flow-through tubs containing 50 L of dechlorinated tap water flowing at a rate of 25 mL/min. A 1 to 4 ratio of sediment to water was used such that 12.5 kg of control or treatment sediment was added to each chamber. The flow-through feed water and drain were maintained at the surface of the water to minimize sediment loss and leaching. In addition, 40 metamorphosed R. marina from south Texas and from south Florida were collected as a mainland reference to provide comparison with the Bermuda toads.
Feeding
Larvae were fed boiled romaine lettuce daily ad libitum. Food was withheld from the toads for 12 h prior to the day of tissue sampling to aid in the histological processing of such small specimens. Uneaten food and fecal material were removed from the test aquaria daily.
Tissue preparation
Test organisms, exposure methodology, and feeding and culture conditions were as described in Test animals and exposure and Feeding sections with the following exceptions. Following the completion of metamorphosis, toads were anesthetized and euthanized in 100 mg/L tricaine methanesulfonate (MS-222). Whole-body weight for each toad was recorded. The presence of splenic and hepatic lesions was evaluated in control, unchallenged, sediment-exposed and in challenged, sediment-exposed organisms. Spleens were aseptically removed, weighed, and prepared for cell isolation in complete amphibian L-15 medium. Cells were plated, and an aliquot of each suspension was stained with trypan blue and counted. Cell suspensions from each individual were then portioned into adjusted aliquots for the various measurements, and assays were performed. Cell concentrations, incubation time and temperature, and mitogen concentrations were modified from standard methods used by Christin et al. 6 in ranids for use in R. marina.
Morphological endpoints
Premetamorphic
Embryolarval survival and the completion of metamorphosis were recorded in each replicate of each treatment daily and as the proportion that completed metamorphosis at the conclusion of exposure (day 75).
Postmetamorphic
Snout–vent length, whole-body wet weight, malformations, and gonadosomatic index (GSI) were determined in each individual toad that completed metamorphosis. At the completion of metamorphosis, individual toads were euthanized using 200 mg MS-222/L (Sigma-Aldrich), weighed to the nearest 10 mg, and digitally photographed (Sony Cyber-Shot DSC-H10) to assess external malformations and measure snout–vent length. Snout–vent length was measured from the digital photographs using SigmaScan Pro (SPSS) to the nearest millimeter. During removal of the spleen, the gonadal tissue was also removed and weighed to determine GSI.
Immunological
Splenocyte cell counts and viability
The total number of splenocytes per milliliter was determined for each sample by placing 1 mL of sample in a Neubauer hemocytometer (Fisher Scientific). Cell viability was determined by evaluating splenocyte membrane permeability using trypan blue in accordance with the methods of Christin et al. 6. A spleen somatic index was determined by dividing the individual spleen weight by wet body weight and expressed as a percentage.
Spleen tissue density
Tissue density was determined by dividing the total cells recovered by the mass of the tissue dissociated to collect the cells. Tissue density represented an indirect indicator of blastogenesis and was considered more meaningful than total cell recovery because it normalized the data set to spleen weight.
Spleen—Lymphocyte proliferation assay
Cultured splenocytes (70 000 cells/0.1 mL L15 with 0.05% mercaptoethanol) were suspended in 24-well plates (Thermo Scientific) with 5 µg/mL of the T-cell mitogen concanavalin A or 2.5 µg/mL of the B-cell activator lipopolysaccharide. The cell-mitogen suspension was incubated for 96 h at 25 °C with 5% CO2. Twenty-four hours prior to the termination of the culture, 0.5 µCi of [methyl-3H]-thymidine (Perkin Elmer; specific activity 20Ci [740 GBq]/mmole, >97%, 250 μCi [9.25MBq]) was added to each well. Following DNA capture, the incorporated 3H-thymidine was counted using a Perkin-MicroBeta scintillation counter, and the results were expressed as counts per minute.
Spleen—Phagocytosis assays
Two specific assays were used to evaluate phagocytosis, a phagocytosis functional assay and an oxidative burst assay. For the phagocyte functional assay, splenocytes (1 × 104 cells/mL of L15) were mixed with a fluorescein Escherichia coli probe (Molecular Probes) for 1 h at 25 °C. The percentage of phagocytes to probe bacteria was 5%. Trypan blue was added to quench the fluorescence of bacteria that were not engulfed by the phagocytes. The fluorescence of engulfed bacteria was then analyzed using a spectrofluorometer (Perkin Elmer LS-55) at excitation/emission wavelengths of 488/520 nm, respectively.
