Relationships between polybrominated diphenyl ethers and transcription and activity of type 1 deiodinase in a gull highly exposed to flame retardants
Abstract
Deca-brominated diphenyl ether (deca-BDE), composed mainly of BDE-209, is subject to usage restrictions in North America and Europe, although global action on its continued use has yet to be undertaken. Relatively large concentrations of polybrominated diphenyl ethers (PBDEs), especially BDE-209 and its higher brominated degradation products, have been reported in tissues of ring-billed gulls (Larus delawarensis) breeding near the densely populated city of Montreal (QC, Canada). There is limited knowledge of BDE-209 biotransformation and toxicokinetics in birds. Deiodinases, a class of enzymes catalyzing thyroid hormone conversion, have been suggested to be involved in BDE-209 debromination in birds. The objective of the present study was to investigate the relationships between PBDE concentrations and type 1 deiodinase (D1) transcription and in vitro activity (microsomes) in livers of Montreal-breeding ring-billed gulls. The ring-billed gulls exhibiting the highest D1 activity in liver microsomes accumulated the greatest liver concentrations of hepta-BDEs and octa-BDEs. Activity of D1 was inversely related to concentration ratios of BDE-209 to octa-BDEs and ∑hepta-BDE. An even stronger inverse relation was found between D1 activity and BDE-209 to ∑nona + octa + hepta-BDE concentration ratios. The messenger ribonucleic acid (mRNA) levels of D1 in gull livers were inversely associated with liver concentrations of ∑octa-BDE. The present study's findings suggest that D1 is potentially involved in BDE-209 biotransformation and accumulation of higher brominated PBDEs in livers of ring-billed gulls. Environ Toxicol Chem 2016;35:2215–2222. © 2016 SETAC
INTRODUCTION
Polybrominated diphenyl ethers (PBDEs) are a class of halogenated flame retardants used in a range of consumer products (e.g., plastics, upholstered furniture, textiles, electronics, and insulation materials) to delay ignition, and thus comply with international fire safety standards. In 2009, the production and use of 2 commercial PBDE formulations (penta-BDE and octa-BDE) were banned under the Stockholm Convention on Persistent Organic Pollutants (POPs) 1. The use of deca-BDE, consisting of >97% of the fully brominated congener BDE-209, was recently (2014) phased out in North America and Europe, although it is still used in many countries in Asia 2.
Bioaccumulation of BDE-209 and several higher brominated PBDE congeners has been reported in tissues of birds worldwide. For instance, in Europe, BDE-209 was found in peregrine falcon (Falco peregrinus) eggs from north (mean: 4.63 ng/g wet wt) and south (mean: 4.79 ng/g wet wt) Sweden 3. Even greater BDE-209 concentrations have been reported in peregrine falcon eggs from North America (up to 136 ng/g wet wt in California 4 and 50.0 ng/g wet wt in the northeast United States 5). In Asia, liver tissue of common kestrels (Falco tinnunculus) from northern China (Beijing area) contained unusually large BDE-209 concentrations (mean: 367 ng/g wet wt) 6. A study of free-ranging chickens (Gallus domesticus) sampled near an e-waste recycling site in China 7 also reported moderate liver concentrations of BDE-209 (mean: 5.20 ng/g wet wt) and 2 of its potential debromination products (BDE-207, mean: 2.0 ng/g wet wt and BDE-196, mean: 0.38 ng/g wet wt). In ring-billed gulls (Larus delawarensis) breeding near the densely populated city of Montreal (QC, Canada), BDE-209 represented 1 of the major PBDE congeners found in liver (mean: 57.2 ng/g wet wt), making up between 10% and 20% of ∑45PBDE congeners 8. Several higher brominated PBDE congeners that are known or putative degradation products of BDE-209 (e.g., BDE-196, BDE-197, BDE-201, BDE-202, BDE-203, BDE-204, BDE-207, and BDE-208) were also detected in livers of these ring-billed gulls 8, 9, as well as in other species of birds from different regions 4, 6, 7.
Sequential debromination was suggested as the main biotransformation pathway for BDE-209 in birds 9-11. For instance, Van den Steen et al. 10 demonstrated that European starlings (Sturnus vulgaris) exposed to BDE-209 released from silastic implants accumulated octa-BDE and nona-BDE congeners (e.g., BDE-197, BDE-206, BDE-207, and BDE-208), which suggested that in vivo debromination of BDE-209 occurred in these birds. In another study conducted by Letcher et al. 11, greater concentrations of BDE-196, BDE-197, BDE-203, BDE-206, BDE-207, and BDE-208 were found in American kestrels (Falco sparverius) fed cockerels (G. domesticus) injected with BDE-209. However, to our knowledge, no information is available on the enzyme system involved in the debromination of BDE-209 in birds. Nonetheless, a study by Chabot-Giguère et al. 9 showed that BDE-209 incubated with ring-billed gull liver microsomes (reduced nicotinamide adenine dinucleotide phosphate was used as cofactor) for 90 min did not yield higher brominated PBDE congeners, suggesting that cytochrome P450 isoenzymes are unlikely to be involved in the biotransformation of BDE-209 in livers of this species. It was postulated in that study, and in another conducted on fish 12, that deiodinases may catalyze bromine removal from BDE-209 in vertebrates.
