Germination and early plant development of ten plant species exposed to titanium dioxide and cerium oxide nanoparticles
Abstract
Ten agronomic plant species were exposed to different concentrations of nano–titanium dioxide (nTiO2) or nano–cerium oxide (nCeO2) (0 μg/mL, 250 μg/mL, 500 μg/mL, and 1000 μg/mL) to examine potential effects on germination and early seedling development. The authors modified a standard test protocol developed for soluble chemicals (OPPTS 850.4200) to determine if such an approach might be useful for screening engineered nanomaterials (ENMs) and whether there were differences in response across a range of commercially important plant species to 2 common metal oxide ENMs. Eight of 10 species responded to nTiO2, and 5 species responded to nCeO2. Overall, it appeared that early root growth may be a more sensitive indicator of potential effects from ENM exposure than germination. The observed effects did not always relate to the exposure concentration, indicating that mass-based concentration may not fully explain the developmental effects of these 2 ENMs. The results suggest that nTiO2 and nCeO2 have different effects on early plant growth of agronomic species, with unknown effects at later stages of the life cycle. In addition, standard germination tests, which are commonly used for toxicity screening of new materials, may not detect the subtle but potentially more important changes associated with early growth and development in terrestrial plants. Environ Toxicol Chem 2016;35:2223–2229. Published 2016 Wiley Periodicals Inc. on behalf of SETAC. This article is a US Government work and, as such, is in the public domain in the United States of America.
INTRODUCTION
Developing screening tools to evaluate the environmental health and safety of engineered nanomaterials (ENMs) represents a significant challenge for regulators because of the unique properties that emerge at the nanoscale 1, 2. Unlike exposure to soluble chemicals, ENM exposure occurs via colloidal suspensions of insoluble particles rather than as true solutions; and as a result, particle suspensions often behave in a manner that may not be addressed in existing ecological testing protocols 3. Confounding the regulatory effort is the lack of knowledge about how these colloidal suspensions interact and change when exposed to organisms in realistic environmental compartments 4, 5. One step in assessing the environmental health and safety of new materials is to evaluate existing approaches to determine the degree to which they are appropriate for testing ENMs or whether they could be modified to accommodate ENMs.
Metal oxides such as nano-titanium dioxide (nTiO2) and nano-cerium oxide (nCeO2) are used in a variety of applications, including coatings such as paints, diesel catalysts, whiteners, and personal care products 6. It is anticipated that these ENMs will reach the environment through a number of pathways, including water (e.g., wastewater-treatment plants), biosolids (i.e., sludge application), and the air, through diesel exhaust and possible degradation of material coatings and paints. Although there is limited information on physicochemical interactions along the exposure pathways, it is important to examine native particles since regulatory decisions are generally based on inherent particle toxicity.
Reports on the effects of nTiO2 and nCeO2 on germination and early growth of vascular plants have been methodologically inconsistent, with high dose amounts; and as such, results have been variable and inconclusive. In addition, studies employing a wide range of test species coupled with a standardized approach have been lacking. Zheng et al. 7 found increased germination in spinach (Spinacia oleracea) seeds at nTiO2 concentrations up to 4000 μg/mL but decreased germination at higher concentrations. No information was provided on the source of the particles or their characterization. Castiglione et al. 8 soaked seeds in ENM suspensions for 24 h and observed that TiO2 (from Sigma-Aldrich) delayed seed germination during the first 24 h in Vicia narbonensis L. and Zea mays L., but germination rate was not different among treatments up to 4000 μg/mL. At the highest concentration, both species exhibited small reductions in root elongation after 72 h 9. Using Evonik P25 nTiO2, Song et al. 9 found no significant effect on germination in tomato (Lycopersicon esculentum L.) when soaked in suspensions for 48 h, rinsed, and followed for 12 d. They also found no differences in tomato root elongation during a 15-d period following germination at nTiO2 exposures up to 5000 μg/mL. Larue et al. 10 found no significant effect of Evonix P25 up to 1000 μg/mL on Triticum aestivum, Brassica napus, or Arabidopsis thaliana germination following 7 d of exposure in hydroponics; however, nTiO2 increased root elongation in Triticum aestivum 11. Feizi et al. 12 found no significant effect on germination rate or root length when Triticum aestivum L. was exposed to either bulk or Evonik P25 nTiO2 on wetted filter paper for 8 d.
