Long-term exposure to gold nanoparticles accelerates larval metamorphosis without affecting mass in wood frogs (Lithobates sylvaticus) at environmentally relevant concentrations
Abstract
Nanoparticles are environmental contaminants of emerging concern. Exposure to engineered nanoparticles has been shown to have detrimental effects on aquatic organisms. The authors synthesized gold nanoparticles (18.1 ± 3.5 nm) and tested their effects on time to and weight at metamorphosis in wood frog (Lithobates sylvaticus) tadpoles, a species known to be sensitive to environmental stressors. Continuous exposure to all concentrations of gold nanoparticles (0.05 pM, 0.5 pM, and 5 pM in particles) for up to 55 d significantly reduced time to metamorphosis by as much as an average of 3 d (p < 0.05). However, exposure to gold nanoparticles had no effect on tadpole mass at metamorphosis. The approximately 18-nm gold nanoparticles used were metastable in dechlorinated tap water, resulting in a change in surface charge and aggregation over time, leading to negatively charged aggregates that were on the order of 60 nm to 110 nm. Nanoparticle aggregation could exacerbate the effect on time to metamorphosis. To the authors' knowledge, the present study is the first report on the effect of engineered nanoparticles of any kind on life-history variables in an amphibian, a taxonomic group that has been declining globally for at least 25 yr. Environ Toxicol Chem 2016;35:2304–2310. © 2016 SETAC
INTRODUCTION
Amphibian populations have undergone a global decline in the past 25 yr 1-3. Potential causes for their demise have included infectious diseases, global climate change, ultraviolet (UV) radiation, and aquatic pollution 4. In the latter case, several studies have shown clear detrimental effects on life-history variables by municipal effluent 5, pesticides and herbicides 6-8, road salt 9, fertilizers 10, heavy metals 11, and active pharmaceutical ingredients 12-15. Among those aquatic contaminants of emerging concern 16-19, engineered nanoparticles have received a considerable amount of recent attention 20-25; but information on their effects on amphibian life cycles is still lacking, even though the tadpole larvae are as likely as any aquatic organism to be exposed to nanoparticles. Indeed, since amphibians have a semiterrestrial life cycle, the adult stages could be exposed to nanoparticles in soil and water.
Relatively few studies of effects by nanoparticles on amphibians have been published; those studies have primarily utilized tadpoles of the African clawed frog (Xenopus laevis) as a model organism using carbon nanotubes, nanoparticles of cerium oxide (CeO2) 26, silver (Ag) 27, or copper(II) oxide (CuO), titanium dioxide (TiO2), and zinc oxide (ZnO) 28-31. Gold nanoparticles (AuNPs) are commonly used in the production of electronics, paints, and cosmetics 32, as well as vehicles in drug delivery 33, 34. Their toxicity has been tested in freshwater crustaceans 35, bivalves 32, 36, and rainbow trout 37, as well as their transfer through food webs 38-41. (See a recent review by Lapresta-Fernandez et al. 42.)
Despite the growing number of applications for engineered nanoparticles, studies measuring environmental concentrations are lacking. Predictions of potential environmental concentrations have been made: estimates for engineered AuNPs originating from consumer products are 0.1 μg/L in water and 5.99 μg/kg in soil 43-45.
Since the widespread use of AuNPs will undoubtedly continue into the future, their persistence in the environment will as well; and studies testing their toxicity in soil, air, and water will be of critical importance. Given the sensitivity of aquatic organisms, including amphibians, to environmental stressors, and in light of their overall global decline, the impact of AuNPs on vulnerable life stages needs to be addressed. The present study represents the first on the toxic effects of AuNPs on any amphibian.