Deiodinases are membrane-bound selenocystein enzymes associated with the endoplasmic reticulum in vertebrates 13. The 3 isoforms of this enzyme (D1, D2, and D3) differ in their activity and distribution in peripheral tissues. Deiodinases catalyze iodine removal from the prohormone thyroxine (T4) to yield the active thyroid hormone triiodothyronine (T3) by outer ring deiodination or its inactive forms, reverse-T3 (rT3) and diiodothyronine (T2), by inner ring deiodination 14, 15. The D1 form has been shown to maintain equilibrium of thyroid hormone levels during altered thyroid events (e.g., thyrotoxicosis and iodine deficiency) 15. It may also play a role in thyroid hormone clearance via formation of T2 through both outer ring deiodination and inner ring deiodination. Because PBDE congeners share some structural similarities with thyroid hormones, a growing number of studies have suggested that deiodinase-mediated debromination of PBDEs may take place in vertebrates. For example, Noyes et al. 16 fed BDE-209 to fathead minnows (Pimephales promelas) for up to 28 d, followed by a 14-d depuration period, and observed changes in deiodinase (D1 and D2) messenger ribonucleic acid (mRNA) levels in liver. It was suggested that D1 mRNA expression levels were up-regulated during the depuration period, whereas D2 mRNA showed a transient up-regulation at day 14 and day 42, and control-like mRNA levels at day 28 16. To our knowledge, no study has explored the linkages between tissue PBDE accumulation and deiodinase expression and activity in birds.
The objective of the present study was to investigate the relationships between liver PBDE concentrations, and transcription and in vitro activity of D1 (a major deiodinase in bird liver) in ring-billed gulls breeding near the metropolis of Montreal. The ring-billed gull is the most abundant gull species breeding in this area of the St. Lawrence River 17. This urban-adapted omnivorous bird is known to forage in a wide array of anthropogenic-related habitats (e.g., waste management facilities and urban areas), and was shown to be exposed to high concentrations of PBDEs, especially BDE-209 18. Moreover, high PBDE exposure in male ring-billed gulls from the Montreal area has been suggested to adversely affect bone (tarsus) mineral density (i.e., bone tissue demineralization) 19, which could be linked, among other factors, to thyroid hormone disruption. Recent findings from our laboratory further showed that PBDE concentrations in ring-billed gulls were positively associated with the ratio of total T4 to T3 levels in plasma, and transcription of several genes involved in the hypothalamic–pituitary–thyroid (HPT) axis (i.e., synthesis, metabolism, transport, and action) 20. We hypothesized that D1 transcription and in vitro activity in livers of ring-billed gulls are related to liver concentrations of BDE-209 and its potential debromination products.
MATERIALS AND METHODS
Sample collection
Fieldwork was carried out in May 2013 on Deslauriers Island, located in the St. Lawrence River, 3.25 km downstream of Montreal. Approximately 44 000 pairs of ring-billed gulls nest on this island annually 21, which makes it the largest colony in eastern Canada. Adult males (n = 8) and females (n = 14) were randomly selected in all areas of the colony and live-captured while incubating using a nest trap triggered from a distance by a remote control. Birds were euthanized by cervical dislocation. The left lobe of the liver was collected within 5 min of euthanasia, and aliquots were stored immediately in liquid nitrogen and RNALater (Qiagen). The right lobe of the liver was kept on ice in a cooler while in the field. In the laboratory, the left lobe of the liver stored in liquid nitrogen was transferred to a –80 °C freezer until microsome preparation (see Hepatic microsome preparation section) and the right lobe was kept in a –20 °C freezer until chemical analysis (see PBDE analysis section). The liver (left lobe) aliquot kept in RNALater was stored at –80 °C until D1 RNA extraction (see Hepatic D1 mRNA transcription analysis section).
Permission to collect ring-billed gulls was granted by the Canadian Wildlife Service (permit no. SC-23), and capture and handling methods were approved by the Institutional Committee on Animal Care of the Université du Québec à Montréal (permit no. 768), and complied with the guidelines issued by the Canadian Council on Animal Care (Ottawa, ON, Canada).
PBDE analysis
Liver samples were analyzed for 31 PBDE congeners. The sample preparation procedures, which included lipid content determination, extraction, and clean-up employed for the analysis of PBDEs in tissues of ring-billed gulls, have been previously described 18, and were applied without modification. Identification and quantification of target analytes were performed using a gas chromatograph (GC) coupled to a single-quadrupole mass spectrometer (MS; Agilent Technologies 5975C Series) operating in the electron capture negative ionization mode (GC/MS-ECNI) 18. The analytical column (15 m × 0.25 mm × 0.10 μm) was a fused-silica DB-5 HT capillary column (J & W Scientific). Quality control and assurance procedures included procedural method blanks and standard reference material (SRM 1947; Lake Michigan fish tissue) for each batch of ten samples. Blank correction of all samples was performed for the following PBDE congeners: BDE-47, BDE-197/204, BDE-206, and BDE-209. Mean (± standard error of the mean [SEM]) internal standard recoveries from ring-billed gull liver, blank, and SRM 1947 samples were as follows: BDE-30 (90.7 ± 2.4%), BDE-156 (92.9 ± 3.6%), and [13C]BDE-209 (57.9 ± 3.7%). Concentrations of PBDEs were quantified using an internal standard approach, and thus all analyte concentrations were recovery-corrected. Method limits of detection (defined as signal-to-noise ratio (S/N) = 3) and method limits of quantification (minimum amount of analyte producing a peak with S/N = 10) were based on replicate analyses (n = 8) of matrix samples spiked at a concentration of 3 to 5 times the estimated detection limit 8.