For the oxidative burst assay, a fluorescent measure of oxidative products of phagocytic cells was based on modified methods of Rosenkranz et al. 7 and Froese et al. 8, utilizing the measurement of the oxidation of dichlorofluorescein diacetate (Sigma-Aldrich) to the fluorescent product dichlorofluorescein. Fluorescence was related to the production of hydrogen peroxide. Using 24-well plates (Thermo Scientific), 1 × 106 cells/mL amphibian phosphate-buffered saline were plated per well with 50 mM dichlorofluorescein diacetate. All plates were incubated at 25 °C for 120 min, and fluorescence was measured using excitation/emission wavelengths of 485/538 nm, respectively. Results were expressed as relative fluorescence units 8.
Peritoneal neutrophils
To determine if innate immune function was compensating for humoral immune dysfunction, peritoneal neutrophils were collected from each toad in accordance with the methods of Froese et al. 8 and Rosenberg et al. 9. A 20-gauge surgical-grade catheter was inserted into the peritoneal cavity, and 10 mL of amphibian phosphate-buffered saline with 25 units/mL heparin was injected. After 2 h, the lavage fluid with the peritoneal neutrophils was removed and peritoneal neutrophils were isolated by centrifugation at 200 g for 15 min at 4 °C. Peritoneal neutrophils were evaluated for viability and functionality as described for the phagocytosis assays in the section Spleen—Phagocytosis assays.
Sediment and tissue residue analyses
For whole-body tissue analyses, 5 newly metamorphosed toads were humanely euthanized (2 g/L MS-222, pH 7.0) per site and homogenized whole. Equal portions were either extracted for total petroleum hydrocarbon or polyaromatic hydrocarbon (PAH) analyses or digested using HNO3 for metals analysis. Total metals (arsenic, cadmium, chromium, copper, iron, lead, nickel, and zinc) were analyzed by inductively coupled plasma spectroscopy 10 with the exception of mercury, which was analyzed by cold vapor 11. Total petroleum hydrocarbons and PAHs were analyzed using gas chromatography–mass spectrometry 12. To express the contaminant results as dry weight, total solids of each sample were measured and the results normalized to the solid fraction of each sample.
Data analysis
The experimental unit for analysis was the replicate. Potential outliers were identified by values that exceed the median plus 3 times the interquartile range (i.e., the difference between the 75th and 25th percentiles). A replicate mean and standard error of the mean were determined for the remainder of the endpoints evaluated. Data sets were initially evaluated for normality (Shapiro-Wilks test) and variance homogeneity (Levene test). Parametric data sets were evaluated using a Mann-Whitney U test. Nonparametric data sets were evaluated using the Wilcoxon rank sum test. Statistical significance was assessed at α = 0.05.
RESULTS
Culture water, sediment, and tissue residue analyses
The physiochemical characteristics of the dechlorinated tap water were as follows: pH 7.5 (7.4–7.6), 8.1 (7.2-8.6) mg/L dissolved oxygen, 874 (732–927) μmhos/cm conductivity, 124 (120–128) mg/L as CaCO3 hardness, 54 (50–58) mg/L as CaCO3 alkalinity, and <0.05 and <0.06 mg/L total residual oxidants and ammonia-nitrogen, respectively. Contaminant characterization of the sediment and metamorphic toad tissue is provided in Table 1 and Table 2. No contaminant residues aside from trace amounts of copper, zinc, and iron, which are naturally occurring, were detected in the control toads (Table 1). Significant PAH and metal residues were found in both the sediments from each pond and the exposed toads (Table 2).
