Determination of silicone rubber and low-density polyethylene diffusion and polymer/water partition coefficients for emerging contaminants
Abstract
There is a growing interest in assessing the concentration and distribution of new nonregulated organic compounds (emerging contaminants) in the environment. The measurement of freely dissolved concentrations using conventional approaches is challenging because of the low concentrations that may be encountered and their temporally variable emissions. Absorption-based passive sampling enables the estimation of freely dissolved concentrations of hydrophobic contaminants of emerging concern in water. In the present study, calibration was undertaken for 2 polymers, low-density polyethylene (LDPE) and silicone rubber for 11 fragrances, 5 endocrine-disrupting compounds, 7 ultraviolet (UV) filters, and 8 organophosphate flame retardant compounds. Batch experiments were performed to estimate contaminant diffusion coefficients in the polymers (Dp), which in general decreased with increasing molecular weight. The values for fragrances, endocrine-disrupting compounds, and UV filters were in ranges similar to those previously reported for polycyclic aromatic hydrocarbons, but were 1 order of magnitude lower for organophosphate flame retardant compounds. Silicone rubber had higher Dp values than LDPE and was therefore selected for further experiments to calculate polymer/water partition coefficients (KPW). The authors observed a positive correlation between log KPW and log octanol/water partition coefficient values. Field testing of silicone rubber passive samplers was undertaken though exposure in the River Alna (Norway) for an exposure time of 21 d to estimate freely dissolved concentration. Some fragrances and UV filters were predominant over other emerging and regulated contaminants, at levels up to 1600 ng L−1 for galaxolide and 448 ng L−1 for octocrylene. Environ Toxicol Chem 2016;35:2162–2172. © 2016 SETAC
INTRODUCTION
Emerging organic contaminants represent a group of compounds spanning a wide range of classes of chemicals with different physicochemical properties 1. While the occurrence and widespread distribution of some of these chemicals have been demonstrated, most of them are not yet regulated. Because personal care products—including fragrances and ultraviolet (UV) filters—and organophosphate flame retardants are heavily used in consumer goods, relatively high rates of emission into the environment are expected 2-4. Some of these chemicals are increasingly gaining attention as a result of their relatively high lipophilicity, low water solubility, and relative stability against biotic degradation. These substances are continuously released into the aquatic environment mainly through treated and nontreated wastewater and landfill effluents 2, as well as recreational activities 5, with minimal control of emissions. Some of these compounds have been shown to be relatively persistent, and their presence in remote environments such as the Arctic has been observed 6. Their continuous emissions to the environment, low (bio) degradation rates, and hydrophobicity (octanol/water partition coefficient [log KOW] > 3) mean they can also be considered as pseudo-persistent organic pollutants 7. As an example, polycyclic fragrances are included in this wide group, all of them with a log KOW greater than 5 (Table 1), and they have been detected at levels up to 2200 ng g−1 (wet wt) in fish taken adjacent to sewage treatment plants 8. Concentrations of these substances can vary spatially and seasonally (ranging from pg L−1 to μg L−1). For example, concentrations up to 1092 ng L−1 have been reported for organophosphate flame retardants—including tributyl phosphate—in a tributary river of the German Bight 1. Up to 1300 ng L−1 of galaxolide and 7301 ng L−1 of octocrylene have been measured in coastal waters from San Francisco Bay (CA, USA) 3 and Oslofjord (Norway) 5, respectively.
| Log Dp | |||||
|---|---|---|---|---|---|
| Compounds | MW | Log KOW | Log KPW measured (L kg−1) | Silicone rubber (m2 s−1) | LDPE (m2 s−1) |
| Fragrances | |||||
| Musk ketone (MK) | 294.3 | 4.3b | 3.05 (0.04) | –10.84 (0.1) | –12.47 (0.3) |
| Musk xylene (MX) | 297.2 | 4.8b | 3.31 (0.03) | –10.72 (0.06) | –12.38 (0.17) |
| Musk tibetene (MT) | 266.2 | 5.9b | 4.15 (0.02) | –10.99 (0.08) | –12.74 (0.13) |
| Musk ambrette (MA) | 268.2 | 5.7b | 3.91 (0.07) | –10.75 (0.02) | –12.23 (0.1) |
| Galaxolide (HHCB) | 258.4 | 5.9b | 3.97 (0) | –10.82 (0.09) | –12.95 (0.05) |
| Tonalide (AHTN) | 258.4 | 5.7b | 4.01 (0.03) | –10.95 (0.13) | –13.00 (0.02) |
| Celestolide (ADBI) | 244.3 | 5.9b | 3.96 (0.008) | –10.85 (0.08) | –12.77 (0.14) |
| Traseolide (ATII) | 258.4 | 6.3b | 4.36 (0.01) | –10.82 (0.09) | –12.90 (0.18) |
| Phantolide (AHMI) | 244.3 | 5.9b | 3.78 (0.002) | –10.66 (0.05) | –12.84 (0.22) |
| Cashmeran (DPMI) | 206.3 | 4.9b | 3.46 (0.09) | –10.68 (0.04) | –12.45 (0.06) |
| Tetramethyl acetyloctahydronaphthalene (OTNE) | 234.3 | 5.28c | 3.63 (0.02) | –10.76 (0.1) | –12.85 (0.15) |
| Endocrine-disrupting compounds | |||||