Hepatic D1 mRNA transcription analysis
Total RNA was extracted from 10 mg to 20 mg of ring-billed gull liver stored in RNALater (kept at –80 °C) using the RNeasy plus mini kit (Qiagen) following the manufacturer's instructions. Each sample was first disrupted in 50 μL of chilled RLT Plus buffer (Qiagen) using a sterile plastic pestle, and then 550 μL of RLT Plus buffer was added and further homogenized. A genomic DNA elimination step was included using a gDNA eliminator spin column (Qiagen) at 12 000 g for 30 s at room temperature. The RNA concentration and purity were estimated using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific). The absorbance ratios (A260/A280) were between 1.9 and 2.1. The RNA integrity was checked against the Experion Automated Electrophoresis System (Bio-Rad) using the Experion RNA StdSens Analysis kit (Bio-Rad). The RNA Quality Indicator values were between 9 and 10. A total volume of 20 μL complementary DNA (cDNA) was prepared using the QuantiTect Reverse Transcription kit (Qiagen) following the manufacturer's instructions, and stored at –20 °C until real-time quantitative polymerase chain reaction analysis (qPCR).
Gene sequences were not available for D1 or the 3 reference genes (see below) for ring-billed gulls. A primer pair designed by Crump et al. 22 for chicken (Gallus gallus) D1 (access number: NM_001097614.1; 5′-TCTTTGTGCTGAAGGTGAAGTGG-3′ [forward] and 3′-AGGTCGGTTATCTCGCATGAAAC-5′ [reverse]; amplicon length 133 bp) and manufactured by IDT was validated for ring-billed gulls as part of a companion study [20]. Primer pairs for the 3 reference genes were designed based on chicken mRNA sequences available at the National Center for Biotechnology Information (NCBI) with Primer-BLAST from the NCBI (Primer3 with Blast 23). The primer pairs used to amplify the selected reference genes (analysis by geNorm and Normfinder in GenEx) were as follows: 5′-ACGGAGAATGTCGTGGTGTT-3′ (forward) and 5′-GCAGTGCCATCACCTGTACT-3′ (reverse), (amplicon length 151 bp) for succinate dehydrogenase complex subunit A, 5′-ACTACAACCACCTGATGCCC-3′ (forward) and 5′-CTTCACGTCTTGCTTTGCGT-3′ (reverse), (amplicon length 101 bp) for TATA box binding protein, and 5′-CATCACAGCAAGCGACACAG-3′ (forward) and 5′-GTACAGAGGTGTGGTTCCCG-3′ (reverse), (amplicon length 114 bp) for ribosomal protein L27. A calibration curve was constructed for each primer pair (starting cDNA concentration: 500 ng, 8 serial dilutions) to estimate PCR efficiencies (values were between 90% and 110%) and limits of detection. The qPCR amplifications were run in 96-well plate, and each reaction was performed in triplicate. Each well consisted of 5 μL cDNA template diluted at 50 ng/μL, 6.5 μL iQ™ SYBR Green supermix (SYBR Green I dye, 25 units/mL iTaqTM DNA polymerase, 0.2 mM of each dNTPs, 3 mM MgCl2, 50 mM KCl, 20 nM Tris-HCl buffer, pH 8.4, 10 nM fluorescein, and stabilizers), 0.26 μL forward and reverse primers (200 nM each), and 0.98 μL diethylpyrocarbonate-treated water. Cycling parameters were 95 °C for 2 min, then 40 cycles at 95 °C for 15 s, and 60 °C for 5 s. Amplification specificity was verified with a melting curve. No-template control was included on each plate. Data acquisition was performed by CFX Manager software (Bio-Rad). Quantification cycle (Cq) values were then imported into GenEx 5.31 Enterprise software (MultiD Analyses), and fold change values were calculated after efficiency correction and normalization applying the comparative threshold method (ΔΔCt) 24 and Cq geometrical mean as reference.
Hepatic microsomal deiodinase activity determination
Hepatic microsome preparation
Ring-billed gull liver microsomes were prepared by differential ultracentrifugation based on previous work [9]. Briefly, 400 mg of liver was thawed on ice and homogenized using a Potter–Elvehjem tissue grinder in a phosphate buffer (0.1 M KH2PO4, 0.1 M Na2HPO4; pH 7.4). The homogenized liver was then transferred to a cool (∼4 °C) polyallomer tube and centrifuged at 9000 g for 15 min. The supernatant (S-9 fraction) was then centrifuged at 100 000 g for 60 min, and the resulting pellets were resuspended in a phosphate–sucrose buffer (0.1 M KH2PO4, 0.1 M Na2HPO4–0.25 M sucrose; pH 7.0). The final microsomal suspension was aliquoted in 2-mL cryovials that were kept at –80 °C until the D1 in vitro activity assay (see section D1 activity assay). The hepatic microsomal protein content was determined following the Lowry method 25.
