Is received dose from ingested soil independent of soil PAH concentrations?—Animal model results
Abstract
Polycyclic aromatic hydrocarbon (PAH) bioavailability from ingested soils will vary between soils; however, the nature of this variation is not well characterized. A juvenile swine model was used to link external exposure to internal benzo[a]pyrene (BaP) and anthracene exposure following oral PAH ingestion of 27 different impacted site soils, soots, or spiked artificial soils. Internal exposure of BaP and anthracene, represented by area under the plasma-time curve, did not relate to soil concentration in impacted site soils, but did relate in spiked artificial soil. Point of departure modeling identified soil PAH concentrations greater than 1900 mg kg−1 as the point where area under the curve becomes proportional to external dose. A BaP internal exposure below 1900 mg kg−1 had an upper 95% confidence interval estimate of 33% of external exposure. Weak relationships between soil:simulated gastrointestinal fluid PAH partitioning and area under the curve values suggest that differences in internal PAH exposure between soils may not be dominated by differences in PAH partitioning. The data seem to best support exposure assessment assuming constant internal PAH exposure below soil concentrations of 1900 mg kg−1. However, because constant internal exposure would challenge several existing paradigms, a bioavailability estimate of 33% of the external exposure is suggested as a likely workable solution. Environ Toxicol Chem 2016;35:2261–2269. © 2016 SETAC
INTRODUCTION
Human exposure to polycyclic aromatic hydrocarbons (PAHs) commonly occurs through ingestion of impacted soil. The absorption and bioavailability of PAHs has been studied extensively (see Ramesh et al. 1 for a thorough review), and it is widely accepted that oral bioavailability of PAHs can differ when present in different media. The observed bioavailability of PAHs varies between different soil types 2-5. These differences arise from contaminant weathering in soil, as well as soil characteristics, which may include soil particle size or chemical partitioning 4-6. However, rarely has a large number of soils differing widely in physical and chemical characteristics and PAH concentrations been fed to a mammal and PAH bioavailability assessed. In the present study we fed 19 soils, 4 artificial soils, and 4 soot samples to juvenile swine.
Once ingested, PAHs transfer from the gastrointestinal tract to the systemic circulation. Transfer of PAHs into the circulation occurs concurrently with lipids 7, and it has been theorized that PAHs are transferred via chylomicron formation within enterocytes and transferred into lymph, which would allow PAHs to bypass the liver and first-pass elimination 8, 9. However, a study done in lymph and bile duct cannulated rats determined that approximately 80% of absorbed PAHs transfer to the circulation via hepatic portal transport, rather than lymph 10. A more recent study confirms these findings, concluding that approximately twice the PAHs entering into the circulation cross through hepatic portal transfer rather than through chylomicron formation and transport through the lymphatic system 11. Thus, the majority of ingested PAHs enters the body via the portal vein, to the liver, and from there to the systemic circulation.
Rapid metabolism of PAHs can confound quantification of PAH uptake following oral exposure. For example, the liver extensively metabolizes PAHs 1. Analyzing unlabeled metabolites in an organism is a very daunting task because PAHs are a family of compounds, that is, there are typically at least 9 to 16 PAHs of interest present in an impacted soil, and each compound can convert into more than 1 metabolite 1. Using 14C-labeled compounds eliminates the complicated analysis necessary for unlabeled compounds; however, a number of factors make 14C analysis unfavorable for use in PAH bioavailability. First, 14C-labeled PAHs may overestimate the risk of PAHs, as most absorbed PAHs metabolize to inert metabolites that excrete quickly 1. In addition, the use of 14C-labeled compounds is limited to spiked soil, and it would be difficult to compare results with those obtained from anthropogenically impacted soils.
Previously, systemic PAH metabolites were thought to be the best estimate of PAH bioavailability 1. These metabolites arise, in a large part, from the action of liver mono-oxygenase enzymes (such as CYP 1A1, CYP 1A2, and CYP 1B1) on PAHs. It was initially assumed that toxic metabolites form in the liver and transport in the systemic circulation to cause peripheral toxicity. However, animal studies using inbred mouse strains observed that circulating metabolites do not cause bone marrow and spleen toxicity 12-14. This is a reasonable observation, because toxic metabolites of PAHs have epoxide groups present on the compound, and thus are highly reactive and would not travel far in the circulation without reacting with epoxide hydrolase or a cellular component. These results should not be taken to imply that the CYP family is unimportant for toxicity, but rather that the first-pass effect in which much of the ingested PAHs are metabolized as the portal vein empties into the liver acts as a detoxification reaction. Thus, the assessment of parent PAHs in the systematic circulation may be a better estimate of nonhepatic toxicity after oral exposure.