| Triclosan (TCS) | 289.5 | 4.76d | 3.02 (0.13) | –10.88 (0.12) | –12.58 (0.16) |
| Methyl triclosan (MTCS) | 303.5 | 5.22c | 3.62 (0.007) | –10.73 (0.07) | –13.03 (0.09) |
| Irgarol | 253.3 | 4.07c | 3.60 (0.01) | –10.61 (0.21) | –12.41 (0.18) |
| Nonylphenol (NP) | 220.3 | 5.9c | 3.49 (0.17) | –10.96 (0.31) | –12.03 (0.23) |
| Octylphenol (OP) | 206.3 | 5.5c | 3.28 (0.17) | –10.88 (0.12) | –11.93 (0.08) |
| UV filters | |||||
| 2-Ethylhexyl methoxycinnamate (EHMC) | 290.4 | 5.77c | 4.77 (0) | –11.16 (0.2) | –14.69 (0.3) |
| 4-Methylbenzylidene camphor (4-MBC) | 254.3 | 4.95c | 3.39 (0.03) | –11.83 (0.12) | –12.77 (0.18) |
| 2-Ethylhexyl salicylate (EHS) | 250.3 | 5.77c | 4.70 (0.09) | –10.83 (0.1) | –12.31 (0.16) |
| Homosalate (HMS) | 262.3 | 5.82c | 4.55 (0.09) | –10.81 (0.04) | –12.73 (0.23) |
| Benzophenone-3 (BP-3) | 228.2 | 3.64c | 3.08 (0.02) | –10.87 (0.13) | –12.03 (0.01) |
| 2-Hydroxybenzophenone (2-OHBP) | 198.2 | 3.47c | 3.04 (0.09) | –10.71 (0.12) | –12.00 (0.07) |
| Octocrylene (OC) | 361.4 | 7.53c | 4.96 (0.11) | –11.36 (0.13) | –14.39 (0.41) |
| Organophosphate flame retardants | |||||
| Tris-isobutylphosphate (TIBP) | 266.3 | 3.71c | 4.68 (0.02) | –11.52 (0.22) | < –15 |
| Tris-n-butylphosphate (TBP) | 266.3 | 4.26c | 5.00 (0.07) | –12.80 (0.2) | < –15 |
| 2-Ethylhexyldiphenylphosphate (EHDPP) | 362.4 | 5.73c | 5.39 (0.12) | –11.38 (0.24) | < –15 |
| Triphenylphosphate (TPP) | 326.2 | 4.7c | 4.94 (0.02) | –14.52 (0.24) | < –15 |
| Tris-2-ethylhexylphosphate (TEHP) | 434.6 | 9.49c | 5.88 (0.2) | –12.03 (0.11) | –14.39 (0.13) |
| Tris-o-tolylphosphate (ToTP) | 368.3 | 6.3c | 5.50 (0.11) | –11.69 (0.23) | –13.61 (0.26) |
| Tris-m-tolylphosphate (TmTP) | 368.3 | 6.3c | 5.77 (0.13) | –11.96 (0.26) | –13.61 (0.18) |
| Tris-p-tolylphosphate (TpTP) | 368.3 | 6.3d | 5.85 (0.08) | –11.91 (0.18) | –13.79 (0.2) |
- a Properties are: molecular weight (MW), logarithm octanol/water partition coefficient (log KOW), measured logarithm polymer/water coefficients (log KPW; L kg−1) for silicone rubber, and estimated logarithm diffusion coefficients (log Dp; m2 s−1) for silicone rubber and low-density polyethylene (LDPE). Values in parentheses represent the error in log units (n = 2).
- b Octanol/water coefficients were obtained from Posada-Ureta et al 40.
- c Octanol/water coefficients were obtained from the Chemspider database 41.
- d Octanol/water coefficients were obtained from the Scifinder database 42.
The assessment of the risk these substances pose to the environment requires knowledge of freely dissolved concentrations in water and sediments, because these drive contaminant movement and bioaccumulation 4. For most of these emerging contaminants, absorption-based passive sampling represents an alternative to traditional sampling. The advantages of passive sampling are the ability to provide time-integrated information for periods of weeks to months and suitably low limits of detection. Because absorption-based passive sampling devices can also be used to measure freely dissolved concentrations in sediments or biota, concentration or activity gradients can be assessed to study the interaction between different environmental compartments 4, 9. So far, different types of passive samplers have been used for monitoring hydrophobic compounds in the environment. These include 2-phase samplers such as semipermeable membrane devices 10, and single-phase polymeric devices made from polydimethylsiloxane 11, polyethylene 12, 13, or silicone rubber 14, 15. Most of them have been used for sampling polycyclic aromatic hydrocarbons (PAHs) 14, polychlorinated biphenyls (PCBs) 16, polybrominated diphenyl ethers, and chlorinated pesticides 12. However, only a handful of studies have focused on UV filters 9, synthetic musks 8, or triclosan by using polyethylene 13, 17. Calibration of polymers used for passive sampling is required to relate freely dissolved contaminant concentrations from masses absorbed in the sampler during exposure. For single-phase polymers, this involves the measurement of polymer/water partition coefficients (KPW) and diffusion coefficients (Dp) for substances of interest 18, 19. Although many calibration studies have been undertaken for substances such as PAHs and PCBs, for low-density polyethylene (LDPE) and silicone rubber 19, 20, calibration data are seldom available for emerging contaminants.
The main goal of the present study was to determine KPW and Dp values in the laboratory for 31 emerging contaminants that include fragrances, UV filters, and organophosphate flame retardants, among others. Because these substances are nonionized in water at ambient pH and have log KOW values > 3, we expected they would be amenable to passive sampling with silicone rubber and LDPE 10. Measurements of KPW and Dp were performed using established methods. The measurement of KPW was undertaken using a cosolvent method 18, while a film-stacking procedure was used to estimate Dp in the polymers 11, 15. In addition, the performance of the newly calibrated passive samplers was evaluated through a field exposure of 21 d in the Alna River (Oslo, Norway).