MATERIALS AND METHODS
Synthesis of AuNPs
Cetyltrimethylammonium bromide (CTAB)-capped AuNPs were synthesized via the seed-mediated approach 46, 47. Briefly, small Au seeds (∼1.5 nm) were synthesized via a reduction of chloroauric acid (HAuCl4) with a strong reducing agent, sodium borohydride (NaBH4), in the presence of CTAB. In a separate flask, 95 mL of CTAB was combined with 5 mL of 10 mM HAuCl4 and 100 μL of 10 mM silver nitrate (AgNO3). To this flask, 550 μL of 100 mM ascorbic acid was added, which changes the color of the solution from reddish-orange to colorless, corresponding to a change in the oxidation state of the Au from +3 to +1. Once the Au solution was reduced, 120 μL of the seed solution was added and the flask gently swirled and then left to sit for at least 2 h. In the resulting red solution, the color is indicative of the formation of AuNPs, and this was then centrifuged at 14 000 g for 20 min. The pellet was collected and resuspended in 18.2 MΩ water to the original volume and then subjected to a second centrifuge cycle. The pellet was then collected and mixed with pellets from other batches. The volumes of AuNPs for this experiment required many 100-mL batches to be synthesized independently and then mixed together to form the final stock solution (500 pM) that would be used for the exposure experiments.
Characterization of AuNPs
Preliminary characterization of the AuNPs was achieved with a Jasco V-670 UV-visible (UV-vis) spectrometer. A symmetrical peak near 520 nm is indicative of high-quality nanoparticles (narrow size distributions and low incidences of nonspherical particles) 48. Batches of nanoparticles that had asymmetric peaks (excessive polydispersity) or peaks that were too far red-shifted (larger particles) were discarded and not included in the mix. The concentrations of the AuNP stock solution were determined with Beer's law and a calculated molar absorptivity based on the absorption maximum. Note that this method returns a concentration of the nanoparticles in molarity where the nanoparticles are the unit of measure. The size distribution of the stock Au solution was determined with a Zeiss EM-109 transmission electron microscope (TEM) operating at 80 keV. Stock solution (5 μL) was placed on a formvar grid and allowed to dry. Images were acquired at ×30 000, and 100 particles were measured using ImageJ (National Institutes of Health).
A 10-fold dilution of the AuNP stock in dechlorinated tap water was characterized with zeta potential, dynamic light scattering, and UV-vis. The zeta potential and dynamic light scattering measurements were acquired on a Malvern Zetasizer Nano ZS-90.
Experimental design
Wood frog (Lithobates sylvaticus) eggs from at least 2 individual egg masses were collected in April 2013 from a series of adjacent vernal ponds in Michaux State Forest (Adams County, PA, USA; 39.91°N, 77.56°W) and immediately transported (20 min) in pond water to the lab at Gettysburg College. Egg masses were placed in 10-gallon aquaria initially supplied with water from their ponds. After 24 h, room-temperature dechlorinated (Stress Coat; Aquarium Pharmaceuticals) tap water was added to the eggs to create a 50:50 mixture of pond water:dechlorinated tap water. Thereafter, egg masses were maintained in 100% dechlorinated tap water (pH 7.5, dissolved oxygen 7.2 ppm, temperature 21 °C, conductivity 772 μS/cm) until hatching. Previous experiments 13 have shown that these eggs and resulting tadpoles survive well in dechlorinated tap water. Hatching from all egg masses occurred spontaneously within 4 d of collection. Tadpoles at Gosner 49 stage 24–25 (viewed under a dissecting microscope) were carefully removed and pooled together before being randomly assigned to experimental aquaria. We used 3-L plastic aquaria as units of replication with 5 tadpoles in each aquarium. Thus, there were 4 aquaria for each treatment group.
We established 5 groups of tadpoles: a control group containing dechlorinated tap water that lacked any AuNPs, a CTAB-only control (50 nm), and 3 treatment groups based on Au concentrations (0.05 pM, 0.5 pM, and 5 pM). Each aquarium had an initial volume of 2000 mL of test medium. When a tadpole metamorphosed, it was removed, and the volume in the aquarium was reduced by 400 mL. In 1 case (1 of the high-concentration aquaria), we removed and did not replace a tadpole that was not developing normally and thus reduced the volume by 400 mL. Fresh medium was added twice per week. At each medium change, tadpoles were carefully netted out and transferred to a holding tank containing dechlorinated tap water for no longer than 2 min while new medium was added to their containers. To 1800 mL of dechlorinated tap water, we added 200 mL of control vehicle or AuNPs at a 10 times higher concentration than the final concentration.