| Site | Samplea | TPH- GRO (mg/kg) | TPH- DRO (mg/kg) | Total As (mg/kg) | Total Cd (mg/kg) | Total Cr (mg/kg) | Total Cu (mg/kg) | Total Fe (mg/kg) | Total Pb (mg/kg) | Total Hg (mg/kg) | Total Ni (mg/kg) | Total Zn (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controlb | Tissue | <0.5 | <0.5 | <0.1 | <0.1 | <0.1 | 22.6 | 43.2 | <0.1 | <0.1 | <0.1 | 0.8 |
| Sediment | <0.5 | <0.5 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | |
| CLO | Tissue | 9.5 | 634.4 | 0.8 | 2.7 | 17.5 | 58.2 | 78.9 | 32.1 | 0.3 | 5.6 | 22.5 |
| Sediment | <0.500 | 104.1 | 5.5 | 4.2 | 40.3 | 87.0 | 4742 | 99.0 | 0.2 | 4.1 | 175.0 | |
| PP | Tissue | 13.3 | 150.3 | 0.4 | 3.8 | 12.6 | 43.3 | 83.1 | 59.7 | 0.4 | 8.7 | 16.4 |
| Sediment | <0.5 | 1926 | 3.9 | 2.1 | 10.5 | 21.0 | 4017 | 106.0 | 0.2 | 4.2 | 107.2 |
- a Tissue represents whole-body composite, n = 20/treatment. Composite sediment sample prepared from 4 equivalent grab samples based on weight.
- b Control tissue = R. marina exposed to laboratory culture water. Control sediment was inert sand mixed with control organic sediment.
- CLO = Cloverdale Pond; PP = Pitman's Pond; TPH-DRO = total petroleum hydrocarbons–diesel range organics; TPH-GRO = total petroleum hydrocarbons–gasoline range organics.
| Control | CLO | PP | ||||
|---|---|---|---|---|---|---|
| Tissue | Sediment | Tissue | Sediment | Tissue | Sediment | |
| Naphthalene (μg/kg) | <10.0 | <10.0 | <10.0 | <10.0 | <10.0 | <10.0 |
| Acenaphthylene (μg/kg) | <10.0 | <10.0 | <10.0 | <10.0 | <10.0 | <10.0 |
| Acenaphthene (μg/kg) | <10.0 | <10.0 | <10.0 | 328 | 138 | 145 |
| Fluorene (μg/kg) | <10.0 | <10.0 | 491 | <10.0 | <10.0 | <10.0 |
| Phenanthrene (μg/kg) | <10.0 | <10.0 | 1206 | 658 | 321 | 235 |
| Anthracene (μg/kg) | <10.0 | <10.0 | 803 | 138 | 234 | 183 |
| Fluoranthene (μg/kg) | <10.0 | <10.0 | <10.0 | 494 | 288 | 108 |
| Pyrene (μg/kg) | <10.0 | <10.0 | 1211 | 567 | 651 | 432 |
| Benzo[a]anthracene (μg/kg) | <10.0 | <10.0 | 269 | 284 | 267 | 124 |
| Chrysene (μg/kg) | <10.0 | <10.0 | 218 | 388 | 144 | 56.0 |
| Benzo[b]fluoranthene (μg/kg) | <10.0 | <10.0 | <10.0 | <10.0 | <10.0 | <10.0 |
| Benzo[k]fluoranthene (μg/kg) | <10.0 | <10.0 | <10.0 | <10.0 | <10.0 | <10.0 |
| Benzo[a]pyrene (μg/kg) | <10.0 | <10.0 | <10.0 | <10.0 | <10.0 | <10.0 |
| Indeno[1,2,3-Cd]pyrene (μg/kg) | <10.0 | <10.0 | 558 | <10.0 | <10.0 | <10.0 |
| Dibenz[a,h]anthracene (μg/kg) | <10.0 | <10.0 | 410 | 134 | 328 | 106 |
| Benzo[g,h,i]perylene (μg/kg) | <10.0 | <10.0 | <10.0 | <10.0 | <10.0 | <10.0 |
- a Tissue samples represents whole-body composite, n = 20/treatment. Composite sediment samples prepared from 4 equivalent grab samples based on weight. Control tissue = R. marina exposed to laboratory culture water. Control sediment was inert sand mixed with control organic sediment.
- CLO = Cloverdale Pond; PP = Pitman's Pond.