D1 activity assay
Measurement of hepatic microsomal D1 activity was performed using an in vitro assay adapted from Noyes et al. 26. Preliminary tests were conducted to determine the optimal reaction time (30 min, 45 min, 60 min, 75 min, 90 min, or 120 min; Supplemental Data, Figure S1) and substrate (unlabeled-T4) concentration (0.25 μM, 0.5 μM, 0.65 μM, 0.75 μM, 1.0 μM, or 2.0 μM; Supplemental Data, Figure S2). The reaction using 1 mg of ring-billed gull hepatic microsomal proteins was found to be linear up to 75 min with a T4 concentration of 0.65 μM. All conditions, including reaction, blank, and control tubes were tested in duplicate. Briefly, reaction and control (A and B) tubes consisted of ring-billed gull liver microsomes (1 mg proteins) in a potassium phosphate buffer (0.1 M KH2PO4, 0.1 M Na2HPO4; pH 7.4) spiked with 0.5 μCi L-[125I]T4 (PerkinElmer) and 0.65 μM of T4. Control A tubes, in which no cofactor was added, were used to monitor for any nonmicrosomal biotic or abiotic transformation, while in control B tubes (i.e., negative controls), propyl-n-thiouracil (Sigma Aldrich) was used as specific D1 inhibitor 12-14 at concentration of 2.5 mM (100% inhibition in gull liver microsomes; see Hepatic D1 activity section and Supplemental Data, Figure S3) with the cofactor. Blank tubes consisted of ring-billed gull hepatic microsomes (1 mg proteins) in potassium phosphate buffer only. The final volume of the reaction was 500 μL for all tubes. The tubes were first preincubated for 5 min at 41 °C (bird body temperature) in a water bath, and the reaction was started by adding 10 mM of the cofactor dithiothreitol (Sigma Aldrich) to all tubes (except for control A) with a 5-μL syringe, and incubated for 75 min. The reaction was terminated by the addition of 500-μL ice-cold methanol (Sigma Aldrich), and the solution was centrifuged (9000 g; 10 min).
The concentrations of 125I-labeled T4 and its deiodinated products [125I]T2 and [125I]T3 (Sigma Aldrich) were determined using a narrow-bore high-performance liquid chromatograph (HPLC; 1200 series; Agilent Technologies) comprised of a degasser, binary pump, and automatic injector. The injection volume was 15 μL. The mobile phase (0.4 mL/min flow rate) was changed along a linear gradient between 0 min and 9 min from 48% methanol to 52% water (0.1% phosphoric acid) to 58% methanol to 42% water (0.1% phosphoric acid), and the final conditions were held for 14 min. A Zorbax Eclipse Plus C-18 column (Solvent Saver HT; 3 × 50 mm; 1.8-μm particle size) was used to separate [125I]T4, [125I]T2, and [125I]T3 (Supplemental Data, Figure S4), which were quantified using a PerkinElmer flow scintillation detector (Radiomatics model 610TR; 125 μL volume; Gamma C-Flow counting cell) with a ProFSA data acquisition package. Continuous peak monitoring was conducted at 230 nm using 400 nm as reference (series 1100 diode-array detector; Agilent Technologies), and an ultraviolet (UV) detector was used to determine retention times for authentic T4, rT3, T3, and T2 standards.
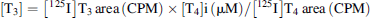
Statistical analysis
Normal distribution of the data was verified using the Shapiro–Wilk test. Because a normal distribution could not be achieved for most variables and because of low sample size, nonparametric tests were used. Wilcoxon tests were performed to determine potential differences in hepatic D1 transcription and activity as well as PBDE concentrations between male and female ring-billed gulls. Spearman rank correlation was used to examine the strength of the relationships between D1 transcription and activity, and PBDE concentrations. For these correlation analyses, combined males and females were used. The D1 relative quantities were log2-transformed to discriminate “higher” and “lower” D1 mRNA levels between individuals (males and females combined) compared with this subpopulation geometric mean D1 relative quantity. This analysis yielded 2 separate groups, a high (n = 11) and a low (n = 11) D1 mRNA transcription group, for which differences in D1 activity and PBDE concentrations were analyzed using the Wilcoxon test. The low D1 mRNA transcription group contained more males (n = 6) than females (n = 5), whereas the high D1 mRNA transcription group contained more females (n = 9) than males (n = 2). Liver concentration (ng/g wet wt) ratios of BDE-209 to ∑nona-BDE, ∑octa-BDE, and ∑hepta-BDE and their combined sums (∑nona + octa + hepta-BDE) were used as markers of sequential BDE-209 debromination, because these had been shown to be the most abundant congener classes in in vivo studies of birds dosed with BDE-209 [10,11] and to correct for differences in liver PBDE concentrations between males and females (Supplemental Data, Figure S5). The statistical package used was JMP 10.0.0 (SAS Institute), and tests were considered to be significant when p ≤ 0.05 or tendencies when 0.05 < p ≤ 0.1.