Tadpoles were cultured until metamorphosis, at which time we recorded the number of days to metamorphosis and the fresh mass of each froglet. Data on time to and mass at metamorphosis were analyzed by one-way analysis of variance (ANOVA) followed by Fisher's least significant difference test. We confirmed normality of data by the Shapiro-Wilks test and homogeneity of variance by Bartlett's test. Null hypotheses were rejected at p < 0.05.
RESULTS
Physical characteristics of AuNPs
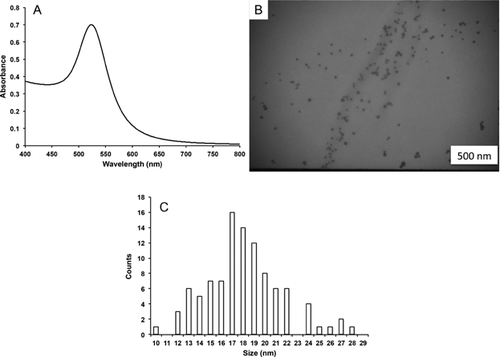
The stock solution of AuNPs displayed a symmetrical absorption peak at 523 nm that is consistent with relatively monodisperse AuNPs (Figure 1A). The TEM images of the particles were consistent with the UV-vis data in that the particles were monodisperse (Figure 1B). An analysis of TEM images of CTAB-capped AuNPs resulted in an average size of 18.1 ± 3.5 nm (see the histogram in Figure 1C).
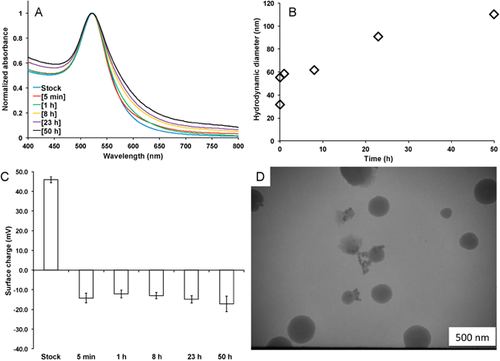
To more accurately characterize the particles that the tadpoles will be experiencing, the physiochemical properties of the AuNPs were monitored over the course of 50 h. Because of the plasmon sensitivity to aggregation, UV-vis spectroscopy is a convenient way to monitor changes in the aggregation state. A 10-fold dilution of the AuNP stock solution in dechlorinated tap water was accompanied by a slight red-shift and broadening of the absorption peak in the UV-vis spectra, as shown in Figure 2A. The shift in the absorption maximum coupled with the peak broadening is indicative of slow aggregation of the AuNPs. Dynamic light scattering was used to further study the kinetics of aggregation. The initial hydrodynamic radius of the AuNPs was approximately 35 nm but quickly increased after mixing with the dechlorinated tap water to approximately 60 nm after 5 min (Figure 2B). Aggregation continued over the 50-h time course, resulting in aggregates that had a hydrodynamic diameter of approximately 110 nm. In addition, the surface charge of the nanoparticles changed from strongly positive (approximately +45 mV) to negative (approximately –10 mV),within the first 5 min of mixing with the dechlorinated tap water (Figure 2C). The surface charge remained fairly stable and negative over 50 h, which is approximately equal to the experimental exposure time. Typically, zeta-potential measurements with a magnitude of less than approximately 20 mV indicate that the particles are metastable, which is consistent with the observed aggregation. As a final confirmation of the particles' aggregation state, TEM was used to examine particle morphology after exposure to dechlorinated tap water (Figure 2D). As seen in Figure 1B, the AuNPs were relatively well dispersed and showed very few aggregates before exposure to dechlorinated tap water. After approximately 50 h, the nanoparticle aggregates were pronounced, and there was evidence of some additional impurity, most likely from the stress coat.
Effect of AuNPs on survivorship and mass at and timing of metamorphosis in tadpoles
All experimental tadpoles survived until metamorphosis. Furthermore, we found no differences in malformations or mortality between treatments of tadpoles exposed to varying Au concentrations and controls.
