In situ and laboratory toxicity of coalbed natural gas produced waters with elevated sodium bicarbonate
Abstract
Some tributaries in the Powder River Structural Basin, USA, were historically ephemeral, but now contain water year round as a result of discharge of coalbed natural gas (CBNG)-produced waters. This presented the opportunity to study field sites with 100% effluent water with elevated concentrations of sodium bicarbonate. In situ experiments, static renewal experiments performed simultaneously with in situ experiments, and static renewal experiments performed with site water in the laboratory demonstrated that CBNG-produced water reduces survival of fathead minnow (Pimephales promelas) and pallid sturgeon (Scaphirhynchus albus). Age affected survival of fathead minnow, where fish 2 d posthatch (dph) were more sensitive than 6 dph fish, but pallid sturgeon survival was adversely affected at both 4 and 6 dph. This may have implications for acute assays that allow for the use of fish up to 14 dph. The survival of early lifestage fish is reduced significantly in the field when concentrations of NaHCO3 rise to more than 1500 mg/L (also expressed as >1245 mg HCO3−/L). Treatment with the Higgin's Loop technology and dilution of untreated water increased survival in the laboratory. The mixing zones of the 3 outfalls studied ranged from approximately 800 m to 1200 m below the confluence. These experiments addressed the acute toxicity of effluent waters but did not address issues related to the volumes of water that may be added to the watershed. Environ Toxicol Chem 2014; 33:2086–2093. Published 2014 Wiley Periodicals Inc. on behalf of SETAC. This article is a US government work and, as such, is in the public domain in the United States of America.
INTRODUCTION
In situ toxicity experiments provide needed information about the ability of fish to survive in the field under real-world conditions 1, 2. Reduced survival of fish may indicate the inability of a watershed to support an optimal population size 1. Therefore, these measurements can indicate impairment at the population level. The placement of sites for these studies can also provide indications about the source(s) of impairment. However, the overall goal when managing aquatic life is to avert or minimize conditions in which the survivability of fish is affected. To scientifically achieve this management objective, laboratory experiments performed in controlled conditions are used to define threshold concentrations in which effects of an element or compound may be observed on aquatic life. These threshold concentrations can then be used to alert managers and site operators about the potential effects of effluents that may be introduced into a water body.
As part of the effort to define threshold concentrations, in situ experiments can provide supporting data for laboratory-derived thresholds. In situ experiments are especially critical when performed at sites where concentrations exceed laboratory-derived thresholds because the ability of fish to survive in real-world conditions can be determined. In this sense, field data are used to ground-truth laboratory-derived concentrations before extensive catastrophic events occur in which populations are affected. In situ experiments provide a link between the laboratory and the field to further assess potential effects in the field.
Experiments performed with site water are another alternative to gather field information. During these experiments, site water is used, but because the experiment is initiated outside of the stream, some physical characteristics such as temperature and dissolved oxygen may be controlled to reduce potentially confounding factors. Stewart 3 used these types of assays when attempting to assess ambient water conditions. Again, these types of experiments can substantiate data gathered from laboratory and in situ experiments because they add further evidence to support or refute laboratory or in situ results.
Age sensitivity to various contaminants has been documented, and guidelines for the assessment of chronic toxicity suggest the use of fathead minnow (Pimephales promelas) less than 48 h old 4, 5. However, current guidelines established in 2002 allow the use of fathead minnow as old as 14 d posthatch (dph) to assess acute toxicity 5, 6. As a result, whole effluent testing conducted to assess the toxicity of potential wastewater discharges is conducted with fish up to 14 dph. However, little research has been conducted on age sensitivities between 2 dph and 14 dph. If the age within this early lifestage affects the acute toxic response, interpretations of whole effluent testing with fish of various ages up to 14 dph could become more complicated and have implications for regulations of wastewaters.
The Powder/Tongue River watersheds are located within the Powder River Structural Basin of Wyoming and Montana. These sites provide a unique opportunity for field assessments, because some tributaries in these watersheds were historically ephemeral but now contain water year round as a result of the discharge of coalbed natural gas (CBNG)-produced waters 7. As such, field sites with 100% effluent water could be studied in these watersheds. Discharged produced water in the Powder/Tongue River has elevated NaHCO3 in the absence of elevated NaCl. Therefore, these sites provide an opportunity to study elevated NaHCO3 in situ. Mixing zones, where tributaries, treated effluent, or untreated effluent flow into the main stem Tongue or Powder River provide another opportunity for field studies in the Powder/Tongue River watersheds. Research related to toxicity in mixing zones is limited. However, toxicity and related gill lesions were elevated in mixing zones that received acid-mine drainage 8. In the experiments performed by Henry et al., 8 toxicity was related to metals that precipitated from solution in the mixing zone. A characterization of mixing zones in the Powder/Tongue River watersheds would provide a needed basis to target toxicity monitoring efforts in these watersheds and beyond. Mixing zone characterizations also provide information about the potential extent of direct acute toxicity. If mixing zones are large, extensive mortality to resident aquatic resources would be expected as untreated effluents enter the main stem of a river.