Very few studies have examined the effects of nCeO2 on germination. Tumburu et al. 13 found enhanced germination in Arabidopsis thaliana exposed to up to 500 μg/mL nCeO2. Lopez-Moreno et al. 14 found reduced germination of soybean at an intermediate concentration of nCeO2, but they dismissed the response in the context of their other findings. In the same study, soybean root elongation increased at all tested nCeO2 concentrations. Other metal oxides have been found to alter germination. Lee et al. 15 found that inhibition in Arabidopsis seed germination by nano-zinc oxide (nZnO) was related to particle size, with smaller particles exhibiting greater inhibition than larger particles.
We examined nTiO2, widely used in paints, sunscreens, and other coatings, because of its ability to diffuse ultraviolet (UV) light, and nCeO2, used in coatings such as paints and in some cases diesel fuel as a combustion catalyst. We used commercial sources for the particles rather than synthesizing them in the laboratory to enhance the comparability of the results. We used a slightly modified standard 10-species germination test 16 for our procedure, imbibing seeds with ENM suspension for 24 h before transferring to a Petri plate, removing root substrates that may interfere with particle interactions, and assessing early root growth and cotyledon development to identify sublethal changes in early development. The present study's experiments were undertaken to 1) determine if uncoated nCeO2 and nTiO2 affect a wide range of commercially important plant species, 2) evaluate the appropriateness of using simplified germination as an endpoint to screen for potential nanoparticle toxicity, and 3) examine in more detail the effects of metal oxide ENMs on early plant growth and development.
MATERIALS AND METHODS
Nanoparticle preparation and characterization
nTiO2
Noncoated nTiO2 P25 Evonik Degussa was used for all experiments. Stock suspensions (1000 μg/mL) were prepared in 1 mM KCl using Milli-Q water. We found that 1 mM KCl increased nTiO2 stability as zeta potential remained stable without a significant change in measured hydrodynamic diameter (measured by dynamic light scattering). Particles were added to diluent, and suspensions were sonicated at 50% power (∼100 W) for 30 min using indirect bath sonication with a cup-horn 17. Subsequent dilutions of 250 μg/mL and 500 μg/mL were made using 1 mM KCl in Milli-Q water.
nCeO2
Noncoated nano-ceria (Mercator) were used for all experiments. Stock suspensions (1000 μg/mL) were prepared in Milli-Q water. Particles were added to diluent, and suspensions were sonicated indirectly as described in nTiO2, above. Subsequent dilutions of 250 μg/mL and 500 μg/mL were made using 1 mM KCl in Milli-Q water.
Suspensions were characterized using a zeta potential analyzer (Brookhaven ZetaPALS) and transmission electron microscopy.
Experimental design
Seeds of lettuce (Lactuca sativa, black seeded Simpson), tomato (Lycopersicon lycopersicum, roma VF), cabbage (Brassica oleracea, late flat Dutch premium), soybean (Glycine max, envoy), carrot (Daucus carota, tendersweet), perennial ryegrass (Lolium perenne, pinnacle), corn (Z. mays, golden bantam), cucumber (Cucumis sativus, straight eight), oat (Avena sativa, common oat), and onion (Allium cepa, hybrid yellow sweet Spanish) were surface-sterilized with 70% ethanol (2–3 min depending on seed size) followed by 50% bleach (5–15 min), rinsed for 1 min multiple times (4–7 times) with MilliQ water, and allowed to air-dry.
The testing procedure was modified slightly from the guidelines outlined in OPPTS 850.4200 16. Briefly, filter paper was used in the Petri dishes instead of sand or glass beads. In addition, light was provided during the test period to examine the potential role of photoreactivity of the nTiO2. Finally, additional developmental measures were made to identify cotyledon development and rate of early root growth in the germinants.