RESULTS
Hepatic PBDE concentrations
Tetra-BDE, penta-BDE, and deca-BDE (BDE-209) were the major congener classes found in ring-billed gull liver (Table 1). The 6 major PBDE congeners detected in both male and female ring-billed gull liver were (in decreasing order) BDE-99, BDE-47, BDE-209, BDE-153, BDE-100, and BDE-207 (Supplemental Data, Figure S5). There was no difference in concentrations of the sum of all 31 PBDE congeners (i.e., ∑31PBDE), ∑di-BDE through ∑penta-BDE, ∑hepta-BDE, and ∑nona-BDE between sexes. However, females tended to have lower ∑hexa-BDE (X2 = 3.40; p = 0.07) and ∑octa-BDE (X2 = 3.15; p = 0.08) concentrations compared to males. Liver BDE-209 concentrations were directly associated with those of ∑hepta-BDE (r = 0.66; p = 0.0007), ∑octa-BDE (r = 0.72; p = 0.0002), ∑nona-BDE (r = 0.98; p < 0.0001), and ∑nona + octa + hepta-BDE (r = 0.95; p < 0.0001) in combined male and female ring-billed gulls. Concentration ratios of BDE-209 to ∑nona-BDE (X2 = 4.48; p = 0.03), ∑octa-BDE (X2 = 7.45; p = 0.006), ∑hepta-BDE (X2 = 5.07; p = 0.02), and ∑nona + octa + hepta-BDE (X2 = 6.73; p = 0.01) were significantly lower in males compared to females. One male exhibited unusually high concentrations of PBDEs, and thus this bird was excluded from further analyses because it was identified as an outlier based on the jackknife test.
| Relative contribution | Mean ± SEM | Range | |
|---|---|---|---|
| ∑3Di-BDE | 0.1 | 0.16 ± 0.03 | <MLOQ–0.41 |
| Tri-BDE | 0.1 | 0.11 ± 0.02 | 0.02–0.39 |
| ∑5Tetra-BDE | 20.6 | 26.3 ± 3.81 | 9.36–68.6 |
| ∑5Penta-BDE | 39.5 | 50.4 ± 7.75 | 11.1–132 |
| ∑4Hexa-BDE | 12.3 | 15.8 ± 3.13 | 3.76–59.3 |
| ∑4Hepta-BDE | 0.5 | 0.62 ± 0.15 | <MLOQ–2.34 |
| ∑5Octa-BDE | 7.4 | 9.49 ± 1.85 | 1.50–32.5 |
| ∑3Nona-BDE | 6.9 | 8.81 ± 1.63 | 2.29–28.9 |
| Deca-BDE | 12.5 | 15.9 ± 1.90 | 2.98–32.5 |
| ∑31PBDE | – | 128 ± 18.6 | 39.8–334 |
- a ∑3Di-BDE: BDE-7, BDE-10, and BDE-15; Tri-BDE: BDE-17; ∑5Tetra-BDE: BDE-47, BDE-49, BDE-66, BDE-71, and BDE-77; ∑5Penta-BDE: BDE-85, BDE-99, BDE-100, BDE-119, and BDE-126; ∑4Hexa-BDE: BDE-153, BDE-139, BDE-140, and BDE-138; ∑4Hepta-BDE: BDE-171, BDE-180, BDE-184, and BDE-191; ∑5Octa-BDE: BDE-196, BDE-197/BDE-204, BDE-201, BDE-203, and BDE-205; ∑3Nona-BDE: BDE-206, BDE-207, and BDE-208; Deca-BDE: BDE-209.
- SEM = standard error of the mean; PBDE = polybrominated diphenyl ether; MLOQ = method limit of quantification.
Hepatic D1 transcription
The D1 gene transcripts were significantly greater in female (1.4-fold) compared to male ring-billed gulls (X2 = 4.19; p = 0.04). No relation was found between D1 mRNA levels and total D1 activity (see Hepatic D1 activity section) in combined males and females. In ring-billed gulls for which D1 mRNA levels were categorized in the low D1 transcription group relative to this subpopulation geometric mean, total D1 activity tended to be greater (1.3-fold) compared to gulls that were categorized in the high D1 transcription group (X2 = 2.18; p = 0.1; Table 2).