Assessing systemic exposure omits exposure that occurs in the gastrointestinal tract and liver, which likely are at the highest risk for ingested PAHs. The greatest incidence of tumor formation occurs in the gastrointestinal tract following oral exposure to PAHs in rodent studies 15, 16. However, assessing PAH exposure to the gastrointestinal tract and liver is very difficult because of the high metabolic capacity of both intestinal and liver cells 1. Assessment of fecal elimination as a bioavailability estimate following oral exposure has been used to address this, but this method also has drawbacks, because gastrointestinal flora can metabolize PAHs without exposure to intestinal cells 2. In addition, the enterohepatic circulation will continually reintroduce metabolites and parent compound to the gastrointestinal tract.
Animals, acting as surrogates for humans, are an excellent means to assess internal exposure of PAHs. Swine have become a popular human exposure model, and have been validated as a model for lead and arsenic 17, 18; they are also gaining popularity for organic compounds like PAHs 4, 5, 19. Swine are an alternative model to rodents because of the similarities between swine and humans in gastrointestinal physiology and intestinal conformation, as well as the cellular makeup of the organs 20. Biochemically, swine aryl hydrocarbon receptor response to agonists like PAHs is very similar in magnitude to that of humans 21.
Soils used for assessing bioavailability in mammals usually come from 1 of 2 sources: impacted sites or spiked soil 1-5, 19. The advantage of using soil collected from impacted sites is that it represents a realistic exposure scenario. However, age and source of the PAHs in the soil may be unknown, and there may be impacts other than PAHs present that could affect bioavailability, such as metals. In contrast, spiked soils are generated in the laboratory, and therefore, the source and condition of amendments can be tightly controlled. The disadvantage to using spiked soil in bioavailability studies is that it does not always accurately represent real-world conditions, as weathering duration in the laboratory does not equal what may be observed in the field, and spiked soil may lack the variability in physical properties that field-collected soils may have.
Assessing internal exposure of parent compounds in an animal that ingests contaminated soils allows us to relate external to internal exposure of PAHs. It is widely assumed that external and internal exposure will follow a linear trend. Bioavailability is the slope of internal to external exposure. However, toxicologists, especially those concerned with mutagens, have long recognized that their dose–response relationships are typically hockey stick–shaped. A hockey stick dose–response relationship comprises a linear and a sublinear component, with the sublinear component typically occurring at lower doses. For example, low doses of a mutagen may cause no adverse effect until an inflection point is reached, where adverse effects increase linearly with dose. Commonly, models such as a benchmark dose or a threshold dose are used for such datasets 22. Both threshold dose and benchmark dose calculations utilize the entire dose–response dataset and interpolate the data to derive a point of departure where the response begins to differ significantly from the control. In other words, this approach allows one to estimate 2 slopes in a biphasic relationship. It is exactly this type of relationship that we observed in the present study. We used plasma concentrations of parent PAHs to calculate bioavailability and elimination/absorption rates. Use of a large dataset is essential to characterize what occurs when mammals ingest PAHs.
MATERIALS AND METHODS
Soils
Artificial soil was prepared and spiked as in Peters et al. 19. Soil spiked with benzo[a]pyrene (BaP) and anthracene resulted in swine exposure of 1 mg/kg, 5 mg/kg, 10 mg/kg, and 20 mg/kg body weight to each compound in 5 g of soil. Soot was provided by the Meyer laboratory group at the Technical University of Denmark. In short, a composite soot sample collected from several wood-burning stoves in a small Danish town near Roskilde was divided into 2 treatment groups, with 1 soot group treated in contaminant traps, whereas the other remained untreated 23. Treated and untreated soot were combined in different ratios to create various PAH concentrations. Soot exposures were designated soot 1, soot 2, soot 3, and soot 4, and contained 100% treated soot, 50% treated and 50% untreated soot, 17% treated and 83% untreated soot, and 100% untreated soot, respectively 24. Soils collected from PAH-impacted sites in the United Kingdom (n = 12), Sweden (n = 2), and Canada (n = 5) were sieved to less than 250 μm and also fed to swine for a total of 4 artificial soil, 19 impacted site soil, and 4 soot exposures. Soil properties and PAH sources are presented in the Supplemental Data, Table S1.