Five replicates of 15 seeds were placed in test tubes for each of 4 nTiO2 and 4 CeO2 treatments (0 μg/mL, 250 μg/mL, 500 μg/mL, and 1000 μg/mL), yielding 20 tubes per species. Small seeds were placed in 1.5-mL tubes and treated with 1 mL treatment suspension (cabbage, carrot, lettuce, ryegrass, onion, tomato), whereas medium-sized seeds (cucumber and oats) and large seeds (soybean and corn) were placed in 15-mL or 50-mL tubes and treated with 10 mL or 20 mL of treatment suspensions, respectively. All tubes were placed on a rocker table and shaken at room temperature (∼25 °C). After 24 h, the seeds were poured onto a 15-cm Petri plate with filter paper (Whatman 2; Whatman International). Additional suspension volumes were added (9 mL to plates with small seeds to total 10 mL, 5 mL to plates with medium and large seeds to total 15 mL or 25 mL, respectively). The 15 seeds per plate were positioned in 1 or 2 lines within the plate, depending on seed size. Plates were then covered and sealed with parafilm. Each plate was randomly positioned on a shelf, angled against a 3.8 cm × 3.8 cm support to encourage linear radicle growth in the growth chamber (Adaptis; Conviron). Temperature was 25 °C, and light cycled in 16:8-h light:dark periods (200–250 μmol m−2 s−1), using bulbs that included wavelengths in the UV range (25% of bulbs) and standard bulbs (75%). Treatments and replicates were randomly placed on a single shelf in the chamber to ensure uniform lighting. The number of seeds germinated (radicle visible), the length of the longest radicle, and the number of radicles >2 cm were recorded daily following plating. The number of seeds with cotyledons showing green and with fully expanded cotyledons also were noted daily. When 65% of the control (1 mM KCl in Milli-Q water) roots had reached 2 cm or when there were no new germinants for 2 consecutive observations, the study was stopped for the respective ENM and the root length for each germinant was measured.
Data were analyzed using regression and analysis of variance. Results were considered significant at p < 0.05 and marginally significant at p < 0.10. An arcsine transformation was used for percentage data.
RESULTS AND DISCUSSION
Nanoparticle characteristics
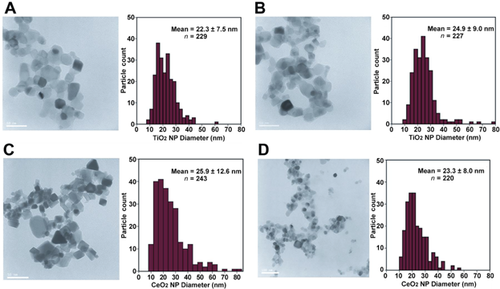
Nano-TiO2 had hydrodynamic diameters ranging from 130 nm to 295 nm and a zeta potential of +19 to 34 (pH <5), while comparable values for nCeO2 particles in suspension were 230 nm to 290 nm and +38 to 44 (pH <5). Transmission electron microscopy was performed on 100-μg/mL and 1000-μg/mL suspensions to bracket the range of concentrations used for toxicity, and particle size varied from 22.3 ± 7.5 nm to 24.9 ± 9.0 nm for nTiO2 and from 25.9 ± 12.6 nm to 23.3 ± 8.0 nm for nCeO2 (Figure 1). These are comparable to sizes reported by the manufacturer.