| Transcription group | |||
|---|---|---|---|
| Variables | Low (n = 11) | High (n = 11) | p value |
| Total D1 activity | 351 ± 19.6 | 278 ± 19.1 | 0.1 |
| Tri-BDE | 0.25 ± 0.05 | 0.18 ± 0.03 | 0.9 |
| ∑5Tetra-BDE | 28.2 ± 4.48 | 24.3 ± 3.15 | 1.0 |
| ∑5Penta-BDE | 53.6 ± 8.73 | 47.2 ± 7.00 | 1.0 |
| ∑4Hexa-BDE | 25.3 ± 4.92 | 14.7 ± 2.25 | 0.4 |
| ∑4Hepta-BDE | 4.56 ± 0.78 | 2.35 ± 0.40 | 0.07 |
| ∑5Octa-BDE | 13.7 ± 2.11 | 5.26 ± 0.97 | 0.006 |
| ∑3Nona-BDE | 29.8 ± 3.36 | 18.8 ± 2.81 | 0.1 |
| BDE-209 | 18.1 ± 1.69 | 13.7 ± 2.06 | 0.2 |
| ∑31PBDE | 157 ± 23.1 | 114 ± 15.5 | 0.4 |
| BDE-209:∑4hepta-BDE | 5.46 ± 0.77 | 7.56 ± 0.94 | 0.3 |
| BDE-209:∑5octa-BDE | 1.64 ± 0.21 | 3.25 ± 0.35 | 0.01 |
| BDE-209:∑3nona-BDE | 0.63 ± 0.02 | 0.72 ± 0.01 | 0.02 |
| BDE-209:∑nona + octa + hepta-BDE | 0.40 ± 0.02 | 0.52 ± 0.03 | 0.03 |
- a See Table 1 footnote for details on congener composition.
- b For explanation of high and low groups, see Statistical analysis section in Materials and Methods section.
- D1 = deiodinase type 1; PBDE = polybrominated diphenyl ether.
Hepatic D1 activity
Radiolabeled [125I]T3 and [125I]T2 were both quantified in liver microsomes (1 mg proteins) of ring-billed gulls incubated for 75 min with [125I]T4 (Supplemental Data, Figure S4). The addition of propyl-n-thiouracil in the assay resulted in the nondetection of [125I]T3 and [125I]T2 (Supplemental Data, Figure S4). Formation rates of [125I]T3 (X2 = 5.07; p = 0.02) and [125I]T2 (X2 = 2.91; p = 0.09) were significantly (or tended to be) lower in female ring-billed gulls compared to males. Total D1 ([125I]T3 + [125I]T2 formation rates) activity in male ring-billed gulls (mean: 377 ± 35.8 pmol/min/g; range: 213–568 pmol/min/g) was significantly higher (1.4-fold; X2 = 5.70; p = 0.02) compared to females (mean: 279 ± 20.1 pmol/min/g; range: 129–491 pmol/min/g).
Relationships between hepatic PBDE concentrations, and D1 transcription and activity
The D1 mRNA levels in liver of combined ring-billed gull males and females were negatively associated with liver concentrations of ∑octa-BDE (r = −0.45; p = 0.03) and individual octa-BDE congeners: BDE-201 (r = –0.49; p = 0.02), BDE-197/BDE-204 (r = –0.45; p = 0.03), although it was not significant for BDE-196 (r = –0.37; p = 0.08). No relation was found between liver D1 mRNA levels and concentrations of other PBDE congener classes or individual congeners. Combined males and females in the low D1 transcription group accumulated significantly greater (2.5-fold) liver concentrations of ∑octa-BDE (X2 = 7.43; p = 0.006); a trend that was also observed for ∑hepta-BDE concentrations in this same group (2-fold greater; X2 = 3.27; p = 0.07; Table 2). Moreover, gulls in the high D1 transcription group exhibited significantly greater BDE-209:∑nona + octa + hepta-BDE concentration ratios (1.3-fold; X2 = 4.84; p = 0.03), which was consistent with BDE-209:∑nona-BDE (1.2-fold greater; X2 = 5.13; p = 0.02) and BDE-209:∑octa-BDE (2-fold greater) concentration ratios (X2 = 6.39; p = 0.01; Table 2).
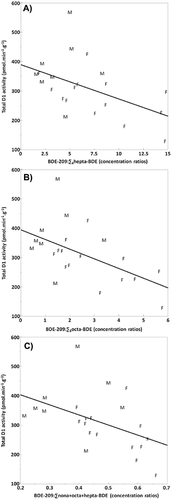
Total D1 activity in liver microsomes of gulls (males and females combined) was inversely related to the ratios of BDE-209:∑nona + octa + hepta-BDE concentrations (r = –0.55, p = 0.008; Figure 1). Consistent inverse relationships were observed for the following concentration ratios: BDE-209:∑octa-BDE (r = –0.48; p = 0.02; Figure 1), BDE-209:∑hepta-BDE (r = –0.47; p = 0.03; Figure 1), and BDE-209:∑nona-BDE (r = –0.35; p = 0.1). A positive relationship, although not significant, was found between total D1 activity and concentrations of ∑hepta-BDE (r = 0.40; p = 0.06) and ∑octa-BDE (r = 0.38; p = 0.08). Moreover, total D1 activity tended to be positively associated with concentrations of individual hepta-BDE and octa-BDE congeners: BDE-180 (r = 0.41; p = 0.06) and BDE-197/BDE-204 (r = 0.39; p = 0.07). No relation was found between total D1 activity, and BDE-209 and ∑31PBDE liver concentrations.