MATERIALS AND METHODS
Theory
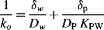 (1)
(1)Equilibrium between the concentration in water and that in the sampler is reached when a sampler is deployed for a sufficiently long period. If that is the case, then KPW is the only parameter needed to estimate freely dissolved concentration. However, when equilibrium is not attained, knowledge of deployment-specific in situ sampling rate is needed. Contaminant uptake rate can be limited by transport in the membrane (generally for chemicals with low KPW or Dp or for very thick polymeric membranes) or by transport through the water boundary layer. For example, PAHs and PCBs are, in most cases, expected to be under water boundary layer-controlled uptake when silicone rubber is used 20, while membrane control of the uptake has been shown for the least hydrophobic PAHs when LPDE is used 24. Performance reference compounds (PRCs) are deuterated chemicals spiked into the polymer prior to deployment that can dissipate from the samplers during exposure. Because of isotropic exchange of chemicals between the polymer and water, first-order PRC dissipation kinetics can be used to assess in situ sampling rates. Models have been developed to extrapolate sampling rates from the dissipation kinetics of a limited number of PRCs. For boundary layer–controlled uptake conditions (e.g., when using silicone rubber), the sampling rate, according to theory, is proportional to Dw2/3, leading to a small decrease in the sampling rate with increasing molecular weight of the compound or KOW 24. It was also shown that an increase in sampling rate can be expected with increasing log KOW for membrane-controlled uptake of the least hydrophobic PAHs into LDPE until the water boundary layer becomes the rate-limiting factor for higher KPW 24. Passive sampler calibration data for substances of interest will inform us on whether (under commonly encountered environmental conditions) passive sampling of these substances is expected to be under the control of membrane, partial, or water boundary layer uptake. This is important because it ensures that the appropriate model to estimate sampling rate from PRC data is used.
Materials and reagents
Methanol, n-pentane, acetone, dichloromethane, and ethyl acetate (purchased from Rathburn) and cyclohexane (purchased from J.T. Baker) were of chromatography quality. Milli-Q water was from an ultrapure water system (Millipore, Option 3; ElgaTM); it was used for rinsing glassware and for the preparation of solutions for the batch experiments. The PTFE centrifuge filters (0.22-µm pore size) were purchased from National Scientific and used to remove interference in the extracts. Anhydrous sodium sulfate was from Panreac. Altesil™ translucent silicone rubber (500 μm nominal thickness) was purchased from Altecweb and LDPE (90-μm thickness) from Brentwood Plastics. Glassware was rinsed with ultrapure water and baked in a muffle furnace at 540 °C before use. Analytical-grade standards were obtained from Dr. Ehrenstorfer, Sigma-Aldrich, LGC Standards, and Chiron (see Supplemental Data, Section S1). Stock solutions of these analytes were prepared in acetone and stored at –20 °C in tightly closed amber vials.
Polymer sheet preparation
Prior to use, the AlteSil silicone rubber was pre-extracted with ethyl acetate in a Soxhlet extractor for 72 h and further immersed in methanol for 1 d to remove impurities; LDPE membranes were precleaned by soaking in n-pentane overnight 16. After that, sheets were wiped with a lint-free tissue and air-dried in a fume hood until the solvent was evaporated completely (overnight).
For measurement of diffusion coefficients, polymer layers were cut into sheets measuring 7 cm × 5 cm for LDPE with a thickness of 90 μm and 6 cm × 2.5 cm for the silicone rubber with a membrane thickness of 500 μm, with all the strips used for the measurements belonging to the same batch. For the KPW experiments, only silicone rubber was used as polymer material, and masses between 0.2 g and 2 g were used. Following cutting, LDPE and silicone rubber sheets were further soaked overnight with n-pentane and methanol, respectively. Polymer sheets to be used unspiked were wiped with a lint-free tissue to remove any solvent and left to dry in a fume hood overnight before storage in a clean glass jar.
A method modified from Booij et al. 25 using methanol–water was used to load contaminants into the spiked sheets for both measurements (Dp and KPW). Polymer sheets were soaked in methanol containing chemicals of interest to obtain a nominal concentration of 300 ng of each single chemical per polymer sheet used for the diffusion coefficients measurements; and different amounts of analytes for the KPW measurements. Initially, a closed amber bottle with 100% of methanol spiked with the target analytes and the polymer sheet was kept agitated for 3 h. Ultrapure water was then gradually added every 6 h to obtain different methanol–water ratios (80/20, 50/50, and 20/80; v/v). Finally, the bottle with the methanol–water solution (20/80; v/v) and the sheet was shaken overnight (total duration: 4–6 d). Differences in contaminant concentrations between spiked sheets were below 5%. Polymer sheets were removed from solution, wiped with a lint-free tissue, and left to dry in a fume hood to ensure no solvent was left (overnight). These sheets were then placed in a clean jar stored at –20 °C until further use.
Diffusion coefficient measurements
Contaminant diffusion coefficients were measured both in LDPE and in silicone rubber using a film-stacking method 26. This method involves bringing into contact a polymer sheet spiked with compounds of interest with unspiked polymer sheets. Diffusion coefficients are estimated from the movement of the chemical into successive superimposed polymer layers over time.