Morphological endpoints
Premetamorphic
The impact of freshwater sediment exposure on premetamorphic R. marina development is presented in Table 3. Survival of developing toads was ≥80% in the controls and each treatment, and exposure to the sediment did not impact survival (Mann-Whitney U, p > 0.05). The frequency of metamorphic completion ranged from 84.9% to 92.3% in the controls and each treatment. No effect of sediment exposure on metamorphic completion in the Cloverdale Pond and Pitman's Pond sediment treatments compared to the respective controls was observed (Mann-Whitney U, p > 0.05).
| Premetamorphic | Postmetamorphic | ||||||
|---|---|---|---|---|---|---|---|
| Test organism location | Media | Survival (%) | Metamorphic completion (%) | Length (cm) | Weight (g) | Malformation (%) | GSIb (%) |
| PP | Control | 81.0 (1.4) | 92.3 (4.5) | 1.14 (0.02) | 0.13 (0.03) | 18.5 (0.3) | 5.3 (0.07) |
| PP | PP | 83.0 (0.5) | 87.9 (5.8) | 1.13 (0.02) | 0.17 (0.01) | 31.3c (0.2) | 4.8 (0.09) |
| CLO | Control | 80.0 (1.8) | 87.8 (5.9) | 1.14 (0.02) | 0.19 (0.03) | 19.4 (0.2) | 4.1 (0.13) |
| CLO | CLO | 83.8 (0.9) | 86.6 (8.6) | 1.08 (0.02) | 0.16 (0.01) | 39.5c (0.2) | 4.2 (0.08) |
| South Florida | Control | 90.0 (2.2) | 88.9 (4.3) | 1.13 (0.07) | 0.15 (0.03) | 0.0 (—) | 5.1 (0.11) |
| South Texas | Control | 91.3 (1.1) | 84.9 (6.7) | 1.16 (0.09) | 0.18 (0.06) | 0.0 (—) | 4.9 (0.08) |
- a Mean with standard error of the mean in parentheses. n = 80, 4 replicates of 20 organisms.
- b Gonadosomatic index = (gonad wt/body wt) × 100.
- c Significantly greater than respective control, Mann-Whitney U test, p < 0.05.
- CLO = Cloverdale Pond; PP = Pitman's Pond.
Postmetamorphic
The effect of freshwater pond sediment exposure on postmetamorphic R. marina development is presented in Table 3. Snout–vent length and wet whole-body weights ranged from 1.08 cm to 1.16 cm and 0.13 g to 0.19 g in the controls and each treatment, respectively. Neither sediment treatment altered postmetamorphic growth relative to the respective control (Mann-Whitney U, p > 0.05). The frequency of external malformation was significantly greater in both Pitman's Pond and Cloverdale Pond toads exposed to their respective site sediments relative to control sediment (Mann-Whitney U, p < 0.05). In addition, the occurrences of external malformation in south Florida and south Texas toads were significantly less than observed with either Pitman's Pond or Cloverdale Pond toads cultured in control medium (Mann-Whitney U, p < 0.05; Table 3). The GSI varied slightly between the Bermuda controls, the Bermuda sediment treatments, and the south Florida and south Texas toads; and GSI values were not significantly different in any of the treatments relative to their respective controls (Mann-Whitney U, p > 0.05).
Immunological endpoints
Spleen characteristics
The immunotoxicological effects of pond sediment exposure on developing R. marina are presented in Table 4 and Table 5. The spleen somatic index values in unchallenged and M. chelonae–challenged toads exposed to the site sediment were not significantly different from those of toads exposed to control sediment (Wilcoxon rank sum, p > 0.05 for both). However, the white pulp content of the spleens from both unchallenged and M. chelonae–challenged toads exposed to the site sediments were both significantly less than their respective controls (Wilcoxon rank sum, p < 0.05 for both).