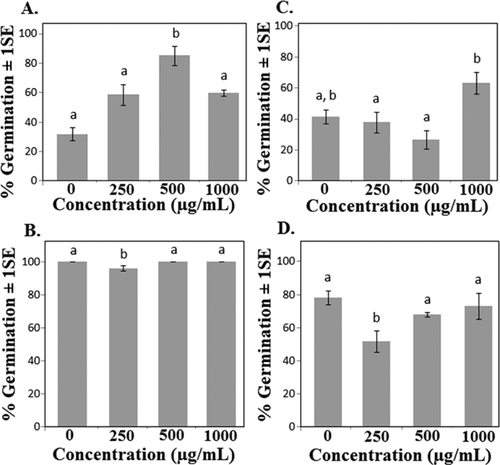
Phytotoxicity of nTiO2
Eight of the 10 species tested responded to nTiO2 through a change in percentage of seeds germinated, timing of cotyledon development, or average root length (Table 1). Germination was significantly altered in 5 of the species, although in some species it was enhanced and in others suppressed (Figure 2). Also, in some cases, germination was accelerated during the first few days of exposure. To test for these differences, germination rate was calculated as the number of days for treated seeds to reach 50% of the final control germination rate. Germination rate significantly increased in cabbage and oats (p < 0.02 and 0.07, respectively; data not shown), and showed a nonsignificant decreasing trend in soybean. Final germination percentages showed both increases and decreases in oats, depending on the concentration (Figure 2). In 3 of the 4 species where germination was affected, the response was observed at lower concentrations but not at the highest concentration tested. A nonlinear dose response also was reported for corn and cabbage in germination and root elongation with citrate-nAg and nZnO treatments using the standard OCSPP 850.4200 protocol 18. Accelerated germination has been observed in plants exposed to multiwalled carbon nanotubes, and the effect may have been related to particle penetration into the seed coat and accelerated uptake of water during early stages of seed germination 19, 20. Although the underlying mechanisms of response reported in the present study are unknown, responses may be related to the photocatalytic activity of TiO2 21, possibly acting on the seed coat. Additional studies will be needed to evaluate the kinetics of particle uptake, through both the seed coat and the developing tissues of leaves and roots. Our past studies indicate that uptake is occurring during this stage, based on changes in gene expression in Arabidopsis thaliana germinants 22.
| TiO2 | CeO2 | |||||
|---|---|---|---|---|---|---|
| Species | Germination | Cotyledon presence | Average root length | Germination | Cotyledon presence | Average root length |
| Cabbage | 0.000 (I) | 0.001 (I) | 0.045 (D) | NS | NS | NS |
| Carrot | NS | NS | NS | NS | NS | NS |
| Corn | NS | NS | 0.001 (D) | NS | NS | NS |
| Cucumber | 0.006 (D) | 0.060 (D) | 0.000 (I) | NS | 0.065 (D/I) | NS |
| Lettuce | NS | NS | 0.016(D) | NS | NS | 0.000 (D) |
| Oats | 0.006 (D/I) | 0.004 (D/I) | 0.072 (D) | NS | NS | NS |
| Onion | NS | NS | 0.020 (I) | NS | NS | 0.026 (I) |
| Ryegrass | NS | NS | NS | NS | NS | 0.029 (I) |
| Soybean | 0.096 (D) | NS | NS | NS | NS | NS |
| Tomato | NS | 0.042 (I) | NS | NS | NS | 0.023 (D) |
- a The letters I, D, and D/I indicate increased, decreased, and both decreased and increased responses following treatment, respectively.
- nCeO2 = nano–cerium oxide; nTiO2 = nano–titanium dioxide; NS = not significant
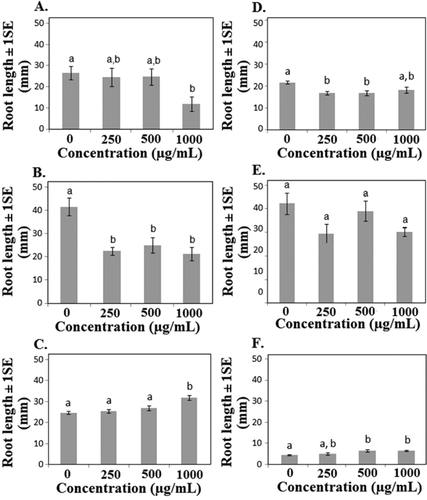
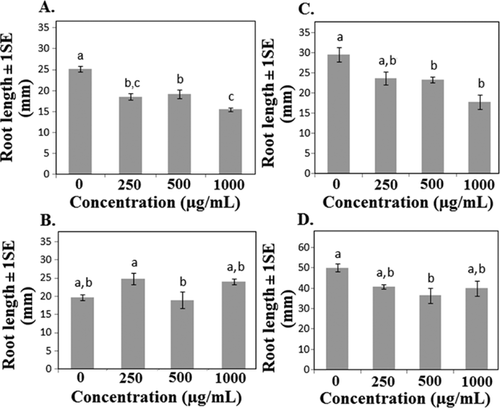