In the present set of experiments, the film stack was composed of 1 spiked sheet sandwiched by 2 unspiked sheets placed on either side. The film stack was then wrapped in clean aluminum foil to minimize contamination and losses of chemicals to the air or other surfaces they may be in contact with and kept firmly in contact by applying sufficient pressure (1 kg cm−2), by placing a determined weight over the stack. The amount of pressure applied was selected to be consistent with previous work by Rusina et al. 11, who used similar pressure when using a film-stack to determine polymer diffusion coefficients for PAHs and PCBs in LDPE and silicone rubber. The experiment was conducted in duplicate and at constant room temperature (20 °C).
The contact times selected for the diffusion coefficients measurements were 4 h for silicone rubber and 12 h for LDPE. These periods were selected in accordance with other studies with other hydrophobic compounds 11. The experiment with LDPE for organophosphate flame retardants was repeated for 24 h. Once the experiment was terminated, sheets were analyzed separately. Unused spiked sheets were analyzed to determine initial concentrations of the analytes. Blanks were also analyzed to measure possible contamination during the extraction or from the manufacturing industry.
Based on Fick's second law describing the diffusion process, an analytical solution to the diffusion equation was developed using the Laplace transform 27, where a finite system and appropriate boundary conditions were considered. In this approximation, the concentration of the compound, thickness of the layers, and diffusion time were taken into account. The estimation of Dp was performed by fitting the model to the experimental data. Best-fit log Dp values were obtained by optimization based on the minimum in the root mean square deviation (RMSD) between measured and estimated concentrations (Supplemental Data, Section S2).
KPW measurements
Polymer/water partition coefficients were measured only for silicone rubber. The cosolvent method (with methanol as the cosolvent) applied by Smedes et al. 18 for determining KPW for PAHs and PCBs was used for our target analytes. In short, the KPW values obtained with 0% methanol (ultrapure water only) are validated against the data obtained using the cosolvent method. Different masses of polymer and amounts of analytes were used depending on the fraction of methanol in the water–methanol mixture used for KPW measurement. Silicone rubber masses used were between 0.2 g and 2 g, and the sizes of the amber bottles used in these experiments ranged from 0.1 L to 2 L, all of them with screw caps (aluminum-lined).
The fraction of methanol in the methanol–water solution ranged from 0% to 50% (v/v), (Supplemental Data, Section S3). The experiments were performed in duplicate. All bottles were shaken for 6 wk 13 using orbital shakers operating at 110 rpm (Heidolph) in amber glass bottles to avoid possible analyte photodegradation and at constant room temperature (20 °C). At the end of the experiment, water and methanol–water solutions and polymer sheets were extracted and analyzed separately to determine KPW values.
Field study
Complementary to the laboratory experiments, a passive sampler deployment was carried out for 3 wk in July 2013 at 1 site (59°54́16″N, 10°47́31″E) in the Alna River, a small river flowing through Oslo before release into the Oslofjord. AlteSil silicone rubber sheets (91–2.5 cm) were used for this river deployment. The silicone rubber strips were transported to the sampling site in a cool box, packed in sealed metal tins 16. Duplicate strips were deployed for 21 d using stainless steel canisters (n = 2 per sampling period), placed approximately 1 m below the water surface and parallel to the water flow. Field control samplers from the same preparation batch were used to evaluate possible contamination during sampler transport and deployment/retrieval operations. After retrieval, the surface of the samplers was cleaned on site with river water, and samplers were packed in sealed metal tins and transported to the laboratory in a cool box. Back at the laboratory, samplers were placed at –20 °C until extraction and analysis. Sampler preparation and extraction procedures were similar to those detailed elsewhere 16. Deuterated PAHs were used as PRCs and included d10-acenaphthene, d10-phenanthrene, d10-fluoranthene, d12-chrysene, and d12-benzo[a]pyrene 14, 16. Preparation controls were also analyzed to determine the initial concentration of PRCs in the sampler prior to exposure and possible contamination during preparation.
Extraction and analysis
Polymer sheets (from laboratory experiments and river deployment) and water solutions (from laboratory experiments) were extracted and analyzed. The polymer sheets were extracted overnight by soaking in n-pentane 16 in 2 consecutive extraction steps (1 g/20 mL and 1 g/65 mL; sampler/solvent, for silicone rubber and LDPE, respectively). Recovery standards were added to the extraction jars during the first solvent extraction step (100 ng tri-n-butylphosphate-d27 and benzophenone-d10). Both extracts were combined and reduced under a gentle stream of N2 to 1 mL or 500 μL, depending on the spiked concentration for the laboratory experiment. Pentane and dichloromethane were tested as extraction solvents. Pentane was selected as extraction solvent as suitable recoveries (>85%) were obtained for all substances of interest.
Methanol–water solutions from the KPW determinations were initially transferred to extraction funnels to ensure we did not extract analytes sorbed to the experimental glass bottles. These solutions were liquid–liquid extracted. For liquid–liquid extraction, the choice of solvent (pentane, dichloromethane, or toluene), the number of consecutive extraction steps required (2 or 3), and final percentage of methanol in solution (10% and 20%, v/v) are extraction parameters that were evaluated prior to extracting real samples. Finally, use of dichloromethane, 3 extraction steps, and 10% of methanol were selected as optimum extraction conditions, enabling recovery of over 70% for most of the compounds (Supplemental Data, Table S1). A batch extraction was performed by vigorously shaking the funnels for 60 s. The 3 extracts were combined, reduced in volume using a gentle stream of N2, and dried with anhydrous sodium sulfate. Finally, the extracts were further reduced to 100 μL. The deuterated standards tri-n-butylphosphate-d27, musk xylene-d15, and benzophenone-d10 (100 ng) were added at the beginning of the liquid–liquid extraction. Mass balances were calculated, and averaged mass balances were 89 ± 9%, 85 ± 10%, and 95 ± 7%, for tri-n-butylphosphate-d27, musk xylene-d15, and benzophenone-d10, respectively. Prior to instrumental analysis, a known amount of internal standard (100 ng of triphenylphosphate-d15, methyl triclosan-13C12, and triclosan-d3) was added to the final extracts to monitor analytical and instrumental variability.