The main goal of the present study was to investigate the survivability of fish in the field, at sites where concentrations of NaHCO3 exceeded threshold concentrations defined in the laboratory and to define the effect of age on the survivability of fish in the field. A second goal of the present study was to investigate the physical extent of the mixing zones at confluence points in the Powder/Tongue River. By characterizing the mixing zone, the in situ toxicity studied in the tributaries could be framed in a larger context of the Powder/Tongue River.
METHODS
In situ and site water experiments
In situ experiments to investigate the acute toxicity of produced waters in the Powder River Structural Basin, Wyoming and Montana, USA, were completed during July–August 2006 and July 2007. Early lifestage fathead minnow and pallid sturgeon (Scaphirhynchus albus; endemic species used in the laboratory experiments 9) were studied during 2006 and 2007, respectively. An additional static renewal experiment with pallid sturgeon was completed in controlled conditions with site-collected water in 2007. In situ experiments were not conducted in the Tongue River because of logistical problems created by the long distances between experimental sites.
Experiments lasted in situ for 96 h. Fish were held in 450-mL cellulose acetate butyrate containers with 149-µm mesh screen covering approximately 40% of the surface area. Each container held 10 fish, and 4 replicate containers were deployed per site. In situ containers were held in place at each site by strapping the individual containers to a vinyl-coated metal dish drying rack that was placed in a perforated (118 L) Rubbermaid high-density polyethylene storage tub. Storage tubs were held in place with stakes, rocks, and rope to prevent loss from potential flash flooding. At 1 site, the depth was not adequate to allow the use of the storage tub, and the dish drying rack was placed directly in the stream channel with a storage tub cover over the top to provide shade.
Experiments were initiated with 2 dph fathead minnow on 26–29 July (experiment 1) and 6 dph fathead minnow on 30 July–02 August, 2006 (experiment 2; “2 dph” and “6 dph” will be used from this point). Newly hatched fish were shipped directly to Buffalo, Wyoming, USA, from Aquatic Biosystems, Fort Collins, Colorado, USA. The brood stock were deemed free of disease and kept in isolation at the Colorado facility. For experiment 1, 2 reference sites and 4 experimental sites were used; Powder River at Moorhead and Clear Creek (reference sites), SA Creek, Upper Beaver Creek, Lower Beaver Creek, and Burger Draw (Figure 1).
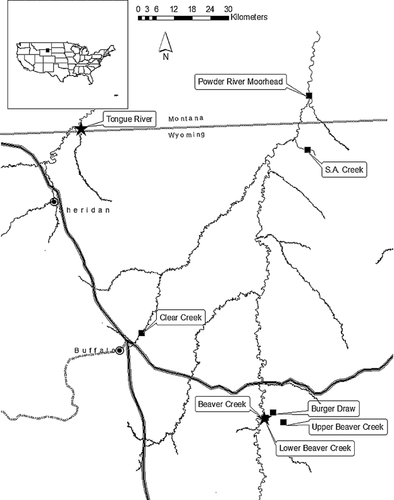
After the completion of the 96-h experiments with 2 dph fish, experiment 2 was initiated with 6 dph fathead minnow in situ at Clear Creek (reference site) Upper Beaver Creek, Lower Beaver Creek, and Burger Draw (experimental sites) with fish from the same source.