The PAHs from the impacted site soils were extracted by an ultrasonication method, and are assumed to represent total extractable PAHs. Briefly, 5 mL of 1:6 toluene:methanol solvent mix was added to 1 g of soil. The slurry was sonicated for 2 h, centrifuged for 15 min at 3000 g, passed through a 0.45-μm filter, and stored at –20 °C until analysis. Anthracene and BaP concentrations in the soot and soils are presented in Table 1.
| Soil | Anthracene (mg kg−1) | Benzo[a]pyrene (mg kg−1) |
|---|---|---|
| WP1 | 27 (2.4) | 18 (1.7) |
| GW5 | 1.1 (0.02) | 4.5 (0.08) |
| Soot 1 | 2 | 10 |
| Soot 2 | 5.5 | 25 |
| Soot 3 | 7.8 | 35 |
| Soot 4 | 9 | 40 |
| BGS 1 | 1.6 (0.07) | 2.5 (0.3) |
| BGS 2 | 11 (0.7) | 56 (2.6) |
| BGS 3 | 12 (0.6) | 55 (2.9) |
| BGS 4 | 11 (0.5) | 61 (2.4) |
| BGS 5 | 5.7 (0.1) | 27 (0.3) |
| BGS 6 | 4.8 (0.2) | 17 (0.2) |
| BGS 7 | 3.4 (0.1) | 9.9 (0.2) |
| BGS 8 | 4.1 (0.04) | 37 (0.4) |
| BGS 9 | 2.6 (0.07) | 22 (1.2) |
| BGS 10 | 17 (3.3) | 41 (8.9) |
| BGS 11 | 11 (0.1) | 48 (0.8) |
| BGS 12 | 144 (5.0) | 290 (5.8) |
| COT 1 | 0.008 (0.003) | 0.12 (0.1) |
| COT 2 | 0.009 (0.004) | 0.014 (0.0005) |
| COT 3 | 0 (0) | 0 (0) |
| COT 4 | 0.013 (0.0008) | 0.009 (0.005) |
| COT 5 | 0.012 (0.0004) | 0.002 (0.0006) |
| Average | 12 (6) | 35 (12) |
- a Standard error in parentheses.
- WP = wood preservation; GW = gas works; BGS = British Geological Survey; COT = Communities of tomorrow.
Intravenous dose
The intravenous dose was prepared by completing a solvent transfer of a PAH calibration standard containing 16 different PAHs (Supelco PAH Calibration Mix, 10 μg/mL in acetonitrile (Sigma-Aldrich) into glyceryl trioctanoate (Sigma-Aldrich). Briefly, 4 1-mL calibration standards were combined, and the acetonitrile was evaporated to near dryness under a stream of high-purity nitrogen gas, after which 10 mL of diethyl ether was added. Approximately half the diethyl ether was evaporated under a stream of high-purity nitrogen gas, 1 mL of glyceryl trioctanoate was added, and the remaining diethyl ether was evaporated.
Swine
Female Landrace cross pigs (7–8 wk of age, ∼20 kg) were obtained and housed at the Prairie Swine Centre in Saskatoon (SK, Canada). Swine were housed in individual pens and allowed 7 d to acclimate prior to exposure. During the acclimation period, staff trained swine to eat a dough ball consisting of flour, molasses, pig chow, and vanilla. Swine were maintained on standard grower ration at 4% body weight and given water ad libitum. Swine were divided into groups (n = 6) and exposed to PAHs by either intravenous or oral routes, as outlined below. Animals were monitored daily during the exposure study by trained animal care staff, and were not observed to suffer ill effects from exposure to PAHs. The present study was reviewed and approved prior to initiation by the University of Saskatchewan Animal Care and Ethics Committee (Animal Use Protocol Number: 20080153) and adhered to the Canadian Council on Animal Care guidelines for humane animal use.