For analysis of samples from the laboratory experiments, capillary gas chromatography separation of analytes was performed on an Agilent 7890N gas chromatograph (GC; Agilent) with a 5975 C mass selective detector (MS; Agilent), using an HP-5MS column (30 m × 0.25 mm inner diameter × 0.25 µm film thickness of 5% phenyl, 95% polydimethylsiloxane), keeping the helium carrier gas flow at 1 mL min−1 and the injection port temperature at 280 °C. Then, 1 μL of sample was injected in splitless mode (Supplemental Data, Section S4). Analyte concentrations in the experimental samples were determined by measuring the peak areas normalized to the internal standard and using external calibration curves for each component. Calibration curves for all compounds were linear in a range between 1 ng mL−1 to 1000 ng mL−1, with R2 values above 0.99 for each compound. The instrumental detection limits ranged between 0.03 ng mL−1 and 1.44 ng mL−1, calculated as the lowest concentrations at which the signal-to-noise ratio was greater than 3 in a standard solution. The analysis of field-exposed silicone rubber samplers was achieved using a GC (SCION 456-GC; Bruker) under the same conditions mentioned above coupled to a triple-quadrupole MS (SCION TQ, Bruker) to achieve a better selectivity (see Supplemental Data, Section S4 for further details on MS/MS). Calibration curves were linear within the range of 5 ng g−1 to 500 ng g−1 for all substances. All data were processed using the Bruker MS Workstation software.
RESULTS AND DISCUSSION
Contaminant Dp in polymers
We assume that diffusion is the only process responsible for mass transfer processes occurring within and between the superimposed polymer sheets used in the present study. Diffusion profiles of contaminant concentrations in the 5 superimposed polymer sheets collected in the film-stacking experiment were obtained for all target compounds (Supplemental Data, Figure S1.1). In general, good correlations were found between experimental and modeled data, demonstrating that the applied model was adequate for estimating diffusion between sheets. Most of the RMSD values were below 20% (for 93% of the analytes) for silicone rubber and less than 15% (87% of the analytes) for LDPE. Mass balances of the target compounds, calculated as the sum of masses measured in the 5 separate layers at the end of the experiment over the amount found in unused spiked sheets, were satisfactory for further interpretation of the data. They were 95% to 100% for organophosphate compounds and 94% to 104% for the remaining substances when silicone rubber was used, whereas a wide range was observed for LDPE (65–111% for most organophosphate compounds and 73–109% for the remaining compounds). The transfer of significant amounts of chemicals from the central layer to adjacent layers demonstrated for most chemicals that the contact times of 4 h for silicone rubber and 12 h for LDPE were sufficient (except for some organophosphate compounds, such as tris-iso-butylphosphate and triphenylphosphate, for which Dp could not be accurately estimated). When the mass in the second sheet was 0 or close to 0 (<20 ng), the model allowed us to estimate Dp but with an RMSD > 30% (i.e., for 2-ethyl-hexyl-4-trimethoxycinnamate [EHMC], or Tris(2-ethylhexyl) phosphate [TEHP] for silicone rubber; and octocrylene or TEHP for LDPE). In these cases, the observed Dp values are likely to be overestimates of true Dp values, possibly because of the nonhomogeneous distribution of these chemicals across the spiked central layer of the polymer stack as a result of their low diffusion coefficients. A heterogeneous distribution of the chemical in the spiked sheet with higher concentration at the edges of the sheet could result in more transfer of these chemicals to the adjacent sheets than if the chemicals were evenly distributed in the polymer sheet.
Estimated log Dp for AlteSil silicone rubber and LDPE are presented in Table 1 together with the log KOW and molecular weight of the emerging contaminants of interest. Figure 1 shows the estimated log Dp as a function of molecular weight. In terms of the first polymer, silicone rubber, for fragrances, selected endocrine-disrupting compounds, and UV filters, the diffusion coefficients covered a relatively narrow range of log Dp values (1 log unit; Figure 1A). Most of them were found in the same range as values published for PAHs for AlteSil silicone rubber 11. For UV filters, 2-hydroxybenzophenone, the least hydrophobic UV filter in the present study (log KOW 3.47) and also with the lowest molecular weight (198.2 g mol−1), had the highest log Dp (–10.71 m2 s−1). The lowest log Dp for UV filters (–11.36 m2 s−1) was for octocrylene, the most hydrophobic UV filter. The log Dp for 4-methylbenzylidene camphor (4-MBC) was lower, but the high relative percent difference between replicate measurements (>12%), indicates that these data may be relatively less robust. The low log Dp of octocrylene may be attributable to the higher molecular size of this molecule compared with the other UV filters. Molecular size has previously been reported to influence the diffusion of chemicals in polymers 11, 28. Log Dp values varied from –11.38 m2 s−1 to –14.52 m2 s−1 for organophosphate compounds, showing a decrease with increasing molecular weight and being in a similar range as those estimated for the UV filters 4-MBC and octocrylene.