Six of the 10 species showed a significant or marginally significant change in average root length in response to nTiO2 exposure, with 4 of the 6 species showing decreased and 2 species showing increased root length (Table 1). Since final root length was measured after a standard period of time, the values shown in Figures 3 and 4 also reflect differences in root growth rate. Nano-TiO2 increased average root length in cucumber and onion, while root length decreased in cabbage, corn, lettuce, and oats (Table 1 and Figure 3). Average root length did not appear to be related to shifts in phenological development (e.g., accelerated germination was not necessarily coupled with increased average root length), and in cucumber decreased germination and cotyledon presence were associated with increased, rather than decreased, root length (Table 1 and Figures 3 and 5). Although mechanisms related to altered root growth are unknown, Ghosh et al. 22 found increased DNA “shearing” in A. cepa roots in response to nTiO2 exposure. Nicotiana tabacum leaves also showed increased DNA fragmentation, although results in the 2 species did not appear to be ENM concentration–dependent 22. Klancnik et al. 23 found changes in mitotic index in A. cepa root tip cells but concluded that nTiO2 had low toxic potential. Titanium dioxide is known to be photocatalytic in the presence of UV light, so the results may reflect responses to generation of reactive oxygen species 21. These free radicals could adversely impact root growth directly or may alter root growth through suppression of pathogenic or beneficial fungi 24, 25. Collectively, the results suggest that effects of nTiO2 on root growth may lead to changes in other important processes.
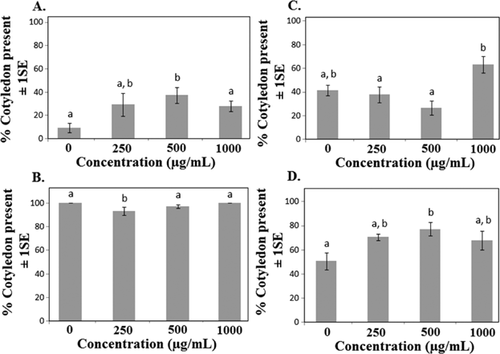
The present results are consistent with the results of others who showed a range of responses following exposure to nTiO2 7-9. Zheng et al. 7 found increased germination rates in response to nTiO2, while Castiglione et al. 8 observed opposite responses in corn (Z. mays) and bean (Vicia narbonensis), with nTiO2 exposure resulting in decreased germination and early development. There were also indications of reduced mitotic index and evidence of potential genotoxic effects in both species. Song et al. 9 found no effect of nTiO2 on germination, root growth, or biomass in tomato (L. esculentum). It appears that nTiO2 may have relatively low toxicity, but conflicting reports in the literature also may be the result of subtle methodological differences among laboratories.
Phytotoxicity of nCeO2
Nano-CeO2 did not significantly alter germination in any of the 10 species tested, and only cucumber showed a marginally significant change in cotyledon development (p < 0.1) (Table 1). Very few studies have examined the effect of nCeO2 in plants. Lopez-Moreno et al. 14 concluded that nCeO2 did not reduce soybean (Glycine max) germination except at 1 intermediate concentration, but there was an increase in root development. Further analysis indicated that nCeO2 caused genotoxic effects in soybean 14. Germination also was unaffected by nCeO2 in rice (Oryza sativa), wheat (Triticum aestivum), and barley (Hordeum vulgare) 26, 27. Tumburu et al. 13 found accelerated germination and early seedling development in Arabidopsis thaliana with nCeO2 treatment. In addition, they found that altered early seedling development was related to changes in gene expression 13.