| LPAb | Phagocytosis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Test organism origin | Media | SSI (%) | White pulp (%) | Tissue density (total cells (×105/g) | Spleen-ocyte viability (%) | LPA, no ConA or LPSc (×103 cpm) | LPA + ConA (×103 cpm) | LPA + LPS (×103 cpm) | PFA (%) | Mean oxidative burst (RFU) | |
| PP | Control | 4.8 (0.2) | 42.0 (3.7) | 1301.7 (340.1) | 76.8 (2.1) | 3.41 (— [0.15]) | 8.14 (2.39 [0.17]) | 6.41 (1.88 [0.20]) | 85.1 (3.6) | 90.5 (1.5) | |
| PP | PP | 5.0 (0.1) | 29.0d (1.9) | 638.9e (95.8) | 74.4 (2.5) | 2.26d (— [0.08]) | 3.99d (1.77 [0.06]) | 3.10d (1.37 [0.09]) | 45.1d (5.0) | 79.2d (2.1) | |
| CLO | Control | 5.2 (0.2) | 46.0 (3.7) | 1600.4 (581.3) | 75.0 (2.7) | 4.04 (— [0.14]) | 7.79 (1.93 [0.15]) | 6.25 (1.55 [0.15]) | 87.1 (2.0) | 90.5 (2.0) | |
| CLO | CLO | 4.7 (0.2) | 31.0 (2.4) | 651.5d (125.0) | 75.6 (4.3) | 2.03d (— [0.04]) | 3.34d (1.65 [0.21]) | 2.97d (1.12 [0.11]) | 36.2d (5.3) | 78.5d (1.7) | |
- a Mean with standard error of the mean, n = 60.
- b Data reported are counts with folds greater than baseline in parentheses and standard error of the mean in square brackets.
- c Baseline, no adjuvant.
- d Significantly less than respective control, Wilcoxon rank sum test, p < 0.05.
- e Significantly less than respective control, t test, p < 0.05.
- CLO = Cloverdale Pond; Con A = concanavalin A; LPA = lymphocytic proliferation assay; LPS = lippolysaccharide; PFA = phagocytosis functional assay; PP = Pitman's Pond; RFU = relative fluorescence unit; SSI = spleen somatic index.
| LPAb | Phagocytosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Test organism origin | Media | SSI (%) | White pulp (%) | Tissue density (total cells (×105/g) | Spleen-ocyte viability (%) | LPA, no ConA or LPSc (×103 cpm) | LPA + ConA (×103 cpm) | LPA + LPS (×103 cpm) | PFA (%) | Mean oxidative burst (RFU) |
| PP | Control | 5.1 (0.4) | 54.0 (4.8) | 3130.7 (599.0) | 68.0 (2.6) | 7.48 (— [0.20]) | 13.70 (1.83 [0.57]) | 11.6 (1.55 [0.30]) | 89.3 (2.0) | 95.6 (1.0) |
| PP | PP | 4.2 (0.6) | 31.0d (1.9) | 473.2d (58.5) | 71.0 (2.9) | 4.96d (— [0.12]) | 6.94d (1.40 [0.38]) | 5.54d (1.12 [0.23]) | 66.6d (2.3) | 80.6d (1.8) |
| CLO | Control | 5.2 (0.4) | 48.0 (3.7) | 1429.7 (183.5) | 70.0 (5.2) | 7.32 (— [0.24]) | 15.50 (2.12 [0.27]) | 12.8 (2.11 [0.32]) | 86.3 (2.3) | 91.5 (0.8) |
| CLO | CLO | 4.3 (0.5) | 33.0d (2.0) | 531.9d (146.3) | 78.0 (3.4) | 3.37d (— [0.36]) | 5.73d (1.70 [0.51]) | 4.42d (1.31 [0.32]) | 61.9d (3.5) | 77.9d (3.2) |
- a Mean with standard error of the mean, n = 60.
- b Data reported are counts with folds greater than baseline in parentheses and standard error of the mean in square brackets.
- c Baseline, no adjuvant.
- d Significantly less than respective control, Wilcoxon rank sum test, p < 0.05.
- CLO = Cloverdale Pond; Con A = concanavalin A; LPA = lymphocytic proliferation assay; LPS = lippolysaccharide; PFA = phagocytosis functional assay; PP = Pitman's Pond; RFU = relative fluorescence unit; SSI = spleen somatic index.
Splenocyte tissue density and viability
Splenocyte tissue densities in unchallenged toads exposed to Bermuda pond sediment were <41% of the respective control toads and <37% of the respective control M. chelonae–challenged toads (Tables 4 and 5). Splenocyte tissue densities in both unchallenged and M. chelonae–challenged toads were both significantly less than in the respective controls (t test, p < 0.05 for both). Splenocyte viability in the toads was consistent among the controls and sediment treatments in both M. chelonae–challenged and unchallenged specimens.