For the second polymer, LDPE, contaminant diffusion coefficients were consistently lower than those found for silicone rubber for all the compounds (Figure 1B). The Dp values for LDPE were lower than in silicone rubber by a factor of 43 to 152 for fragrances, 11 to 200 for endocrine disrupters, 9 to 3000 for UV filters, and >3 to >3000 for organophosphate compounds. Lower contaminant diffusion coefficients in LDPE than in silicone rubber have also been reported for PCBs and PAHs (up to 2 orders of magnitude lower in some cases 11, 15). In any case, most of the log Dp measured for target analytes (fragrances, endocrine disruptors and some UV filters) are found in the same range as values published for PCBs (Figure 1B). Two UV filters, octocrylene and EHMC, presented significant lower log Dp values. Organophosphate compound diffusion coefficients in LDPE (–13.6 m2 s−1 to –14.4 m2 s−1) were in the range of those found by Hale et al. 12 for organochlorine pesticides such as aldrin, dieldrin, endrin, heptachlor, or heptachlor epoxide (Figure 1B). However, the experimental exposure time did not appear to be long enough to observe significant movement of some of the organophosphate compounds from the spiked LDPE sheet to adjacent layers. We therefore calculated that log Dp for tri-isobutylphosphate (TIBP), tributylphosphate (TBP), ethylhexyl diphenyl phosphate (EHDPP), and triphenylphosphate was below –15 m2 s−1 (no diffusion between sheets was observed). The diffusion of these substances in LDPE appears restricted. This may in part explain the much more significant accumulation of many organophosphate compounds in silicone rubber passive samplers compared with LDPE that was observed when samplers were deployed next to each other under identical exposure conditions in the River Alna 16.
Overall, silicone rubber—with the lowest glass transition temperature of the 2 polymers—exhibits the highest permeability for gases and organic chemicals 29, so diffusion coefficients are consistently higher than those for LDPE for emerging contaminants (well over 1 order of magnitude in most cases). The Dp value depends not only on the properties of the polymer but also on the nature and physicochemical characteristics of the diffusing molecule. Rusina et al 11 showed that the slope of log Dp-molecular weight is consistently steeper for PAHs than for PCBs, indicating that the structure of the chemical affects its diffusion. In this respect, significant correlations of log Dp with molecular weight were found for some groups of compounds (Supplemental Data, Figure S1.2.). This descriptor, however, appears not to be sufficient to predict values of log Dp in some cases. Differences in the molecular structure of substances of interest, their rigidity, and their cross-sectional diameter could be responsible for the observed differences in log Dp values. Our grouping of chemicals does not necessarily assemble structurally related chemicals, and the possibility of finding relationships with molecular weight or other simple descriptors is limited. For example, log Dp is expected to decrease with increasing molecular weight 11, which can certainly be observed for UV filters (they span a sufficiently wide range of molecular weight, from 198.2 g mol−1 for 2-hydroxybenzophenone to 361.4 g mol−1 for octocrylene) but not for fragrances (it is much more challenging to observe such relationships because their range of molecular weights is much narrower than for UV filters). In addition, other descriptors—such as surface area (previously reported by Rusina et al. 11), solubility, or certain geometric parameters, for example, taking into account the number of freely rotating bonds—were tested, but no relationships were found. Furthermore, it should be noted that the available data for emerging compounds is limited.
Silicone KPW measurements
 (2)
(2)
The log KPW values obtained using ultrapure water are reported in Table 1, and those from the cosolvent method (using the intercept of the linear regression of log KPW with the volume fraction and the mole fraction of methanol in water) are given in the Supplemental Data, Table S2. The relative percent difference values of duplicate KPW measured (n = 2) were on average 5.9% and below 30% for all compounds except for the organophosphate TEHP and the endocrine-disrupting compounds octylphenol and nonylphenol, which presented a relative percent difference higher than 40%. These results were then compared with data from the cosolvent method. To our knowledge, no polymer/water partitioning data have been reported for these compounds for silicone rubber. All emerging contaminants of interest had log KPW > 3 (Table 1). Thus, log KPW values for fragrances are from 3.05 (for musk ketone) to 4.36 (for traseolide). The log KPW for methyl triclosan (3.62) is higher than that measured for the parent compound triclosan (3.02), which was also observed for LDPE 13. As expected from their respective hydrophobicities, the log KPW for nonylphenol is slightly higher than for octylphenol. The log KPW values of UV filters range from 3.08 for benzophenone-3 to a value close to 5.0 for octocrylene. The log KPW values for the organophosphorus compounds are generally higher than those found for other emerging contaminants evaluated in the present study (from 4.68 for TIBP to 5.88 for TEHP).