Even though nCeO2 did not affect germination in any of the species examined, it was found to alter average root length and, hence, root growth rate in 4 of 10 species tested (Table 1). Average root length was decreased in 3 of the 4 species showing a response to nCeO2, while the response in onion roots showed significant main effects, although variation among treatments was high (Figure 4). Coupled with the results from nTiO2 exposures, average root length appeared to be more sensitive to the ENM exposure than did germination, with 8 of the 10 species showing a change in average root growth in response to either nTiO2 or nCeO2 (Figures 3 and 4). It is possible that developing roots are susceptible to metal ENM exposure and may be a useful early indicator of potential toxicity. Lopez-Moreno et al. 14 found that nCeO2 increased root elongation in soybean at all levels tested, including 4000 μg/mL, which was 4 times greater than the highest concentration used in the present study. In the same study, nZnO increased root elongation at the lowest concentrations but significantly reduced root elongation at higher concentrations, suggesting different modes of action for the 2 metal oxide ENMs. Zinc is a plant micronutrient that is toxic at high concentrations, which may explain the differential responses at different concentrations 14.
Species and particle differences
Lettuce, onion, ryegrass, and tomato were the only species that responded to both ENMs; and only 1 species, carrot, did not respond to either ENM (soybean showed only a marginal response in germination to nTiO2). The differential sensitivity exhibited among species to the 2 chemically different ENMs suggests that the mode of action of the 2 types of ENMs is different, possibly related to the greater photocatalytic activity in TiO2 compared with CeO2. Different modes of action are supported by other experiments in our laboratory and in other species, where exposure to nTiO2 and nCeO2 altered different genes in Arabidopsis thaliana 13. Similarly, a recent study using 5 different types of metal-based ENMs also noted differential responses in Escherichia coli 2. Additional research will be necessary to fully identify how these 2 ENMs differ in altering early plant growth and development.
In 3 of the 4 species showing a significant germination response to TiO2, the degree of response was not concentration-dependent (Figure 2). One explanation is that the standard germination test may not be a sensitive indicator of nanoparticle effects in plants. Similarly, the timing of the development of cotyledons generally followed a similar pattern to germination; that is, under those treatments where germination occurred earlier, earlier development of cotyledons also occurred, regardless of ENM concentration. However, the nonlinear responses observed in the present study and others may indicate the shortcomings of using a test designed for soluble chemicals to test for toxicity of particles across a range of concentrations in suspension 18, 28. Unlike toxicity tests employing soluble substances, the metal oxide ENM test media we used do not behave as solutions; and therefore, it can be difficult to interrogate the nature of the dose–response relationship. Comparable particle suspensions under a range of environmental conditions may differ in agglomeration rate, surface charge, and particle interactions with test subjects at the surfaces of cells 4, 5, 29, 30. The literature suggests that, even with the use of similar experimental protocols, different results may occur because the high physicochemical reactivity of the ENMs can be very responsive to small differences in preparation procedures among studies. Because both the ENMs used in the present study had similar hydrodynamic diameters, transmission electron microscope diameter, and surface charge, additional research will be needed to determine whether bimodal responses are related to physicochemical interactions or the result of varying modes of action of ENMs across a range of types of living cells 13 or test species used 18, 28, 31.
The present results suggest that nTiO2 and nCeO2 do not cause widespread acute toxicity during germination and early growth of these 10 agronomic species. However, lengthening the observation period typically used in germination tests, as done in the present study, does provide additional information regarding early developmental shifts that may lead to altered growth during later stages of plant development. Future work on evaluating the direct transfer of toxicity tests designed for “traditional chemicals” for use with ENMs needs to examine in more detail the effects of particle size, solution pH, and zeta potential of particle suspensions on tissue responses in general and on seed germination in particular. Better understanding of these physicochemical relationships and how they correlate with responses in early plant development will help link traditional toxicity test results with plant growth and development under more natural conditions. In addition, studies examining the uptake of these particles by the seed coat, developing roots, and leaves will be needed to elucidate specific mechanisms of response. Such information will provide insights on how to develop early indicators of response that may lead to changes at broader scales, allowing better prediction of how ENMs may interact in terrestrial ecosystems.
Acknowledgment
The present study was funded by the US Environmental Protection Agency. It has been subjected to the agency's peer and administrative review, and it has been approved for publication as a US Environmental Protection Agency document. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. The authors declare no competing financial interest.
Data availability
Readers may contact C.P. Andersen ([email protected]) or G. King ([email protected]) for original data files.