DISCUSSION
PBDE concentrations
Hepatic BDE-209 concentrations in Montreal-breeding ring-billed gulls were generally greater relative to those reported for several species of birds from Europe 3, 27, but were within the same concentration range as reported for birds of prey, for example, peregrine falcons in North America [5]. However, BDE-209 concentrations in ring-billed gulls were lower than in several avian species from Asia 4, 6, 7, where no usage restriction on deca-BDE has thus far been undertaken 2. Moreover, PBDE concentrations and congener patterns found in liver of ring-billed gulls collected in 2013 differed slightly from those collected by Gentes et al. [8] from the same breeding colony in 2010. More specifically, mean BDE-209 concentrations in gulls in the present study were lower and contributed less to ∑45PBDE concentrations (13.7%) compared with 2010 (25%) 8. One potential explanation would be that these differences are attributed to the over-representation of males in the 2010 sample (6 females and 22 males, with no sex-specific means reported) relative to the present study (14 females and 8 males). In fact, because ring-billed gull sample collection was carried out between 1 d and 28 d post egg-laying in both these years, females may have transferred a significant PBDE burden to their eggs [28]. Interestingly, liver concentrations of higher brominated congeners (∑hepta-BDE and ∑nona-BDE, and BDE-209) in female ring-billed gulls did not differ from those of males, whereas ∑hexa-BDE and ∑octa-BDE concentrations tended to be lower in females compared to males. This general PBDE pattern difference between sexes was consistent with the in ovo transfer dynamics for PBDEs reported elsewhere that showed preferential deposition of lower brominated PBDEs into eggs compared with higher brominated congeners (28, 29). Furthermore, in a recent study 18, differences in foraging site preferences (i.e., time spent in waste management facilities) led to greater BDE-209 concentrations in plasma of male ring-billed gulls breeding in the Montreal area, but not in females, which suggests that foraging behavior may in part explain these sex-specific differences in liver PBDE profiles.
Concentrations of hexa-BDE, octa-BDE, and nona-BDE congeners that accumulate in ring-billed gull liver can be influenced by environmental exposure to BDE-209 degradation products originating from abiotic (e.g., UV light and heat) and biotic processes (e.g., bacterial activity and metabolism in vertebrates) 30, 31. For example, exposure of BDE-209 to UV light was shown to yield hexa-BDEs and traces of other lower brominated congeners by photolytic debromination 30. Nonetheless, hexa-BDE, octa-BDE, and nona-BDE congeners are also found in trace amounts in octa-BDE and deca-BDE commercial mixtures 32. Hence, a portion of the higher brominated PBDE burden in ring-billed gull liver resulting from dietary and/or atmospheric exposure can originate, at least in part, from these sources. However, because ring-billed gulls accumulate a series of potential BDE-209 debromination products (e.g., BDE-196, BDE-197, BDE-201, BDE-206, BDE-207, and BDE-208) [8,9; present study] that are rarely detected in tissues of aquatic organisms such as fish collected from the St. Lawrence River near Montreal 33, further suggests that nondietary exposure may be more important than dietary exposure in explaining the bioaccumulation of these higher brominated congeners in this species 18. This is partly supported by a study of Gentes et al. 18, who found positive associations between the time spent foraging (determined using miniature GPS data loggers) in waste management facilities where large amounts of PBDE-containing products are discarded, and BDE-209 concentrations in plasma of male ring-billed gulls. Alternatively, in vivo biotransformation of BDE-209 could also have occurred in ring-billed gull (e.g., debromination pathways mediated by hepatic deiodinases).
Hepatic deiodinase transcription and activity
Type 1 deiodinase in liver of vertebrates is implicated in both outer ring and inner ring deiodination of thyroid hormones, which leads to the formation of the 3 main deiodinated products: T3, rT3, and T2. The D1 possesses greater substrate affinity for rT3 and T3 relative to T4 16. The HPLC chromatograms of ring-billed gull hepatic microsomes incubated with T4 (and dithiothreitol as cofactor) showed that 2 peaks corresponding to T3 and T2 were formed in the 75-min in vitro assay. An rT3 peak was identified in a few samples, although values were below the method detection limit for quantification in all samples. This suggests that both outer ring and inner ring deiodination took place in this in vitro assay; however, no information on the relative contributions of these 2 iodine-removal pathways could be obtained. Propyl-n-thiouracil was used as a specific inhibitor of D1 16, which has been shown to be the major deiodinase type present in bird liver, followed by much smaller activities of D2 and D3 13, 34. Because D3 is also capable of inner ring deiodination (formation of rT3 from T4, and T2 from T3) 13, 14, and because no T3, rT3, or T2 peaks were detected in liver microsome incubates in which propyl-n-thiouracil was added, this confirms that both outer ring and inner ring deiodination were catalyzed by D1 in the present assay, but not by D2 or D3. The absence of an rT3 peak above the quantification limit in all samples may be explained by the greater affinity of rT3 to deiodinases relative to T4 16, which would have resulted in its nearly complete degradation during the 75-min assay.
Both hepatic D1 activity and mRNA levels differed between male and female ring-billed gulls. Differences in hepatic D1 activity and mRNA expression between sexes have been observed in some mammalian models. For example, Miyashita et al. 35 showed that after orchidectomy, male rats exhibited significantly lower D1 mRNA levels and activity. The D1 activity and mRNA concentrations reached control levels in the orchidectomied rats after testosterone injection. However, no difference in D1 mRNA levels and activity was observed after β-estradiol (E2) administration or ovariectomy in female rats. A more recent study by Šošić-Jurjević et al. 36 also showed that orchidectomy of young rats resulted in a decrease in D1 activity and thyroid-stimulating hormone levels, and that testosterone administration resulted in a recovery of D1 activity and thyroid-stimulating hormone levels to control levels. However, there is to our knowledge no information available on the underlying mechanisms that link testosterone levels, D1 activity, and the regulation of the HPT axis in rats or any other vertebrates. Regardless, these studies suggest that sex hormones, and more specifically testosterone, may play a role in the regulation of D1 in liver of rats, which may also be the case for birds.