Exposure study
To maximize the data generated by each pig, swine experienced multiple exposures to PAHs in dose media. This was done by exposing swine to a single dose of PAHs, either through oral (i.e., soil or soot) or intravenous exposure, generating a 48-h plasma time course, and allowing a 7-d washout period before subsequent exposure. Swine were dosed over a period of 5 wk, and euthanized at the end of the experiment.
Oral exposure
Swine were given approximately 5 g of soil or 7 g of soot in a dough ball consisting of flour, molasses, pig chow, and vanilla. The soil or soot was added to the dough ball along with addition of the flour. Swine were allowed to eat the dough ball passively, and generally consumed it in less than 1 min.
Intravenous exposure
Swine were moved to a dedicated intravenous dose area and restrained with a hog snare and handling board. A 38-mm, 20-gauge catheter was inserted into an ear vein following topical application of lidocaine to numb the skin. The intravenous dose media containing PAHs (1 mL) were injected through the catheter, and the catheter was flushed with saline. The catheter was removed immediately after the injection was completed, and pressure was applied to the injection site until bleeding had stopped. After bleeding had ceased, the animals were returned to their individual pens.
Blood collection and analysis
Whole blood was collected from the jugular vein of swine at 0 h, 2 h, 4 h, 6 h, 8 h, 12 h, and 24 h post exposure into heparinized vacutainers. Four swine were sampled per sampling event, with rotatation of pigs sampled in each event, to reduce physical trauma to the animals as a result of restraint and blood collection, and data were pooled for analysis. Blood was stored at 4 °C until plasma separation by centrifugation (170 g for 15 min), and plasma was stored at –20 °C until extraction. Plasma was extracted by solid phase extraction, as in James et al. 4, and stored at –20 °C until analysis.
High-pressure liquid chromatography
Plasma and soil extract were analyzed by high-pressure liquid chromatography coupled with fluorescence detection (HPLC-FD) using an Agilent 1260 Infinity system. A 10-μL aliquot of extract was injected on an Agilent PAH Pursuit column (3-μm particle size, 100-mm length, and 4.6-mm inner diameter) guarded by an Agilent MetaGuard 3-μm C18 4-6 mm column. The column was kept at 25 °C during use by a column heater. Run time was set at 30 min, and HPLC grade water and acetonitrile were used as the solvents. The initial solvent gradient was 60:40 acetonitrile:water, with a linear shift to 90:10 acetonitrile:water between 0 min and 20 min. The 90:10 acetonitrile:water gradient was maintained for 5 min, and then the gradient was returned to 60:40 acetonitrile:water for 5 min to re-equilibrate the column for the next sample. The Agilent 1260 system was equipped with multisignal acquisition; therefore the excitation wavelength was set at 260 nm, whereas the 4 fluorescence detectors were set for emission wavelengths of 350 nm, 420 nm, 440 nm, and 500 nm, respectively.
Quality assurance and control
Plasma collected at the 0-h time point was analyzed and used to correct the analytical results from the plasma of the PAH-exposed swine. Duplicates, blanks, and spikes were also completed as part of the quality assurance and quality control process. The average percentage deviation of analytical duplicates for swine samples was 18%. Average spike recovery from plasma during the solid phase extraction process was 70%, and from the ultrasonication extraction process for soil it was 94%. The HPLC-FD was calibrated using dilutions of an external standard consisting of 10 μg/mL of each PAH, and the calibration was updated daily. Limits of detection for the HPLC-FD were 0.97 ng/mL for anthracene and 1.74 ng/mL for BaP. Plasma concentrations were corrected for partitioning of PAHs into whole blood components, and the average recovery for anthracene and BaP in plasma compared to whole blood was 42% and 43%, respectively.
To determine whether the washout period of 7 d was adequate to allow metabolic processes to return to baseline levels between exposures, 1 group of swine was exposed to the same soil in week 1 of exposure, as well as week 5, and calculated bioavailabilities were compared. No statistically significant difference was observed between exposure weeks (data not shown).