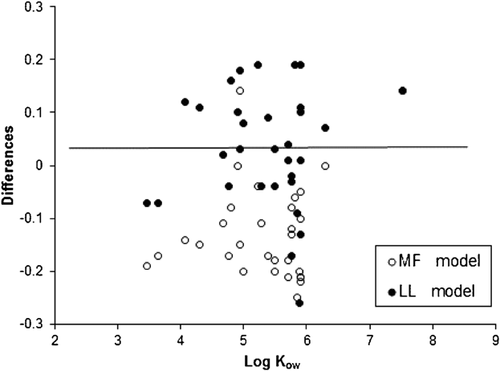
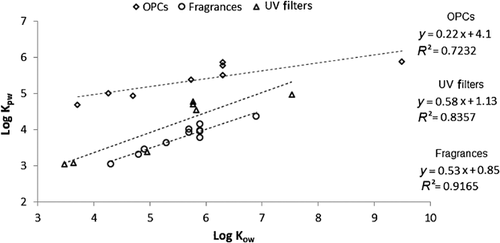
Linear regressions of measured log KPW with log KOW were evaluated. Correlations were found for most groups of chemicals. For most of the substances of interest in the present study, log KPW values for AlteSil silicone rubber are lower than their respective log KOW values. An example for fragrances, UV filters, and organophosphate compounds is given in Figure 3. The log KOW explained 91% of the variation in log KPW for fragrances, 83% for UV filters, and 72% for the organophosphate compounds. For these groups, the slope of the linear regression was below 1 (between 0.2182 and 0.5579). Values of log KPW were lower than those of log KOW, up to a factor of 1.6 for TEHP. This has been reported for other organic compounds such as PAHs 19. It is therefore advised that when KPW values for new emerging substances within these classes of chemicals are needed, it is best not to rely on predicted values of log KPW but instead to measure them. This is particularly important for chemicals with relatively low KPW values, which reach equilibrium rapidly, greater uncertainty in the KPW estimation leading to increased degree of uncertainty in estimated water concentrations. In addition, few or no studies have been undertaken to measure silicone rubber/water partition coefficients for these emerging substances. Semipermeable membrane devices have previously been used to sample UV filters, but no sampler/water partition coefficients were measured 9. A comparison of our silicone rubber results with some literature data for other polymer samplers shows that silicone rubber partition coefficients are generally lower than published values for triclosan, methyl triclosan, nonylphenol, and octylphenol for polyethylene 13, and reported values for nitro musks, such as musk xylene and musk ketone, and polycyclic musks, for instance, galaxolide and tonalide in semipermeable membrane device samplers 8.
Water boundary layer and polymer resistance
The AlteSil silicone rubber calibration data (Dp and KPW) obtained in the present study can now be used to assess the domain of application of this polymer for passive sampling purposes. We can assess how commonly used PRCs (e.g., deuterated PAHs) and sampling rate models 16, 20, 34 can be applied to estimate freely dissolved concentrations for our emerging contaminants. The mass transfer of an analyte from or to water from a passive sampling device is governed by diffusion through the water boundary layer and sorption to and diffusion into the polymer 21. The relative significance of both mass transports, external (through the water boundary layer) and internal (through the polymer), should be taken into account for the application of passive samplers 35. Boundary layer–controlled uptake is generally assumed for silicone rubber for PAHs, PCBs, and other more hydrophobic substances 20 because resistance to mass transfer in the polymer is expected to be negligible in comparison with that in the water boundary layer for these substances (Equation 1). We therefore need to assess whether the uptake of emerging contaminants under commonly encountered environmental conditions is also under boundary layer–controlled uptake.
Using Equation 1, we can evaluate the relative differences in mass transfer resistances calculated for the polymer layer, Ip (1/kpKPW, with kp being the mass transfer coefficient for the polymer layer, from our calibration data) and for the boundary layer Iw (1/kw, with kw being the mass transfer coefficient of the water boundary layer, from boundary layer thickness and contaminant diffusion coefficients in water). For typical environmental exposures of passive samplers in the aquatic environment, the thickness of the water boundary layer can vary from 10 µm to 1 mm 36, depending on the hydrodynamic conditions. To calculate the water diffusion coefficient (Dw), we used the US Environmental Protection Agency on-line tool 37 based on the estimation method of Hayduk and Laudie 38, where the LeBas molar volumes of the compounds and the viscosity of the water are considered. The log Ip was calculated using the half-thickness (0.25 mm) of the silicone rubber sheets for δm, because the compounds diffuse from both sides of the polymer sheet. Figure 4 shows a comparison of log Iw (δw/Dw) for δw (water boundary layer thickness) of 10 μm and 1000 µm with log Ip (δp/DpKPW). The analysis shows that for most emerging compounds, the mass transfer resistance in the polymeric membrane is lower than that in the water boundary layer (10 µm) and that only 2 such compounds are found above this water boundary layer (4-MBC and triphenylphosphate). The uptake into silicone rubber is therefore boundary layer–controlled (Ip < Iw) for most of the compounds under study. For some of these compounds, however, mass transfer resistances in the membrane and in the water boundary layer are closer. These compounds include benzophenone-3 or 2-hydroxybenzophenone, for which the uptake could be membrane-controlled. Because these chemicals exhibit relatively low KPW values (Table 1), we estimated the exposure time needed to obtain equilibrium between the contaminant concentration in a sampler (500-μm-thick sheet) and that in water. For these analytes, the time required to reach equilibrium (>90%, an approximation of the mass transfer coefficient model, Equation 1), estimated based on measured KPW, the surface area/thickness of the sampler and the sampling rate was less than 1 wk (e.g., 4.6 d for benzophenone-3 and 3.3 d for 2-hydroxybenzophenone). Based on the range of KPW values measured in the present study (Table 1), we expect that a substantial number of other emerging contaminants will be sampled at equilibrium or close to equilibrium when samplers are deployed for periods of weeks or months. If equilibrium is not reached, we show that for most substances and commonly encountered water turbulences, sampling will be under boundary layer control. The PRC dissipation data provide us with an estimate of boundary layer thickness 20, 34 and taking into account an exposure adjustment factor that, in turn, can be used to calculate sampling rate for the emerging contaminants under boundary layer–controlled uptake. (The thickness estimated from the PRCs exposed in our sampling in the River Alna was lower than 350 µm, and in that case the mass resistance for all the compounds, except triphenylphosphate, was lower than in the water boundary layer.) The full mass transfer coefficient model (Equation 1) can be used to estimate sampling rate for substances for which the resistance to mass transfer in the membrane should be taken into account.