Lymphocyte proliferation assays
The effects of pond sediment exposure on lymphocyte proliferation in unchallenged and M. chelonae–challenged R. marina are presented in Tables 4 and 5. Baseline lymphocyte proliferation assay assessment (no adjuvant) indicated that lymphocyte proliferation was less in the sediment-exposed toads regardless of M. chelonae challenge compared to their respective controls (t test, p < 0.05 for both). Addition of concanavalin A or lipopolysaccharide increased lymphocyte proliferation in each control and sediment treatment regardless of M. chelonae challenge compared to their respective baseline (t test, p < 0.05 for both). However, although the adjuvants increased lymphocyte proliferation relative to the baseline, proliferation in toads exposed to sediment was markedly less than that in their counterpart controls regardless of challenge status (t test, p < 0.05).
Phagocytosis
Evaluation of the impact of sediment exposure on phagocytic activity using the phagocytosis functionality assay and oxidative burst measurements is detailed in Tables 4 and 5. Based on the phagocytosis functionality assay and oxidative burst measurements, phagocytic activity was reduced in toads exposed to sediment relative to their respective controls regardless of challenge status (t test, p < 0.05 for each).
Peritoneal neutrophils
No change in peritoneal neutrophil and lymphocyte counts was observed in either control sediment–exposed or site sediment–exposed toads (t test, p = 0.135 and 0.153, respectively). Peritoneal neutrophil and lymphocyte counts in control sediment–exposed and site sediment–exposed toads were 83.3 ± 5.6% and 3.6 ± 1.3%, 81.1 ± 2.9% and 4.2 ± 1.5% (Pitman's Pond), and 78.2 ± 7.8% and 6.6 ± 2.8% (Cloverdale Pond), respectively.
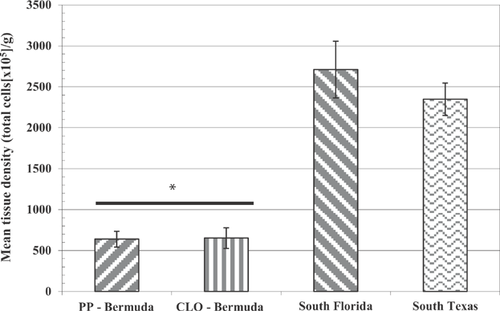
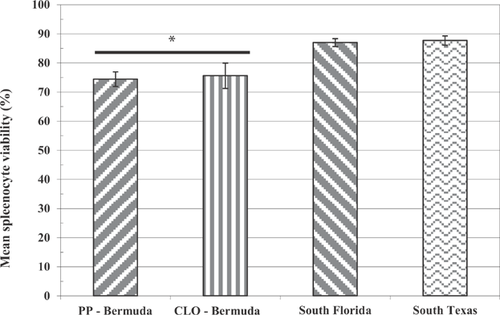
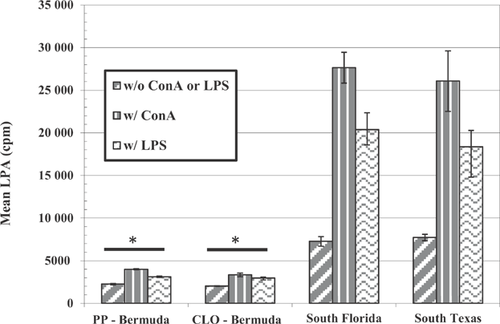
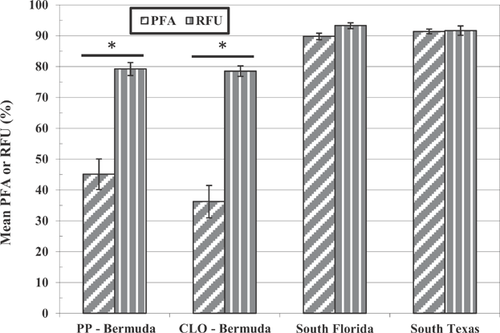
Mainland US R. marina
Comparisons of the splenocyte tissue density, splenocyte viability, lymphocyte proliferation, and phagocytic activity in mainland R. marina collected from southern Florida and Texas to unchallenged toads exposed to pond sediment from Bermuda are illustrated in Figures 1 through 4. Splenocyte tissue density in the Bermuda specimens (Figure 1; t test or Mann-Whitney U test, p < 0.05 for each comparison) and, to a lesser extent, splenocyte viability (Figure 2; t test, p < 0.05 for each comparison) were significantly less than what was found in the mainland toads from the United States. In addition, lymphocyte proliferation in the Bermuda toads was significantly reduced regardless of adjuvant administration relative to proliferation in mainland US toads (t test or Mann-Whitney U test, p < 0.05 for each comparison; Figure 3). Phagocytic activities in the Bermuda toads, regardless of method used or site, were also reduced compared with the phagocytic activities measured in US mainland toads (t test or Mann-Whitney U test, p < 0.05 for each comparison; Figure 4).