Survival was monitored every 24 h, and water samples were collected daily from all sites. Water samples were placed on ice and filtered each evening with a 0.45-µm Millipore filter. Subsamples for cation analyses were acidified to less than pH 2.0 with ultrapure nitric acid and were held at 4 °C during storage and shipment for analyses. Anion samples were collected into bicarbonate rinsed containers and stored at 4 °C during storage and shipment. Alkalinity, hardness 10, 11, and pH were measured each evening (Table 1). Water temperature, conductivity, and dissolved oxygen were measured manually at each site at least once daily, and monitored remotely with YSI Data Sonde loggers at Powder River at Moorhead, Clear Creek, SA Creek, and Lower Beaver Creek every 30 min. Mean temperatures (with standard error in parentheses) were recorded as follows Powder River at Moorehead 27.4 °C (2.3 °C), Clear Creek 21.6 °C (3.2 °C), Lower Beaver 21.9 °C (3.4 °C), Upper Beaver 20.3 °C (2.4 °C), Burger Draw 21.9 °C (4.7 °C), and SA Creek 27.6 °C (2.5 °C). Minimum and maximum temperatures recorded at 4 sites with data loggers were Powder River at Moorhead 21.3 °C to 31.2 °C, Clear Creek 15.1 °C to 29.5 °C, Lower Beaver 14.1 °C to 32.0 °C, and SA Creek 16.2 °C to 30.0 °C. Mean dissolved oxygen was 7.6 mg/L or higher at all sites measured with hand-held meters, and data loggers did not reveal measurements less than 5.0 mg/L. Mean conductivity was 857 µS/cm at Clear Creek and 3616 µS/cm at Powder River at Moorhead, 1 of the 2 reference sites. Mean conductivity at the experimental sites were Lower Beaver 2843 µS/cm, Upper Beaver 2612 µS/cm, Burger Draw 3649 µS/cm, and SA Creek 2620 µS/cm. Mean pH ranged from 8.3 at Powder River at Moorhead to 9.3 at Lower Beaver. Additional water chemistry characterization can be found in Farag and Harper 12. Ammonia was measured onsite daily with a Hach test kit (Table 1). Additional trace metal scans were performed on 1 subsample of water from each site (data not presented, concentrations were below chronic criteria established for the protection of aquatic life). In 2007, 2 dph pallid sturgeon were transported from the Montana Fish Wildlife and Parks Miles City, Montana Fish Hatchery, to Buffalo, Wyoming. Pallid sturgeon at 2 dph, 4 dph, and 6 dph were exposed in experiments at Clear Creek (reference site), Upper Beaver Creek, Lower Beaver Creek, and Burger Draw (experimental sites) with the same methods employed with fathead minnow during 2006. The water quality monitoring design and methods were the same as described for our 2006 in situ experiments (Table 2, trace metal scans not presented); however, in addition to performing onsite ammonia assays with a Hach test kit, water samples were collected and acidified for ammonia analyses in the laboratory with the US Environmental Protection Agency 350.1 methodology 13.
| In situ exposure site | Alkalinity (mg/L as CaCO3) | HCO3− (mg/L) | SO4 (mg/L) | Ca (mg/L) | Mg (mg/L) | Na (mg/L) | K (mg/L) | Total ammonia (mg N/L) |
|---|---|---|---|---|---|---|---|---|
| Powder River at Moorhead | 202 | 196 | 1767 | 197 | 185 | 660 | 32 | <0.01 |
| (11)a | (10) | (36) | (49) | (43) | (162) | (11) | (–)b | |
| N = 5c | N = 5 | N = 3 | N = 4 | N = 4 | N = 4 | N = 3 | N = 5 | |
| Clear Creek | 198 | 192 | 285 | 74 | 52 | 68 | 5 | 0.05 |
| (12) | (11) | (12) | (13) | (9) | (5) | (0.2) | (–) | |
| N = 8 | N = 8 | N = 5 | N = 6 | N = 6 | N = 6 | N = 5 | N = 1 | |
| Lower Beaver | 1926 | 1740 | 75 | 10 | 27 | 765 | 20 | 0.05 |
| (68) | (128) | (1) | (0.4) | (0.4) | (5) | (0.20) | (0.03) | |
| N = 9 | N = 9 | N = 5 | N = 6 | N = 6 | N = 6 | N = 5 | N = 5 | |
| Upper Beaver | 1759 | 1646 | 257 | 16 | 24 | 664 | 20 | 0.5 |
| (66) | (64) | (211) | (0.4) | (0.2) | (6) | (0.4) | (0.1) | |
| N = 9 | N = 9 | N = 5 | N = 6 | N = 6 | N = 6 | N = 6 | N = 5 | |
| Burger Draw | 2535 | 2315 | 27 | 15 | 28 | 1003 | 41 | 1.5 |
| (135) | (136) | (9) | (2) | (1) | (7) | (2) | (0.3) | |
| N = 9 | N = 9 | N = 5 | N = 5 | N = 5 | N = 5 | N = 5 | N = 5 | |
| SA Creek | 1364 | 1245 | 395 | 17 | 45 | 605 | 16 | 0.13 |
| (53) | (44) | (18) | (0.6) | (0.6) | (18) | (2) | (0.04) | |
| N = 5 | N = 5 | N = 3 | N = 4 | N = 4 | N = 4 | N = 3 | N = 5 |
- a Standard error in parentheses.
- b No calculation of standard error was performed.
- c Sample size.
| Site | Alkalinity (mg/L as CaCO3) | HCO3– (mg/L) | SO4 (mg/L) | Ca (mg/L) | Mg (mg/L) | Na (mg/L) | K (mg/L) |
|---|---|---|---|---|---|---|---|
| Clear Creek | 145 | 165 | 322 | 84 | 36 | 58 | 5 |
| (17)a | (16) | (41) | (8) | (3) | (6) | (0.4) | |
| N = 5b | N = 5 | N = 5 | N = 5 | N = 5 | N = 5 | N = 5 | |
| Lower Beaver | 1419 | 1513 | 289 | 16 | 35 | 681 | 19 |
| (55) | (59) | (33) | (1) | (1) | (17) | (0.5) | |
| N = 4 | N = 4 | N = 4 | N = 4 | N = 4 | N = 4 | N = 4 | |
| Upper Beaver | 1468 | 1626 | 163 | 16 | 29 | 650 | 20 |
| (26) | (11) | (10) | (1) | (0.5) | (14) | (0.5) | |
| N = 4 | N = 4 | N = 4 | N = 4 | N = 4 | N = 4 | N = 4 | |
| Burger Draw | 2348 | 2561 | 56 | 14 | 23 | 943 | 29 |
| (47) | (67) | (11) | (0.2) | (0.5) | (17) | (0.2) | |
| N = 5 | N = 5 | N = 5 | N = 5 | N = 5 | N = 5 | N = 5 |
- a Standard error in parentheses.
