Identification of interspecific differences in phase II reactions: Determination of metabolites in the urine of 16 mammalian species exposed to environmental pyrene
Abstract
Interspecific differences in xenobiotic metabolism are a key to determining relative sensitivities of animals to xenobiotics. However, information on domesticated livestock, companion animals, and captive and free-ranging wildlife is incomplete. The present study evaluated interspecific differences in phase II conjugation using pyrene as a nondestructive biomarker of polycyclic aromatic hydrocarbon (PAH) exposure. Polycyclic aromatic hydrocarbons and their metabolites have carcinogenic and endocrine-disrupting effects in humans and wildlife and can have serious consequences. The authors collected urine from 16 mammalian species and analyzed pyrene metabolites. Interspecific differences in urinary pyrene metabolites, especially in the concentration and composition of phase II conjugated metabolites, were apparent. Glucuronide conjugates are dominant metabolites in the urine of many species, including deer, cattle, pigs, horses, and humans. However, they could not be detected in ferret urine even though the gene for ferret Uridine 5′-diphospho-glucuronosyltransferase (UDP-glucuronosyltransferase, UGT) 1A6 is not a pseudogene. Sulfate conjugates were detected mainly in the urine of cats, ferrets, and rabbits. Interestingly, sulfate conjugates were detected in pig urine. Although pigs are known to have limited aryl sulfotransferase activity, the present study demonstrated that pig liver was active in 1-hydroxypyrene sulfation. The findings have some application for biomonitoring environmental pollution. Environ Toxicol Chem 2014; 33:2062–2069. © 2014 SETAC
INTRODUCTION
Interspecific differences in the biotransformation and elimination of drugs and other xenobiotics are typically complex, making it difficult to predict the adverse consequences of environmental pollutants 1. Studies on interspecific differences in xenobiotic metabolism of various animals, such as domesticated livestock, companion animals, and captive and free-ranging wildlife, are becoming of increasing interest to ecotoxicologists 2. The best-known species difference in phase II metabolism of xenobiotics is the biotransformation of drugs and structurally related phenolic compounds by glucuronidation in domestic cats. Slow glucuronidation of acetaminophen and acetylsalicylic acid (aspirin) accounts for the slow clearance and extreme sensitivity of the Felidae family to the adverse effects of these drugs in comparison with dogs and most other mammalian species 3, 4. In addition, other enzymes reduce susceptibility to toxins (e.g., glutathione-S-transferase in the mouse affords tolerance to the food-borne hepatocarcinogen aflatoxin B1 5).
Studies on experimental exposure in vivo represent the best approach to characterizing interspecific differences in kinetics, metabolic pathways, and specific toxicological effects; yet, they are usually difficult to characterize in domesticated livestock, companion animals, and captive and free-ranging wildlife. However, genomic investigation and in vitro experiments (e.g., enzyme kinetics analysis, cell culture experiments) are recognized as powerful tools for predicting these differences. A previous report on genomics-based phylogenetic analysis of uridine 5′-diphospho-glucuronosyltransferase 1A6 (UGT1A6; i.e., the principal gene regulating the biotransformation of phenolic compounds) indicates that this gene occurs as a pseudogene throughout Felidae 6 and in other species such as the brown hyena (Hyaena brunnea) and the northern elephant seal (Mirounga angustirostris) 7. This analysis predicts that the chemical sensitivity of these species, which do not express UGT1A6, is high. On the other hand, gene-based phylogeny is not always correlated with in vivo results. For example, ferret UGT1A6 is not a pseudogene, yet glucuronidation of acetaminophen in ferrets is relatively slow in comparison with other species 8. Hence, both in vitro (genetic and enzymatic analysis) and in vivo studies are necessary to predict interspecific difference in xenobiotic metabolism.
Polycyclic aromatic hydrocarbons (PAHs) are environmental pollutants that can evoke carcinogenic and endocrine effects in humans and other animals. They are formed and emitted as a result of the incomplete combustion of organic material. They may also be released into the environment through the disposal of coal tars and other coal-processing wastes, petroleum sledges, and other wood preservative wastes. The biomarkers of PAH exposure have been widely reported. The induction of mRNA and protein expression levels of cytochrome P4501A (CYP1A) and ethoxyresorufin-O-deethylase activity are well studied 9. Moreover, the metabolites of these compounds, especially in urine, are available for use as biomarkers in humans. The most commonly assayed urinary PAH metabolites are 1-hydroxypyrene and pyrene-1-glucuronide because of their relative ease of measurement 10.