Pharmacokinetic parameter estimations
Area under the curve
Area under the curve calculations were completed on the plasma concentration time course for each compound in individual pigs. The area under the curve calculations are assumed to represent the total body exposure to a compound following an oral dose. The area under the curve was calculated to the 24-h time point with the MESS package 25 in statistical program R 26 using the trapezoidal rule.
Absorption and elimination rates
Absorption (ka) and elimination (ke) rates were calculated for each compound in each soil group as factors of the absorption and elimination slopes in the plasma concentration time course. Flip-flop kinetics were observed; therefore, the absorption rate was calculated from the slope of the elimination phase of the log plasma concentration time course. The elimination rate constant was calculated from the elimination phase of the intravenous exposure. Both rates were calculated using PKSolver, an open-source Microsoft Excel add-in 27. Data for individual swine were pooled for each exposure group, as blood samples were not collected from each pig at all time points. Thus, standard error was not calculated for absorption and elimination rate constants.
Bioavailability
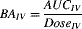 (1)
(1) (2)
(2)Point of departure calculations
Threshold effect level
Threshold effect level values were calculated using a piecewise linear model. This model defines a linear relationship for both the low and high part of the dose–response curve, as well as an unknown knot point at the threshold dose. A significance level of α = 0.05 was assumed, so 95% upper and lower confidence intervals were calculated for the threshold by bootstrap analysis. The 95% lower confidence interval is typically reported as the point of departure. This model is available as part of the SiZer package 28 for the statistical software program R 26. The initial slope of the line below the point of departure, along with 95% confidence intervals of the slope, is also calculated with this model.
Benchmark dose
Benchmark doses are calculated by fitting models to the dose–response data and using a predetermined response level, commonly 10% from background, to select a benchmark dose. The benchmark dose values were determined using the US Environmental Protection Agency (USEPA) Benchmark Dose Software Ver 2.5. This software contains 30 different models that can be used to calculate a benchmark dose. The exponential model for continuous data was chosen for these data, because it provided the best fit. The lower bound 95% confidence limit on the benchmark dose was also calculated, and this value is typically reported as the point of departure. The exponential model does not calculate a sublinear slope for the data.
RESULTS AND DISCUSSION
Swine anthracene and BaP area under the curve values following a single exposure to real world soils did not demonstrate a relationship with soil concentration of PAHs (Figure 1; anthracene: r2 = 0.14, p = 0.54; BaP: r2 = 0.13, p = 0.56). The highest soil concentration of anthracene and BaP (BGS 12) was not included in the regression, because it exhibited excessive leverage. Total soil anthracene doses ranged from 0.04 μg to 724 μg and averaged 66 (standard error [SE] = 32) μg, whereas total BaP doses ranged from 0.01 μg to 1450 μg and averaged 188 (SE = 64) μg. Because no relationship was found between impacted site soils and internal exposure, average anthracene and BaP area under the curves were calculated. Anthracene area under the curves ranged from 0.61 μg h L−1 to 14.4 μg h L−1 and averaged 3.6 (SE = 0.6) μg h L−1, whereas BaP area under the curves ranged from 1.0 μg h L−1 to 7.2 μg h L−1 and averaged 2.6 (SE = 0.4) μg h L−1.
Soot is often considered a PAH source in soil and may have different toxicokinetic parameters than soil, because soil characteristics may influence chemical desorption and uptake into the organism. The BaP and anthracene concentrations in soot corresponded to the range seen in the impacted site soils (Table 1), so area under the curve values were included in the regression analysis. Soot data were not included in the figures, but they corroborate with the values presented for impacted site soil (data not shown).
In contrast to real world soils, BaP and anthracene area under the curves generated from swine exposed to spiked artificial soil had a strong relationship with soil concentration (Figure 1). Linear regressions completed for both anthracene and BaP demonstrated that area under the curve has a high dependence on soil concentration (anthracene: r2 = 0.99, p = 0.007; BaP: r2 = 0.95, p = 0.02). The strong linear relationship at high doses indicates that absorption in the swine model was not limited by concentration of PAHs in soil. Bioavailability of anthracene and BaP was very low from spiked artificial soil, and was found to be 0.7% and 0.5%, respectively. Absolute bioavailability was also determined, and calculated as 1.2% for anthracene and 0.7% for BaP.