- b Sample size.
In addition to the in situ experiments completed in the field in 2007, a simultaneous experiment was completed with site water in more controlled laboratory conditions. Water was collected at Clear Creek (reference site), Upper Beaver Creek, Lower Beaver Creek, and Burger Draw (experimental sites) and held in insulated 22 L containers. Four replicates of 5 fish were exposed to water from each site in 530 mL containers. The containers were kept in a water bath that fluctuated slightly with the room temperature (16 °C–22 °C), and water in the containers was replaced every 12 h.
Water samples intended for laboratory analyses were kept refrigerated in Buffalo, Wyoming, USA, and transported in ice to the US Geological Survey, Columbia Environmental Research Center, Jackson Field Research Station, Jackson, Wyoming (herein referred to as Jackson Field Research Station). Samples were then sorted and shipped in ice to the State of Montana Department of Health and Human Services Environmental Laboratory for anion and cation analyses. For both experiments in 2006, the survival data were analyzed with the Toxstat software package 14. A t test was performed between the reference sites (experiment 1). Because no difference was observed, the data were pooled and a one-way analysis of variance was performed followed by a Bonferroni means comparison. Because 1 reference site was used during experiment 2, no pooling was necessary. To compare the survival of 2 dph against 6 dph fathead minnow at sites t tests were used. All data met homogeneity and normality assumptions without transformations. In 2007, a one-way analysis of variance was performed followed by a Bonferroni means comparison for 4 dph and 6 dph pallid sturgeon in situ, and 4 dph pallid sturgeon static-renewal experiments. All means were tested with a statistical criterion of p < 0.05.
Potential differences in mean ammonia concentrations derived with field assays of chilled samples and the EPA method 350.1 laboratory assays 13 were defined with t tests in the Toxstat software package with a statistical criterion of p < 0.05. No transformations were necessary because all data met homogeneity and normality assumptions without transformation.
Mixing zone and site water experiments
Mixing zones were characterized at 3 locations of the Powder/Tongue River watersheds: downstream from the confluence of Beaver Creek and the Powder River; downstream from the untreated produced water diffuser in the Tongue River near Decker, Montana; and downstream from the treated produced water diffuser (Higgins Loop Treatment, an ion exchange) near Decker, Montana. These sites were chosen to characterize 3 types of discharge: no treatment of a stream that often contained 100% effluent, no treatment but a diffuser was used to enhance mixing, and water that was treated before discharge. These sites vary from the in situ experiments that were designed to define survivability in tributaries that primarily contained produced water.
Rhodamine WT dye was added at a constant drip into Beaver Creek and into the treated and untreated produced water. At the Powder River, transects were spaced at 4 m, 8 m, 16 m, 32 m, and 804 m below the confluence of Beaver Creek. At the Tongue River, transects were spaced 15 m, 30 m, 60 m, 90 m, and 1200 m downstream from the diffusers for treated and untreated discharge. The dye concentration was measured in the water at evenly spaced locations (0.15 m–3 m) across the river, and spacing was dependent on the width of the river. The number of locations across the width ranged from 5 to 11 locations, and the width of the river at site locations ranged from 3 m to 33.4 m. At each point along the transect, dye concentrations were measured with a YSI–600 oms sonde, equipped with a 6130 Rhodamine WT optical probe. The concentration of the injected dye solution was measured in reference to a dilution series of the stock solution 15.
Toxicity experiments were performed to assess the toxicity of waters from the mixing zone. Site water experiments were completed with water collected from the Powder River and from Beaver Creek near the confluence with the Powder River, in Johnson County, Wyoming. Water also was collected from the Tongue River and 2 diffuser discharge points into the Tongue River in Big Horn County, Montana; 1 site contained treated (Higgins Loop ion exchange) CBNG-produced water, the other site contained untreated CBNG-produced water; water was collected immediately above their discharge point into the Tongue River. Water collected from all sites was placed in acid-washed, 20-L high-density polyethylene containers, which were chilled and transported to the Jackson Field Research Station. The waters were used to complete 96-h static renewal experiments with 2 dph fathead minnow from September to October 2007.