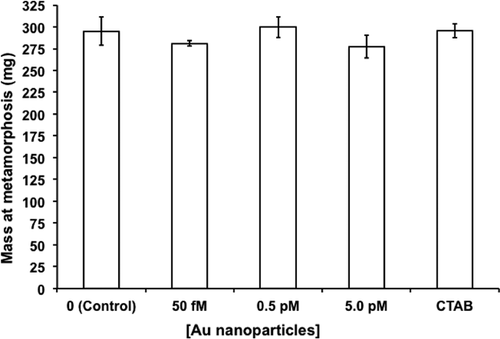
Tadpole mass at metamorphosis did not differ significantly across treatments (one-way ANOVA, F4, 15 = 0.76, p = 0.56; Figure 3). Mean values ranged from 277.45 mg in 5 pM AuNPs to 299.83 mg in 0.5 pM AuNPs. By contrast, AuNP concentration significantly shortened mean time to metamorphosis (one-way ANOVA, F4, 15 = 6.86, p < 0.002; Figure 4). All 3 pairwise comparisons of AuNP concentration versus water control were significantly different (p < 0.01 for 0.05 pM and 0.5 pM vs control, p < 0.05 for 5 pM vs control). Tadpoles exposed to 0.5 pM AuNPs took on average 42.53 d to metamorphose, whereas water controls averaged almost 46 d. Furthermore, tadpoles in the 0.5-pM group metamorphosed significantly faster than those in the CTAB control (p < 0.05).

DISCUSSION
Ecotoxicological studies of AuNPs on aquatic organisms have included those on uptake and depuration in freshwater crustaceans 35, root uptake by freshwater macrophytes 50, oxidative stress and lysosomal membrane instability in marine mussels 36, mortality in freshwater algae and oxidative stress in freshwater bivalves 32, hepatocyte cytotoxicity in rainbow trout 37, and trophic transfer 38-41.
Recent studies have documented the toxic effects of engineered nanoparticles on amphibians. Mouchet et al. 51 found that very high concentrations (10–500 mg/L) of double-wall carbon nanotubes blocked the gills and digestive tract of African clawed frogs (X. laevis). Exposure to iron(III) oxide (Fe2O3), TiO2, ZnO, and CuO nanomaterials caused developmental abnormalities in X. laevis embryos at high (1000 mg/L) concentrations; and CuO and ZnO nanomaterials caused gastrointestinal and spinal abnormalities at 3.16 mg/L and inhibited growth at 10 mg/L 30. Bacchetta et al. 28 exposed embryos of X. laevis to CuO2, TiO2, and ZnO nanoparticles at concentrations up to 500 mg/L and found significant developmental malformations of the gut. The ZnO nanoparticles caused the most serious lesions to the intestinal lining, some of which penetrated underlying tissues. Bour et al. 26 reported mortality of X. laevis tadpoles at high (100 mg/L) and growth inhibition at moderate (1.0 mg/L) concentrations of CeO2 nanoparticles. Salvaterra et al. 52 found significant disruption in a number of biochemical endpoints, including catalase and lactate dehydrogenase levels, in Pelophylax perezi tadpoles exposed to titanium silicate nanoparticles.
In the present study, we found that exposure to AuNPs affects the timing of metamorphosis in wood frog tadpoles. Time to metamorphosis of tadpoles exposed to the medium Au concentration (0.5 pM) was significantly shortened compared with both water-only and CTAB control groups. Our lowest-observed-effect concentration (LOEC, 0.05 pM) significantly shortened the time to metamorphosis compared with the water-only control without affecting tadpole mass. Our mass values at metamorphosis are in agreement with those recorded in our lab by Carfagno and Fong 13 for wood frog tadpoles. Previous studies on the effects of toxicants on the timing of metamorphosis have shown reduced body mass concomitant with early metamorphosis in X. laevis tadpoles when exposed to antidepressants 14. The authors of that study suggest that antidepressants induced a reduction in foraging behavior and food intake and that tadpoles may have been chemically “tricked” into early metamorphosis. Other studies have shown accelerated metamorphosis in cricket frog tadpoles exposed to pesticides 53 and in wood frog tadpoles exposed to road salt 9.