DISCUSSION
Results from the present study demonstrated that toads raised from early tadpole stage 20 through metamorphosis in the presence of pond sediments from Bermuda had reduced splenic white pulp content, splenocyte tissue densities, lymphocyte proliferation, and phagocytic activity compared with toads from the same clutch raised in control sediment. Exposure to the Bermuda site sediments also reduced the capacity of the toads to respond to an M. chelonae challenge. No effects on spleen somatic index or splenocyte viability were noted in either treatment scenario. Compared with the 2 North American toad populations, a marked reduction in splenocyte tissue density, lesser but significant reduction in splenocyte viability, and reduction in lymphocyte proliferation and phagocytic activities were observed in Bermuda toads raised in either control or site medium. Although the induction of lesions by M. chelonae appeared to be only slightly less severe in the North American R. marina, the North American toads responded to infection markedly better in terms of lymphocyte proliferation than Bermuda R. marina. Based on an assessment of peritoneal neutrophil viability and functionality, peritoneal neutrophils did not compensate for splenocyte dysfunction. Therefore, R. marina from Bermuda were functionally immunocompromised. Because R. marina in Bermuda are a captive population, the degree of inbreeding may be partially reflected in the general lower immune function compared with mainland US R. marina. Further toxicogenomic work is currently being performed to determine the degree of genetic isolation of the Bermuda population; however, the results from the present study clearly demonstrate that exposure to freshwater pond sediments from 2 Bermuda ponds reduced immune function compared to control exposure. These sediments contained elevated levels of metals and PAHs, which were detected in tissues from the exposed toads, sometimes at levels well above those detected in the sediments. Overall, a distinct relationship between immune function and environmental factors in Bermuda was identified in the present study.
Immune dysfunction is the latest effect identified in several biota from Bermuda. Bacon et al. 5 and Fort et al. 13, 14 described the presence of deformed adult, juvenile, newly metamorphosed, and larval R. marina across the island. Developmental deformities have also been identified in endemic killifish (Fundulus bermudae) 3, mosquitofish (Gambusia affinis) 3, and diamondback terrapins 15 in Bermuda. More recently, Fort et al. 4 report reproduction and endocrine dysfunction in surrogate fish, fathead minnow (Pimephales promelas) and killifish (Fundulus heteroclitus), exposed to freshwater and marine sediment from Bermuda. In an effort to determine the role of metals and PAHs in the sediments from Bermuda on developmental, reproductive, endocrine, and immune processes in developing R. marina, a sediment spiking study was performed (D.J. Fort and J.P. Bacon, unpublished data). The results suggested that both PAHs and metals play a role in the adverse effects identified including immune function.
As opposed to urodele amphibians that specifically utilize innate defense mechanisms to provide rapid but nonspecific protection from pathogens, anurans utilize both acquired (humoral) and innate immune defense processes 8, 16. Because both processes are important for maintaining health, it is necessary to evaluate both when considering immune function. The innate defense mechanisms of urodele and anuran amphibians include peptides, natural killer cells, and phagocytic cells 15. Christin et al. 6 described the use of the lymphocyte proliferation assay following adjuvant administration to monitor acquired immune function, whereas Froese et al. 8, Brousseau et al. 17, and Johnson et al. 18 each described the use of phagocytosis assays, including relative functional assays and oxidative burst measurements to assess innate defense mechanisms. Based on the results from the present study, acquired spleen-based immunity was compromised, but innate immune compensation from peritoneal neutrophils was observed.