Newly hatched fathead minnow were shipped directly to the Jackson Field Research Station from Aquatic Biosystems, Fort Collins, Colorado, USA. At the Jackson Field Research Station, fish were acclimatized to Tongue River water and held at 20 °C.
Static-renewal experiments with the site waters were completed in 1-L glass beakers filled with 750 mL site water and maintained in a water bath at 20 °C. The fish were exposed for 96 h, and the experimental water was replaced every 24 h; the replacement water was allowed to equilibrate to the exposure temperature in the water bath overnight. Water from Beaver Creek, untreated CBNG-produced water, and treated CBNG-produced water were experimented with at 100%, 75%, and 50% concentrations. Beaver Creek water was diluted with Powder River water to prepare the proper concentrations, and the produced waters were diluted with Tongue River water to prepare the proper concentrations. Waters collected from the Powder and Tongue Rivers served as reference waters for the experiments. Water temperature and dissolved oxygen were monitored twice daily, and water samples were collected daily for water chemistry from the 100% (undiluted) and 50% (diluted) treatments (Table 3).
| Site | Alkalinity (mg/L as CaCO3) | HCO3− (mg/L) | SO4 (mg/L) | Ca (mg/L) | Mg (mg/L) | Na (mg/L) | K (mg/L) | Total ammonia (mg N/L) |
|---|---|---|---|---|---|---|---|---|
| Powder River | 179 | 179 | 858 | 144 | 69 | 374 | 15 | 0.08 |
| (4)a | (4) | (37) | (5) | (3) | (18) | (1) | (0.06) | |
| N = 3b | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | |
| Tongue River | 236 | 232 | 536 | 64 | 26 | 41 | 4 | 0.03 |
| (15) | (17) | (493) | (2) | (16) | (17) | (0.4) | (–)c | |
| N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | N = 2 | |
| Beaver Creek | 1233 | 1097 | 354 | 13 | 29 | 553 | 16 | 0.16 |
| (76) | (34) | (151) | (–) | (–) | (–) | (–) | (–) | |
| N = 3 | N = 3 | N = 3 | N = 2 | N = 2 | N = 2 | N = 2 | N = 2 | |
| Beaver Creek, 50% | 638 | 604 | 586 | 33 | 49 | 499 | 15 | NMd |
| (–) | (–) | (–) | (–) | (–) | (–) | (–) | (–) | |
| N = 2 | N = 2 | N = 2 | N = 2 | N = 2 | N = 2 | N = 2 | (–) | |
| Treated | 89 | 89 | 309 | 106 | 1.8 | 106 | 1.4 | 0.13 |
| (2.1) | (2.1) | (24) | (3.3) | (0.5) | (9.9) | (0.1) | (0.02) | |
| N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | |
| Treated, 50% | 199 | 195 | 221 | 81 | 16 | 63 | 2.8 | NM |
| N = 2 | N = 2 | N = 2 | N = 2 | N = 2 | N = 2 | N = 2 | (–) | |
| Untreated | 1560 | 1420 | 97 | 9.7 | 5.6 | 678 | 8.5 | 2.0 |
| (55) | (25) | (23) | (1.2) | (0.8) | (22) | (0.4) | (0.4) | |
| N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | N = 3 | |
| Untreated, 50% | 864 | 784 | 110 | 14 | 20 | 381 | 6.6 | NM |
| N = 2 | N = 2 | N = 2 | N = 2 | N = 2 | N = 2 | N = 2 | (–) |
- a Standard error in parentheses.
- b Sample size.
- c No standard error is presented when sample size was ≤2.
- d Not measured.
RESULTS
In situ experiments
Concentrations of NaHCO3 were elevated at all experimental sites except the reference sites (856–3504 mg/L; Tables 1 and 2). In 2006, ammonia concentrations were largest at Burger Draw and averaged 1.5 mg/L to 2.0 mg/L in 2006, and 1.17 mg/L in 2007. Ammonia concentrations did not exceed 0.5 mg/L at any other sites. Ammonia concentrations in samples measured in the field were greater than acidified samples measured in the laboratory. Mean total ammonia (mg/L) measured 0.01 mg/L at Clear Creek for both laboratory and field, 0.1 mg/L laboratory and 0.4 mg/L field for Lower Beaver, 0.12 mg/L laboratory and 0.29 mg/L field for Upper Beaver, and 1.05 mg/L laboratory and 1.17 mg/L field for Burger Draw. Differences in ammonia concentrations were not significant between the methodologies at Burger Draw, Lower Beaver, and Clear Creeks; however, differences were significant at Lower Beaver Creek (p < 0.05).