Analysis of the biphasic external to internal exposure relationship
The shift from no relationship between area under the curve and dose in impacted site soils to a strong linear relationship between area under the curve and dose in spiked soils may occur because of biochemical interactions between uptake and soil PAH concentration. The PAHs are taken up concurrently with lipids 7, and thus may be conveyed to the systemic circulation via chylomicrons in the gastrointestinal tract. In addition, PAHs could sporadically adsorb to other components in the gastrointestinal lumen, like food, which would reduce oral PAH absorption 7. The dose swine were exposed to in the spiked artificial soil experiment may have been high enough to overwhelm this adsorption, leading to linear PAH absorption, whereas in the impacted site soil, a much higher proportion could have bound to these other components, limiting the extent of absorption. Alternatively, if absorption is occurring because of a nonsaturable process (i.e., a pseudo–first-order kinetic), then the very high concentrations in the spiked soils may have resulted in enough PAHs being absorbed to overwhelm the excretion mechanisms.
Point of departure modeling of area under the curve versus soil concentration indicated that area under the curve did not increase until soil concentration values greatly exceeded those typically seen in anthropogenically PAH-impacted soil. The point of departures calculated using the USEPA Bench Mark Dose Software were 10 700 mg kg−1 and 4500 mg kg−1 for anthracene and BaP, respectively. Alternatively, piecewise regression resulted in point of departures of 7500 mg kg−1 for anthracene and 1900 mg kg−1 for BaP. This analysis would suggest that below these concentrations, there is a limited link between external dose and internal exposure. However, there are limitations associated with the data generated from spiked artificial soil, namely, weathering time. Soil collected from impacted sites had experienced significant weathering time prior to collection, whereas the spiked artificial soil had not. In addition, the 10-fold gap between impacted site and spiked soil concentrations was very large, and thus may skew the point of departure models. Unfortunately, it was not possible to identify a field soil with greater than 300 mg BaP kg−1 soil, and thus there is a large gap of contaminant concentrations in our dataset, that is, our top field soil had 300 mg BaP kg−1 and our lowest laboratory spiked soil was 4000 mg BaP kg−1. This gap arises because the laboratory-spiked soils were designed to assess toxicological effects within swine and thus used high concentrations of BaP to induce toxicity such as micronuclei 29.
Toxicokinetic parameters of PAHs ingested with soil
Like area under the curves, anthracene absorption rate constants (ka) calculated in swine exposed to impacted site or spiked artificial soils did not relate to soil concentration, whereas BaP ka values had a significant regression, with a weak relationship (Figure 2; anthracene: r2 =.01, p = 0.64; BaP: r2 = 0.26, p = 0.03). Absorption rate constants calculated for spiked artificial soils remained fairly constant, and the range of calculated values did not differ greatly from those calculated for impacted site soils (anthracene spiked soil: 0.18–7.6 h−1; BaP-spiked soil: 0.08–7.4 h−1; anthracene real soil: 0.003–4.8 h−1; BaP real soil: 0.02–12.6 h−1). Average ka values calculated for combined artificial and impacted site soils were 0.5 (SE = 0.2) h−1 and 1.4 (SE = 0.4) h–1 for anthracene and BaP, respectively.

Swine ka values calculated for both BaP and anthracene compare with the range of PAH ka values available in the literature for rodents. Absorption rate constants reported in both rats and mice, for BaP, pyrene, and phenanthrene, range from 0.69 h−1 to 18.8 h−1 2, 30, 31. The highest reported ka of 18.8 h−1 was found in rats exposed to 4 mg kg−1 pyrene in a study consisting of a range of doses from 2 mg kg−1 to 15 mg kg−1, and this value was much larger than the other reported ka values for other doses from the same study 30. If we exclude this value, the highest reported ka value is 5.0 h−1, from Withey et al. 30. Furthermore, Kadry et al. 2 reported similar ka values for phenanthrene between exposure media after oral exposure from neat compound, as well as spiked clay and sand (0.69–1.4 h−1). Elimination rate constants calculated following intravenous exposure for both anthracene and BaP were found to be 5.3 h−1 and 3.7 h−1, respectively.