The objectives of the present study were to construct an in vivo indicator to characterize phase II metabolism in domesticated livestock, companion animals, and captive and free-ranging wildlife, and to establish effective biomarkers to help predict the ecotoxicological effects of PAH exposure in these species. Urine was selected for analysis as it is readily available and easy to collect from various animals. Pyrene, a PAH composed of 4 benzene rings, is a frequently encountered environmental pollutant. Therefore, urinary metabolites of pyrene are often used as a nondestructive biomarker of PAH exposure. Biotransformation of pyrene is a necessary step in its excretion via urine. Pyrene metabolism involves the formation of 1-hydroxypyrene, a phase I metabolite that undergoes phase II metabolism by conjugation to yield more water-soluble forms 10. The biotransformation of pyrene to 1-hydroxypyrene occurs because of the cytochrome P450 (CYP) family of enzymes, especially the CYP1A subfamily; and the biotransformation of 1-hydroxypyrene to various conjugated metabolites occurs because of phase II conjugation enzymes such as uridine 5′-diphospho-glucuronosyltransferase (UGT) and sulfotransferases (SULT). Urinary 1-hydroxypyrene has been employed in various studies as a biomarker of exposure to PAH in developing and industrial countries 11. Interestingly, a previous study identified interspecific differences in pyrene metabolites 11-15. Not only 1-hydroxypyrene but also various pyrene-conjugated metabolites have been identified in aquatic crustaceans, snails, crabs, fish, amphibians and reptiles, rats, and humans 16-21. These results indicate that characterization of the dominant pyrene metabolites in various animals is a necessary step in establishing the biomarkers of environmental exposure to PAH. In addition, characterization of urinary pyrene metabolites is considered a good predictor of species differences in phase II xenobiotic conjugation reaction.
We performed in vivo exposure experiments with rats to establish the method of purification and identify urinary metabolites using pyrene as a model compound. Characterization of phase II conjugation, especially in glucuronidation and sulfation in domesticated livestock, companion animals, and captive and free-ranging wildlife, was carried out on the basis of this method. Subsequently, urine samples from 16 mammalian species were collected, and interspecific differences in phase II reaction were predicted using environmental intake of pyrene. This is the first study to offer comparative information on phase II conjugation reactions in several mammalian species.
MATERIALS AND METHODS
Chemicals
Pyrene, 1-hydroxypyrene, methanol (high-performance liquid chromatography [HPLC] grade), ethyl acetate, sodium hydroxide, sulfuric acid, and acetonitrile (HPLC grade) were purchased from Kanto Chemical. Sulfatase (limpets, type V; 34 U/mg), β-glucuronidase (bovine liver, type B-1; 1240 U/mg), β-glucosidase (almonds; 3.4 U/mg), bovine serum albumin, and 3′-phosphoadenosine 5′-phosphosulfate were obtained from Sigma-Aldrich. The 6-hydroxychrysene used as an internal standard was purchased from AccuStandard. Acetic acid, formic acid, sodium phosphate, diethylamine, potassium dichromate, and ammonium acetate solution were purchased from Wako Pure Chemical Industries.
Animals
Urine samples from mammals in Japan and Thailand were collected. Samples were obtained from cattle (n = 9), deer (n = 14), horses (n = 13), elephants (n = 10), rabbits (n = 2), dogs (n = 2), guinea pigs (n = 2), hedgehogs (n = 2), cats (n = 2), ferrets (n = 2), pigs (n = 3), humans (nonsmokers, 25–35 yr old) (n = 3), tapir (n = 1), chimpanzee (n = 1), and bear (n = 1) (Table 1). Voided urine samples were collected in the morning directly into a clean container. Samples were then rapidly transferred to storage at −20 °C to reduce bacterial growth prior to analysis.
| Animals (n) | Genus/species | Race/breed/strain | Age (yr) | Gender |
|---|---|---|---|---|
| Deer (14) | Cervus nippon yesoensis | Sika deer | 1–6 | 10 males, 4 females |
| Cattle (6) | Bos tarurus | Holstein Friesian | 2–4 | Females |
| Rabbit (2) | Oryctolagus cuniculus | Crossbreed | 2–3 | 1 male, 1 female |
| Horse (13) | Equus ferus | Thoroughbred | 7–20 | Males |
| Beef cattle (3) | Bos primigenius | Brahman bull crossbreed | 2–4 | Females |
| Dog (2) | Canis familiaris | Golden retriever | 5–6 | Males |
| Guinea pig (2) | Cavia porcellus | Cavies | 2 | Males |
| Hedgehog (2) | Atelerix albiventris | African pygmy | 1 | Males |
| Cat (2) | Felis domesticus | Domestic short hair | 2–3 | 1 male, 1 female |
| Ferret (2) | Mustela putorus furo | Domestic | 5 | Males |
| Pig (3) | Sus domesticus | Large white | 4 | Females |
| Human (3) | Homo sapieus | Asian | 28–32 | 2 males, 1 female |
| Elephant (10) | Elephas maximus | Asian elephant | 10–40 | 2 males, 8 females |
| Tapir (1) | Tapirus indicus | Malayan tapir | 20 | Male |
| Chimpanzee (1) | Pan troglodytes | Common chimpanzee | 16 | Male |
| Bear (1) | Ursus arctos yesoenis | Hokkaido brown bear | 12 | Male |
Male Wistar rats (Rattus norvegicus, 9 wk old, n = 3; SLC) were maintained according to the guidelines of the Hokkaido University Institutional Animal Care and Use Committee. Rat body weight (average ± standard deviation) was 248 ± 10 g. Rats were kept at 24 ± 1 °C under a 12-h light and 12-h dark cycle and given food and water ad libitum.