Survival of 2 dph fathead minnow in 2006 was 77% and 78% in the Powder River at Moorhead and the Clear Creek sites, respectively. Because no significant difference in survival was seen between the 2 sites, the data were combined to create a pooled reference (78% as pooled reference) for further comparisons. Survival was significantly less at all experimental sites (p ≤ 0.05) when compared with the pooled reference (Table 4) during experiment 1. Survival of 6 dph fathead minnow was 90% at Clear Creek (reference site) and was not significantly different at any experimental site.
| Experiment | Site | Age | Percentage survival at 96 h |
|---|---|---|---|
| 1 | |||
| Powder River at Moorhead | 2 dphb | 77 | |
| Clear Creek | 2 dph | 78 | |
| Pooled Reference | 2 dph | 78 | |
| SA Creek | 2 dph | 24* | |
| Burger Draw | 2 dph | 37* | |
| Upper Beaver | 2 dph | 11* | |
| Lower Beaver | 2 dph | 49* | |
| 2 | |||
| Clear Creek | 6 dphc | 90 | |
| Burger Draw | 6 dph | 73 | |
| Upper Beaver | 6 dph | 75 | |
| Lower Beaver | 6 dph | 75 |
- a Two experiments were performed with fathead minnows of different ages.
- b Two dph at the initiation of experiment 1.
- c Two dph at the initiation of experiment 2.
- * p < 0.05 compared with pooled reference or Clear Creek.
- dph = days posthatch.
Survival of 2 dph pallid sturgeon was low at all sites including the reference in 2007, and the experiment was terminated at 24 h. The experiment was restarted with 4 dph fish, and survival at Clear Creek (reference site) was 95%, whereas survival in all experimental sites was 0% (Table 5). Survival of 4 dph pallid sturgeon in a static-renewal experiment was similar to the in situ experiment, with reduced survival of fish exposed to produced water (0% to 15%), and 90% survival of fish held in Clear Creek reference water. An experiment completed with 6 dph fish provided similar results when compared with the 4 dph fathead minnow, although time to death was longer with the older fish (data not presented).
| Experiment | Site | Age | Percentage survival at 96 h |
|---|---|---|---|
| In situ | |||
| Clear Creek | 4 dpha | 95 | |
| Lower Beaver | 4 dph | 0* | |
| Upper Beaver | 4 dph | 0* | |
| Burger Draw | 4 dph | 0* | |
| Static renewal | |||
| Clear Creek | 4 dph | 90 | |
| Lower Beaver | 4 dph | 10* | |
| Upper Beaver | 4 dph | 15* | |
| Burger Draw | 4 dph | 0* | |
| In situ | |||
| Clear Creek | 6 dphb | 80 | |
| Lower Beaver | 6 dph | 0* | |
| Upper Beaver | 6 dph | 0* | |
| Burger Draw | 6 dph | 0* |
- a Four dph at the initiation of experiment.
- b Six dph at the initiation of experiment.
- * p < 0.05 compared with reference site (Clear Creek).
- dph = days posthatch.
Site water/mixing zone experiments
In the Powder River at the Beaver Creek confluence, dye concentrations, which represented the portion of the stream composed of Beaver Creek discharge, were greatest on the bank closest to the input source. At the first transect, dye concentrations ranged from 122 µg/L near the inflow of Beaver Creek to 0 µg/L on the opposite stream bank. At 16 m below the confluence of Beaver Creek, dye concentrations across the width of the river ranged from 48 µg/L to 4.4 µg/L, and at 804 m below the confluence of Beaver Creek dye concentrations ranged from 23 µg/L to 7 µg/L.
At the untreated water outflow into Tongue River near Decker, Montana, dye concentrations 15 m downstream from the diffuser ranged from 55 µg/L to 0 µg/L, and were most concentrated in measurements taken closest to the diffuser, whereas 3 measurements located farthest from the diffuser outfall contained little or no dyed produced water. At 30 m, dye ranged from 39 µg/L to 0 µg/L across the river; at 60 m, dye concentrations ranged from 25 µg/L to 0 µg/L; and at 1200 m, the dye ranged from 19 µg/L to 6 µg/L.
At the treated water outflow, dye concentrations measured 15 m downstream from the diffuser ranged from 30 µg/L to 0 µg/L, and the dye was most concentrated in the center of the stream channel near the diffuser outfall. Points along the width of the river that were nearest to both stream banks contained little or no dyed produced water. At 30 m, dye concentrations across the width of the Tongue River ranged from 37 µg/L to 0.6 µg/L, and at 1200 m, dye concentrations ranged from 12 µg/L to 9 µg/L.
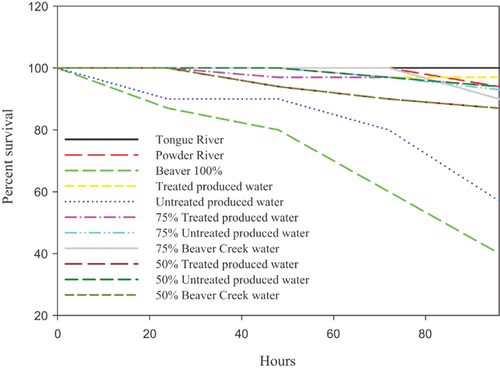
Survival of 2 dph fathead minnow exposed to 100% Beaver Creek water and 100% untreated produced water was reduced significantly when compared with Powder River and Tongue River reference sites. Survival of all other mixtures was not significantly different from the reference sites (Figure 2).