Timing of metamorphosis is of special importance for those species that breed in ephemeral habitats 54, such as those of the wood frog. Trade-offs between the timing of larval metamorphosis and the growth and survival of postmetamorphic froglets are well documented. The advantages of such trade-offs depend on environmental conditions such as harsh weather, pond desiccation, food availability, competition, and predation 53, 55. Shorter time to metamorphosis may confer advantages by allowing tadpoles to avoid becoming trapped in a shrinking ephemeral water body or by escaping aquatic predators or intensifying competition 55-57. The disadvantage of accelerated metamorphosis could be in the production of smaller and less mature froglets, which are subject to greater predation risk 58. Smaller size at metamorphosis could lead to reproductive delays and smaller size at sexual maturity 55. Exposure to toxicants in combination with natural stressors, such as competition, predation, and environmental fluctuations, has been shown to affect life-history variables in aquatic organisms including anurans 59-62.
Metamorphosis in anurans is controlled by hypothalamic release of corticotropin-releasing hormone, which in turn regulates the release of thyroid hormone 63. Silver nanoparticles have been shown to disrupt thyroid hormone signaling in X. laevis during metamorphosis 27. In addition, Hinther et al. 64 reported that gene expression of thyroid hormone transcripts was altered by both nanosilver (≥2.75 mg/L) and quantum dots (≥0.22 mg/L) in bullfrog tadpoles. Furthermore, other types of nanoparticles have been shown to impact thyroid hormone regulation in aquatic vertebrates. Titanium dioxide nanoparticles, in combination with the flame retardant polybrominated diphenyl ether decaBDE, have been shown to disrupt the hypothalamic–pituitary–thyroid system in larval zebrafish 65. Miao et al. 66 found that TiO2 nanoparticles enhanced the uptake of lead and decreased the levels of thyroid hormone in zebrafish larvae. Mouchet et al.'s 51 observation of carbon nanotubes in the digestive tract of X. laevis tadpoles supports an ingestive mode of uptake. The present finding of accelerated time to metamorphosis is likely not related to reduced food intake 14 since there was no difference in mass at metamorphosis. How AuNPs impact the timing of metamorphosis is unknown; but elucidation of the mechanisms of action requires knowledge of the route of uptake, and we are currently investigating this in several different anuran species.
It has been shown that positively charged nanoparticles tend to be more toxic than those that are negatively or neutrally charged 67. Although we did not observe any acute toxicity with the nanoparticle dosages utilized in the present experiment (1.2 trillion particles/tadpole), it is important to note that the surface charge influences both the aggregation state and the surface chemistry that the organism/cell experiences. Previous studies have established that AuNPs aggregate in a variety of media and that the uptake was not correlated to the size of the aggregates (67.1–178.8 nm) for the amphipod Gammarus pulex 68. Nonetheless, it is of critical importance to understand that the identity of the nanoparticles characterized in the lab under tightly controlled conditions will not be the same as that of nanoparticles introduced into a different chemical environment, which changes how the particles will impact a particular ecosystem. The present results show that the nanoparticles do indeed undergo some degree of aggregation in the dechlorinated tap water used in the present experiments; and even in the relatively controlled situation in which these experiments were conducted, there was a complexity to the suspending medium that was out of our control. There is a time component to the aggregation, and the physiochemical identity of the particles experienced by the organisms at any given moment is transient, which is likely to be an accurate model for particle behavior in the natural environment.
There are no measured environmental concentrations for AuNPs, but the predicted concentrations in water are on the order of 0.1 μg/L 44. Thus, our LOEC of 0.05 pM (calculated to be 2.5 μg/L) is in the range of environmentally predicted concentrations and lower than the effective concentrations of AuNPs investigated in many published studies. Most studies of nanoparticle toxicity on aquatic organisms have utilized short-term (days) exposure. Such short-term exposure limits the types of questions than can be asked as well as the type of organisms that can be tested. To our knowledge, our exposure time (up to 55 d) is the longest continuous exposure time used for AuNPs tested on any aquatic species; thus, we addressed questions concerning important life-history variables of longer-lived species or species with an extended larval life.
Acknowledgment
We thank T. Bury for help with the experiments and the Office of the Provost of Gettysburg College for funding. The experimental protocols were approved by the Gettysburg College Institutional Animal Care and Use Committee. We also thank 2 anonymous reviewers for helpful comments.
Data availability
Data, associated metadata, and calculation tools may be requested from the corresponding author ([email protected]).