Although the effects of pesticide exposure on immune function in cane toads were not implicated in Bermuda, these studies demonstrate the effects of environmental contaminants on immune function. A variety of environmental stressors, including physical stressors 19 and environmental contaminants 20-23, have been shown to cause a modulation in the immune system of anurans. Christin et al. 6 exposed juvenile leopard frogs (Lithobates pipiens) to a mixture of 6 pesticides (atrazine, metribuzin, aldicarb, endosulfane, lindane, and dieldrin) for 21 d, and then challenged them with a parasitic nematode, Rhabdias ranae. Exposure to the mixture significantly reduced lymphocyte proliferation but did not alter phagocytosis or the total number of splenocytes at the conclusion of the exposure. These results suggested that pesticides have the capacity to impair immune function of frogs and affect their ability to deal with parasitic infection.
Albert et al. 24 examined the relationship between dietary exposure to DDT and dieldrin and immunosuppression in the northern leopard frog (L. pipiens). Immune function was measured before, during, and after a 10-wk exposure phase. Adaptive and innate immunity responses were measured during each phase. Exposure to 75 ng DDT/g body weight or 2.1 ng dieldrin/g body weight resulted in significant immunosuppressive effects on antibody production and secondary delayed-type hypersensitivity. The low-dose results demonstrated that environmentally relevant concentrations of pesticides were capable of weakening the immune response of L. pipiens. Gilbertson et al. 25 utilized 3 assays, immunoglobulin M–specific antibody response, zymozan-induced chemiluminescence of whole blood, and delayed-type hypersensitivity, to evaluate the impact of pesticide exposure on humoral, innate, and cell-mediated immune endpoints. Malathion (990 ng/g wet wt), DDT (923 ng/g wet wt), and dieldrin (50 ng/g wet wt) reduced antibody response, increased delayed-type hypersensitivity reactions, and reduced oxidative burst. In addition, the present study found significant differences in immune function between frog populations in pesticide-exposed and pesticide-free locations. Overall, exposure to these pesticides caused both stimulation and suppression of immune function in field populations of frogs.
Most recently, Cary et al. 23 demonstrated that environmentally realistic concentrations of polybrominated diphenyl ethers were capable of altering immune function in frogs. However, further study is needed to determine how these alterations impact disease susceptibility in L. pipiens. Of perhaps more relevance to the present study, Rosenberg et al. 9 described the effects of lead on the immune system in Bufo arenarum. Phagocytic and lytic functions of blood cells collected from lead-injected toads and incubated with suspensions of Candida pseudotropicalis were impaired. Decreases in phagocytic activity were correlated with increased plasma lead levels.
CONCLUSIONS
Results from the present study demonstrated that R. marina raised in the presence of pond sediments from Bermuda had reduced immune function relative to toads from the same cohort raised in the presence of control sediment. Exposure to the pond sediments also reduced the capacity of the toads to respond to M. chelonae challenge. Compared with the 2 North American toad populations, toads from Bermuda were markedly immunosuppressed. Based on an assessment of peritoneal neutrophil viability and functionality, peritoneal neutrophils did not compensate for splenocyte dysfunction. Toads exposed to Bermuda sediment bioaccumulated metals and PAHs from the sediment during exposure. Although differences in immune function between Bermuda cane toads and US mainland cane toads are influenced by genetics, the results from the present study clearly demonstrate that exposure to metals and PAHs in sediment from Bermuda had an immunosuppressive effect on splenic immune function in R. marina.
Acknowledgment
This is Contribution #240, Bermuda Biodiversity Project, Bermuda Aquarium, Natural History Museum and Zoo, Department of Conservation Services. Funding for the present study was provided by the Bermuda government's Department of Environmental Protection, the Atlantic Conservation Partnerships, and the Bermuda Zoological Society (to J.P. Bacon and D.J. Fort). The present study was also supported by grants from the National Institute of Environmental Health Sciences (ES09098), the US Environmental Protection Agency (R825760), and the Virginia Community College System Professional Development (to D.W. Linzey). The authors also thank M. Outerbridge for assistance in collecting the sediment samples. The present study was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals.
Data availability
For access to the data associated with the present study, please contact Douglas J. Fort at [email protected].