Ammonia concentrations were below US Environmental Protection Agency (USEPA) water-quality criteria for all exposures and dilutions except the untreated CBNG-produced water (Table 3) 16. However, ammonia concentrations were sufficient in the untreated produced water (mean = 2.0 mg/L) to potentially contribute to mortality.
DISCUSSION
The value of in situ experiments as reviewed by Chappie and Burton 17 cannot be understated for use in watersheds such as the Tongue and Powder River where NaHCO3 is discharged. The limitations of field experiments, including a lack of control for some physical variables, such as temperature and turbidity, are precisely what give the experiments strong applicability. The daily fluctuations created by the diurnal cycle are a natural part of life in a prairie stream. The in situ experiments performed during the present study were completed during midsummer low-flow conditions, when days were long and fluctuations in temperature are most dramatic. Though care was taken to minimize lethal extremes (shade was provided and chambers were suspended in the water column but out of strong current), the natural cycle provided the setting that fish must endure in natural conditions.
Laboratory-derived threshold concentrations for NaHCO3 9 were validated in the field. The concentrations of NaHCO3 in the various sites studied were above 1749 mg/L (or expressed as 1245 mg HCO3−/L; the likely toxic ion), a concentration similar to or above the determined lethal concentration that will cause 50% mortality or detrimental effect (LC50) of 1356 mg NaHCO3/L and 1749 mg NaHCO3/L for pallid sturgeon and fathead minnow, respectively 9. For example, the concentration of NaHCO3 at Lower Beaver was 2505 mg/L, Upper Beaver was 2310 mg/L, and SA Creek was 1850 mg/L during fathead minnow experiments. The concentrations at Lower and Upper Beaver were 2194 mg/L and 2276 mg/L, respectively, for the pallid sturgeon experiments. The field experiments were substantiated further with the site water experiments where percentage survival was similar between both types of experiments. Untreated produced water was the dominant constituent of water at these sites and likely reduced survival.
However, when the produced water was treated with Higgins Loop technology, the acute toxicity of the water was removed. The Higgins Loop exchanges sodium for hydrogen ions 18, and as a result, the NaHCO3 was reduced to 195 mg/L (89 mg HCO3−/L). This reduction accounted for the reduced toxicity. It also should be noted that the concentration of ammonia in the untreated produced water was reduced after treatment from 2.0 mg/L to 0.13 mg/L.
Age and species sensitivity were also documented in response to NaHCO3 in the field. Newly hatched 2 dph fish were the most sensitive age for fathead minnow, and mortality was significantly greater when compared with the reference sites. The same species exposed as 6 dph fish were less sensitive, and differences in survival between experimental and reference sites were not significant. Pallid sturgeon were more sensitive than fathead minnow, and 0% survival occurred in both 4 dph and 6 dph pallid sturgeon at experimental sites. Therefore, pallid sturgeon had greater overall sensitivity to NaHCO3, and these results substantiate laboratory data 9.
Johnson also completed in situ experiments with fathead minnow during the summer of 2006 (L.A. Johnson, 2006, Master's thesis, University of Wyoming, Laramie, WY). The researcher did not observe decreased survival at a site approximately 4 km upstream from the Upper Beaver site used during the present study, although concentrations of NaHCO3 were greater than the LC50 defined previously with fathead minnow 9, 19. However, Johnson used fish that were 12 dph to 14 dph. The Johnson results are similar to the lack of survival effects observed when 6 dph fathead minnow were used in the present study.
Others have documented age sensitivity to various contaminants 4, and USEPA guidelines for the assessment of chronic toxicity 5 suggest the use of fathead minnow less than 48 h old. However, current guidelines established in 2002 allow the use of fathead minnow as old as 14 dph to assess acute toxicity 5, 6. The present study suggests that sensitivity during early fathead minnow development is not only important among distinct lifestages (e.g., early lifestage, juvenile, adult), but also exists within a relatively short time span of 4 d during the early lifestage. This finding is intuitive if we consider the rapid development of early lifestage fathead minnow that hatch approximately 4 d postfertilization. Johns documented changes in gene expression related to growth and sexual differentiation during the first 30 d post fertilization for fathead minnow with significant changes in 6 of the 9 genes studied (S.M. Johns, 2009, Master's thesis, Purdue University, West Lafayette, IN, USA). Many of those changes were noted during the first 2 dph. Devlin and Nagahama 20 also noted that fathead minnow were most sensitive to stress during the short time between hatch and exogenous feeding. Based on the literature and the results of the present study, operators, managers, and researchers may wish to take care when interpreting and comparing results gathered with various aged fish less than 14 dph.