Field application
The performance of 500-μm-thick AlteSil silicone rubber passive samplers for monitoring emerging contaminants was assessed through 21-d deployment of replicate samplers in the River Alna. The Alna is an urban river where we expect to find relatively high concentrations of many of the emerging contaminants studied. Details of the estimation of sampling rates and freely dissolved contaminant concentrations are provided in the Supplemental Data (Section S5, Figure S3). Most emerging contaminants of interest were not detected in preparation and field control strips. Only tetramethyl acetyloctahydronaphthalene (OTNE), octocrylene, nonylphenol, galaxolide, and EHMC were found in detectable concentrations in preparation and field control samplers. This is generally not an issue because these compounds are typically found at high concentrations in impacted environments. As an example, masses of galaxolide found in samplers exposed to Alna River water were on the order of 100 μg, while in preparation and field control samplers these were 60 ng and 200 ng, respectively. This may be a more significant issue when pristine environments are sampled.
As expected, a number of analytes including triclosan and benzophenone-3 with log KPW below 3.5 would be expected to have reached equilibrium within this 21-d exposure. The more hydrophobic compounds, that is, octocrylene, OTNE, and galaxolide would have remained in the linear phase of uptake. The relative percent differences in estimated Cw measurements (from duplicate samplers) were below 23% for all substances.
Analysis of the samplers revealed the presence of 21 emerging contaminants. It is not surprising to find many of these emerging substances in the Alna River, because it receives contamination from wastewater discharges (stormwater overflow), landfill effluents, and urban surface runoff, which can all be sources of these emerging organic pollutants to the river 2. Overall, galaxolide has the highest accumulated masses (116 μg). Concentrations for the Alna River are summarized in Table 2. The levels of the polycyclic fragrances tonalide and galaxolide were 51 ng L−1 and 1600 ng L−1, respectively. These compounds are widely used in Europe, with continuous input into the environment. Levels found in the present study are of the same order of magnitude as previously reported 3, 39. One of the fragrances also measured at high concentrations (up to 147 ng L−1) was OTNE. For UV filters, the highest concentrations were found for benzophenone-3 and octocrylene (108 ng L−1 and 448 ng L−1, respectively). These compounds have also been dominant compounds in others studies in Norway 39. Other UV filters, such as (4-MBC, ethyl hexyl salycilate, and EHMC, were found at concentrations in the same range as previously published by other authors in the north of Europe 9, and at a lower concentration than in other studies in Oslofjord area, where selected sampling sites were bathing areas affected directly by swimmers 5. The transformation product of triclosan, methyl triclosan, was found at levels up to 0.2 ng L−1, in accordance with values measured by other authors 13. Other compounds, such as nonylphenol isomers and tris-n-butyl phosphate, widely used in consumer goods, were also detected (Table 2). For some of the emerging contaminants (e.g., UV filters), weather conditions and season can significantly affect their use of and discharge to the environment. Sampling in the summer, as was done in the present study, is likely to result in higher UV filter concentrations in water than during other seasons.
| Compounda | Cw (ng L−1)b |
|---|---|
| AHTN | 51 (9) |
| HHCB | 1600 (11) |
| OTNE | 147 (6) |
| MK | 9 (6) |
| MT | 3 (16) |
| TCS | 9 (12) |
| MTCS | 0.2 (3) |
| NP | 169 (13) |
| Irgarol | 1.4 (0.2) |
| OC | 448 (23) |
| HMS | 18 (19) |
| EHS | 6.3 (13) |
| EHMC | 4 (22) |
| BP3 | 108 (10) |
| 4-MBC | 24 (3) |
| TIBP | 15 (18) |
| TBP | 99 (12) |
| ToTP | <LOQ |
| TmTP | 2 (0.6) |
| TpTP | 1 (4) |
| TPP | 170 (15) |
- a See table one for definitions of abbreviations.
- b Relative percent difference in parentheses.
- LOQ = limit of quantitation of the analytical method.
CONCLUSIONS
The present study presents the first assessment of the use of silicone rubber samplers for monitoring a wide range of emerging contaminants (31), mostly personal care products with log KPW values ranging between 3.02 and 5.88. Polymer calibration data—namely, contaminant diffusion coefficients in the polymer and KPW values—are provided and allow the use of AlteSil silicone rubber for the measurement of freely dissolved concentrations of these emerging contaminants in water or even sediments. Because we measured higher contaminant diffusivities in silicone rubber than in LDPE, we decided to focus further efforts on calibrating the silicone rubber, as we expected an overall lower resistance to mass transfer for this polymer than for LDPE. Particularly if KPW values for LDPE are substantially higher than for silicone rubber, LDPE could still be a useful polymer for sampling these chemicals. The low diffusion coefficients and membrane control of the uptake in some cases can make modeling the uptake of chemicals into the polymer more complex than for silicone rubber. For most of these substances, sampling is expected to be under boundary layer–controlled conditions by using silicone rubber. Because KPW estimated values for most of these compounds are not very high, equilibrium sampling could be undertaken with exposures of weeks to months. The field deployment in the River Alna in Oslo (Norway) demonstrated that exposure of 21 d was suitable for the detection and quantification of many of these substances in urban surface waters.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.3390.
Acknowledgment
We express our gratitude to S. Sal Bregua (Instituto Español Oceanografía) for her technical support with the R software. The present study was supported by 2 Spanish regional research projects (RNM 5417 and RNM 6613), with the help of a grant from the Spanish Ministry of Education, Culture and Sport, and also by the Norwegian Institute for Water Research (NIVA) through its Strategic Program on contaminants of emerging concern.