No relationship was found in ring-billed gulls from the present study between hepatic D1 activity and its transcription. Riese et al. 37 suggested that the absence of a relation between D1 mRNA expression and activity in female mice could be attributed to a reduced translational efficiency for D1 (and other selenoproteins) in females as a result of E2 action because ovariectomized females presented male-like hepatic D1 mRNA transcription levels associated with increased hepatic D1 activity. Because the thyroid axis is well conserved among vertebrates, it could be suggested that similar mechanisms leading to a mismatch in D1 transcription and activity levels in ring-billed gull liver may be at play. Moreover, a number of post transcriptional and post translational events have been identified for D2 in human and chicken. For example, alternative splicing, the presence of specific sequence motifs (selenocystein insertion sequence [SECIS), and 5′ or 3′ untranslated regions regulating the expression of D2, could also occur for D1 38. Because the regulation of deiodinase mRNA levels and activity are dynamic, those events could lead to a mismatch between transcription and translation [39, which may partially explain the lack of association between D1 activity and mRNA transcription levels in ring-billed gulls. Alternatively, the absence of a relationship could be attributed to the different matrices used for their determination. Hepatic microsomes were used to determine D1 in vitro activity, whereas whole (homogenized) liver was used for D1 mRNA quantification. Although D1 is primarily located in the endoplasmic reticulum membrane, a small amount of D1 can also be found in the cell membrane, as demonstrated, for example, in rats 40.
Relationships between PBDEs and D1 mRNA transcription and activity
Hepatic ∑nona-BDE, ∑octa-BDE, and ∑hepta-BDE concentrations were greater in combined ring-billed gull males and females in which liver D1 transcription was lower as well as in individuals that exhibited greater hepatic microsomal total D1 activity. Furthermore, concentration ratios of BDE-209 to ∑nona-BDE and ∑octa-BDE were greater in individuals in which D1 mRNA transcription levels were larger. Moreover, total D1 activity was inversely related to concentration ratios of BDE-209 to ∑hepta-BDE, ∑octa-BDE, and ∑nona-BDE. However, no relation was found between liver concentrations of BDE-209 and D1 mRNA transcription and total D1 activity in ring-billed gulls. As suggested by Noyes et al. 16 in a fish study, compensatory mechanisms can modulate deiodinase expression following long term exposure to BDE-209 in response to decreased plasma thyroid hormone levels. However, in that study, changes in deiodinase mRNA levels did not translate into changes in deiodinase activity 12. Because the ring-billed gulls have been chronically exposed in their environment to high concentrations of BDE-209 and other PBDEs 8, 18, perturbations of their HPT axis may have been elicited. In fact, a study from our research group on Montreal-breeding ring-billed gulls from the same colony uncovered multiple relationships among circulating thyroid hormone levels, mRNA levels of several thyroid-related genes, and PBDE concentrations 20. Hence, compensatory mechanisms of the HPT axis could explain, at least in part, the absence of an association between total D1 activity and liver BDE-209 concentrations in ring-billed gulls.
CONCLUSIONS
The present ring-billed gull study confirmed that D1 is the major deiodinase involved in thyroid hormone deiodination in bird hepatic microsomes. Major putative BDE-209 degradation products (e.g., BDE-196, BDE-197, BDE-201, BDE-206, BDE-207, and BDE-208) were detected in ring-billed gull liver, and a number of relationships were found between their concentrations (and ratios to BDE-209 concentrations) and liver D1 transcription and activity. However, despite these significant relationships, the present study design could not confirm that D1-mediated debromination of BDE-209 took place in ring-billed gull liver. Moreover, even though D1 is the main deiodinase expressed in bird liver (microsomes), it should also be considered that D2 and D3 may play a role similar to that of D1 with respect to BDE-209 biotransformation in the nonmicrosomal fraction of hepatocytes or in other peripheral tissues. Ongoing work in our laboratory is aiming to investigate the biotransformation capacity of ring-billed gull hepatic microsomes toward BDE-209 using an in vitro assay targeting deiodinases, and the potential effects the coincubation of hepatic microsomes with BDE-209 may have on T4 deiodination rate in this assay.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at DOI: 10.1002/etc.3372.
Acknowledgment
Funding for the present study was provided by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant to J. Verreault. Supplemental funding was provided by Environment Canada's Chemical Management Plan to M. Houde. The authors thank L. Wang (Université du Québec à Montréal) for assistance with chemical analysis and M. Douville (Environment Canada) for mRNA transcription analysis. The authors also extend their appreciation to F. St-Pierre (Université du Québec à Montréal) for fieldwork assistance.
Disclaimer
The authors declare they have no conflicts of interest.
Data availability
Data can be accessed by contacting the corresponding author ([email protected]).