Elimination rate constant estimates for both anthracene and BaP in swine compare with published rodent ke values; however, the swine ke values tended to fall on the high end of the range reported for rodents. Published pyrene, BaP, phenanthrene, and BaP ke values for both rats and mice range from 0.02 h−1 to 1.3 h−1 2, 13, 30-32]. Two of these studies contain BaP ke values, with widely variable results reported: 0.12 h−1 by Ramesh et al. 32, and 1.3 h−1 by Uno et al. 13. These 2 studies were conducted in different species (rats and mice, respectively), which may account for the variability in reported ke values. As with oral exposure, swine ke values after an intravenous exposure exceeded published values. Elimination rate constants found in the literature for intravenous exposure of pyrene and BaP range from 0.173 h−1 to 3.5 h−1 30, 33-35. Moir et al. 35 evaluated the kinetics of BaP in rats over a range of doses, and reported ke values ranging from 0.98 h−1 to 2.85 h−1, the maximum of which is similar to the BaP ke for swine in the present study. In addition, Lipniak-Gawlik 34 investigated the influence of other PAHs on pyrene kinetics, and demonstrated that mixtures of PAHs may affect the toxicokinetics of a compound.
Physiological explanation of low dose responses
Differences in intravenous and oral exposure elimination kinetics provide the clue to explain why external exposure is not linked to internal exposure at low PAH concentrations. The differences between intravenous and oral exposure kinetics suggest that flip-flop kinetics are occurring. Flip-flop kinetics occur when the gastrointestinal absorption rate is slower than the elimination rate of a compound 36. Flip-flop kinetics reduce systemic parent compound exposure, because the compound metabolizes at a faster rate than it is absorbed 37. Work previously published by Withey et al. 30, and Viau et al. 38 reports that elimination kinetics of pyrene following intravenous and oral exposure do not differ significantly. However, both studies used a liquid carrier (a saline/emulphor mix and a glucose/emulphor mix, respectively) for the oral exposure of pyrene. In the present study, the soil matrix may limit the gastrointestinal absorption rate of PAHs compared with other exposure media, and therefore one observes flip-flop kinetics. In addition to limiting absorption rate, the soil matrix could also limit the extent of PAH absorption by binding PAHs too tightly to be released in the short transit time through the gastrointestinal tract.
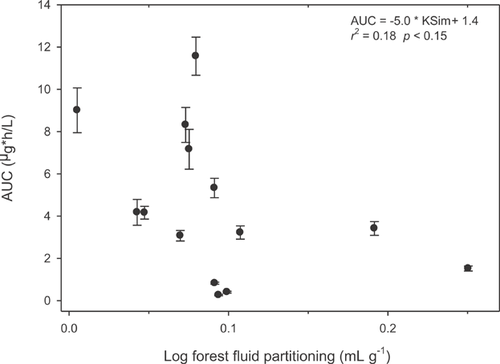
Anthracene area under the curves weakly related to anthracene partitioning from soil in simulated intestinal fluid (r2 = 0.18, p = 0.13, Figure 3). The fraction of BaP absorbed (area under the curve normalized to dose) also relates weakly with Forest fluid partition coefficients 39. Both anthracene and BaP demonstrate negative relationships with Forest fluid partitioning coefficients—as the partitioning coefficient increases, the area under the curve and fraction absorbed decrease. Partitioning coefficients represent the ratio of compound in soil to compound in fluid; therefore, partitioning coefficient increases signify a greater proportion of compound remaining in soil, rather than fluid. Thus, increases in simulated gastrointestinal fluid partitioning values indicate a stronger affinity of the compound, whether anthracene or BaP, to the soil particles, and explain the negative relationship with area under the curve.