Ammonia concentrations exceeded USEPA recommended maxima in Burger Draw in 2006 (1.32 mg/L at pH 9.0) and may have contributed to mortality of fathead minnow 16. Elevated concentrations of ammonia in Burger Draw also were documented by Smith et al. 21 when the researchers calculated lethal concentrations of ammonia in June 2004. Smith et al. 21 also cautioned about the existence of a diel cycle for ammonia in which concentrations would be greatest in mid to late afternoon and the smallest in predawn hours. Samples collected during the present study were collected late morning to late afternoon and thus, generally should have documented the largest concentrations of the diel cycle.
At all other experimental sites, ammonia concentrations were not above chronic criteria concentrations, and survival statistically was reduced when compared with the reference site. This indicates that although ammonia may have played a part in the mortality at Burger Draw, other toxicants were the likely causative agent at all other experimental sites for 2 dph fathead minnow, and 4 dph and 6 dph pallid sturgeon. To further support this conclusion, water samples collected from Beaver Creek had 0.16 mg/L total ammonia during the 2007 mixing zone analyses (compared with 0.4 mg/L in the 2006 in situ experiment), and survival was decreased in the experiment completed with the 2007 water (Table 3, Figure 2).
Ammonia concentrations were measured with a Hach test kit, and in the laboratory with the USEPA 350.1 methodology 13 on water samples that had been collected and acidified for ammonia analyses. The concentrations were smaller in samples measured in the laboratory, with a significant difference between samples collected at Lower Beaver Creek. However, the mean concentrations determined by both methodologies were below USEPA recommended maxima.
The mixing zone appeared to persist 804 m below the main stem of the Powder River and the confluence of Beaver Creek, and 1200 m downstream from the discharge of untreated and treated produced water into the Tongue River. However, the area containing concentrated untreated produced water was limited to locations immediately downstream from the confluence of Beaver Creek in the Powder River, and the diffusers in the Tongue River. In the laboratory, dilution of untreated produced water resulted in a rapid amelioration of toxicity to 2 dph fathead minnow. Survival of fathead minnow was not significantly different from reference waters when untreated outflow and water from Beaver Creek were diluted to 75% of the source water. In the Powder River below Beaver Creek, and the Tongue River below the diffusers, the plume of water exceeding more than 75% produced water was limited during low-flow conditions. If the low volume of produced water and the relatively large quantity of dilution water remain constant, the acute toxicity from individual discharges of produced water will be limited. The effects of chronic exposures and the cumulative effects from multiple sources of produced water would likely occur at concentrations lower than 75% produced water, but were not addressed in these field experiments.
In summary, the combination of in situ experiments, static-renewal experiments performed simultaneously with in-situ experiments, and static renewal experiments performed with site water in the laboratory in the present study demonstrated that CBNG-produced water reduces survival of fathead minnow and pallid sturgeon. Age affected survival of fathead minnow, but pallid sturgeon appear more sensitive, and survival was adversely affected at 4 and 6 dph.
These results support the laboratory findings and LC50 determinations that will cause 50% mortality or detrimental effects defined in Harper et al. 9 and by Mount et al. 19. Therefore, the survival of early lifestage fish, especially those younger than 6 dph, is likely reduced significantly in the field when concentrations of NaHCO3 rise to more than 1749 mg/L. Measurements were not made of Na/K adenosine triphosphatase in fish from experiments completed in situ, but the data from Farag and Harper 22 suggest that a significant decrease in Na/K adenosine triphosphatase may have affected the ability of the 2 dph fish to survive in the field.
The present study results also determined that treatment with ion exchange or dilution of untreated water increased survival in the laboratory. Both of these situations reduced ammonia in addition to concentrations of NaHCO3. Finally, the mixing zones of the 3 outfalls studied ranged from approximately 800 m to 1200 m below the confluence, and the areas within these mixing zones with acutely lethal concentrations of NaHCO3 (as defined by the presence of concentrated dye) are limited and variable within each zone. These experiments addressed the acute toxicity of effluent waters being added to the main stem rivers but did not address issues related to the volumes of water that may be added to the watershed.
Acknowledgment
Private landowners were gracious in allowing access to their property, especially J. Iberlin and T. Harriet, who allowed access repeatedly over the years. Fidelity Exploration and Production allowed access to their property during sample collection from their outflow. The field experiments could not have been conducted without the assistance of the Wyoming Game and Fish Department and Permitting Office, who worked beyond traditional business hours to complete the approval process. Mixing zone studies could not have been completed without T. Cleasby, US Geological Survey, who provided assistance in the field and with data interpretations. L. May, US Geological Survey, provided assistance with data quality assurance and table construction. Funds were provided by the US Bureau of Land Management and the US Environmental Protection Agency.
Disclaimer
Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government.