Options for the risk assessment of contaminated soils
We propose 4 PAH exposure options, as detailed in Table 2: 1) assume 100% bioavailability; 2) assume constant internal exposure below 1900 mg kg−1; 3) assume <100% (e.g., 33%) bioavailability below 1900 mg kg−1; or 4) model internal exposure through area under the curve versus soil characteristic relationships. The most conservative, but least likely scenario, method of exposure assessment for PAH-impacted sites assumes that 100% of ingested PAHs transfer into an organism. The incremental lifetime cancer risk is calculated by multiplying the external compound dose to the appropriate cancer slope factor. Thus, if we assume 100% bioavailability and the average BaP concentration of our soils, the incremental lifetime cancer risk is 2.3 × 10−5 for adults and 3.9 × 10−4 for toddlers. As demonstrated in the present study, as well as others, PAH bioavailability can vary widely depending on dose media, and may lie below 1% in soil 1, 19. Therefore, this method may result in extremely elevated risk values for impacted sites.
| Option | Pros | Cons |
|---|---|---|
| 100% Bioavailability | Simple, conservative | May overestimate risk |
| Constant AUC | Simple, likely realistic | Not site-specific |
| Constant (<100%) bioavailability | Simple | Highly dependent on spiked soil concentrations, may not be accurate |
| Soil:fluid partitioning | Site specific | More complex, weak relationship, more labor intensive |
- AUC = area under the curve.
The second exposure assessment option assumes that humans absorb a constant amount of PAHs in contaminated soil, irrespective of the contaminant level in these soils. Toxicity studies for PAHs base the reference dose value on the external exposure the model organisms received, rather than on what reaches the systemic circulation. These studies exposed animals to PAHs in food 15, 16, and Ramesh et al. 1 demonstrates that PAH bioavailability from food is near 100%. Thus, if we assume that internal dose is not linked to external dose, then using the average BaP area under the curve value from the present study, corrected for assumed adult and toddler soil ingestion rates 40, the incremental lifetime cancer risk is 6.2 × 10−6 for adults and 1.6 × 10−6 for toddlers. Although assuming a constant internal dose is very simple, it does not incorporate site-specific variations, and thus may not accurately represent risk.
The third exposure assessment option calculates bioavailability as the slope of the internal-exposure dose curve at environmentally relevant soil concentrations. At these low concentrations, this slope is often termed the sublinear portion because it is not significantly different from 0. Piecewise regression of this sublinear portion indicates that the 95% confidence interval of bioavailability estimates for environmentally relevant soil concentrations range from 2.5% to 33% for BaP and 0% to 36% for anthracene. If we use this BaP bioavailability estimate to calculate incremental lifetime cancer risk for toddlers and adults from the average soil concentration used in the present study, the incremental lifetime cancer risk values are 1.3 × 10−4 and 7.6 × 10−6 for toddlers and adults, respectively. However, the point of departure approaches are highly sensitive to the spiked soil doses, the limitations of which were discussed previously in Analysis of the biphasic external to internal exposure relationship. Thus, our estimate of 33% soil BaP concentration becoming bioavailable may be too inaccurate to use at a contaminated site.
The fourth, and final, option is to use partitioning to estimate internal exposure of PAHs to humans. For example, in Figure 3, there is a weak relationship between partitioning and area under the curve. This approach can derive internal exposure estimates from site-specific soils but requires site-specific data. In addition, the observed relationships between partitioning coefficients and internal exposure in swine were very weak, and thus the bioavailability estimate calculated from the slope of the line may be inaccurate.
CONCLUSIONS
Analysis of swine anthracene and BaP toxicokinetics demonstrated that PAH soil concentration does not influence internal exposure of PAHs. This contradicts the common assumption in risk assessment that risk relates linearly to the soil concentration, and therefore external dose, of a compound. There appears to be a point of departure in soil concentrations where internal exposure and external dose become related. The use of 2 different point of departure models indicated that area under the curve and soil dose were only linked at soil concentrations much larger than those typically seen in PAH-impacted soils found in the environment. Thus it may be reasoned that humans are exposed to a constant internal dose of PAHs, regardless of external dose. We hypothesize that this occurs because of limited absorption coupled with rapid elimination, leading to a reduced amount of circulating compound. Because the present study measured parent PAHs in systemic circulation as an indication of internal exposure, decreases in circulating compound would lead to a decreased apparent internal exposure. However, the present study design cannot speak to the risk of exposure of the gastrointestinal lining, because PAH exposure to this tissue occurs during the absorption phase, independent of their systemic circulation.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.3384.
Acknowledgment
The present study was supported by an NSERC Discovery Grant to S.D. Siciliano.
Disclaimer
None of the authors have competing financial interests to declare.
Data availability
Data are available on request to S. Siciliano, corresponding author ([email protected]).