Exposure experiment
Rats were allowed to fast for 24 h before exposure. Pyrene was dissolved in 100% propylene glycol and administered by oral gavage at a dose of 4 mg/kg body weight. The maximum exposure per rat was less than 1000 µL. Rats were then kept in a metabolic cage for 24 h for urine collection. Extraction followed the protocol described below.
Liver sample collection
Liver samples were collected from rats and pigs. Three rats were euthanized with carbon dioxide, and then livers were removed and perfused with cold 1.15% KCl to remove the blood. Samples were flash-frozen with liquid nitrogen and kept at −80 °C until analysis. Five livers from male large white pigs (Sus domesticus) were collected from a slaughterhouse in Hokkaido, Japan, immediately flash-frozen in liquid nitrogen, and then stored at −80 °C until analysis.
Preparation and purification of urine samples
Aliquots of urine (5–500 mL) were acidified (to pH ∼6.8) with 10 mM formic acid before extraction. The extraction process was modified from the procedures of Stewart et al. 22 and Strahm et al. 23. The acidified samples were loaded onto an Oasis WAX plus solid-phase extraction cartridge (50 mg; Waters) with 6-hydroxychrysene added as an internal standard. Cartridges were conditioned with methanol (10 mL) and MilliQ water (10 mL). The loaded samples were washed with sodium hydroxide solution (0.1 M, 5 mL), sodium phosphate buffer (0.1 M, pH 7.4, 5 mL), and Milli Q water (5 mL), and then cartridges were dried under vacuum. The target analytes were sequentially eluted with methanol/ethyl acetate solution for fraction 1 (1:1 v/v, 10 mL), with methanol/10% formic acid solution for fraction 2 (9:1 v/v, 10 mL), or with methanol/Milli Q water/diethylamine solution (17:2:1 v/v, 10 mL) and methanol/ethyl acetate/diethylamine solution (50:50:1 v/v, 2 mL) for fraction 3. All 3 fractions were reduced to 1 mL under a gentle nitrogen flow, and 5 µL was used for analysis by HPLC with fluorescence detector.
Pyrene metabolite analysis
Samples were analyzed on an HPLC 20A series (Shimadzu) with a fluorescence detector (RF-1AXL; Shimadzu), equipped with an ODS column (ODS-120T 2.1 mm × 300 mm; Tosoh). The analysis was modified from HPLC methods of Beach et al. 18 and Ueda et al. 20. Mobile phase A consisted of 10 mM ammonium acetate buffer (pH 5.0), and mobile phase B was a methanol/acetonitrile/water solution (38:57:5, v/v/v). The solvent gradient was as follows: 10% mobile phase B at 0 min to 2 min, followed by a linear gradient to 100% mobile phase B at 2 min to 35 min, and then 100% mobile phase B at 35 min to 45 min. Solvent flow rate was 0.5 mL/min, and column temperature was 45 °C. Excitation and emission wavelengths for the fluorescence detector were 343 nm and 385 nm, respectively. The recovery rate of each metabolite in each fraction of rat urine was determined.
Pyrene metabolite identification
To identify pyrene metabolites, urine of an exposed rat was used for identification and quantification analysis, as performed in our previous study 20. Each metabolite was collected from a fraction collector (FRC-10A; Shimadzu) that was connected to the HPLC system. Each separated peak was then identified by an electrospray ionization (ESI) ion-trap mass spectrometry (MS) detector (LTQ Orbitrap; Thermo Fisher Scientific). The ESI conditions were full scan (mass to charge ratio [m/z] 80–750) in negative mode with an ion source voltage of −5.0 kV and an ion source temperature of 300 °C. In addition, each collected metabolite was identified by deconjugation. Deconjugation was performed using the method described by Ikenaka et al. 16. The enzymes sulfatase, β-glucuronidase, and β-glucosidase were dissolved in 0.1 M sodium acetate buffer, and the pH was adjusted to 5.0 with acetic acid. Enzyme concentrations were 10 U/mL, 4000 U/mL, and 17 U/mL, respectively. Aliquots (30 µL) of each sample containing pyrene metabolites were mixed with 270 µL of buffer, and then deconjugation enzyme (200 µL) was added. For the control, the same volume of bovine serum albumin (1 mg/mL) was used and subjected to the same conditions as the deconjugation enzymes. All samples were incubated at 37 °C for 8 h, and then 500 µL of methanol was added to stop the reaction. After centrifugation at 12 000 g for 10 min, the deconjugated solutions were analyzed by HPLC with a fluorescence detector.
Quantification of pyrene metabolites
Concentrations of the pyrene metabolite compounds were estimated from the fluorescence peak areas of the 1-hydroxypyrene standard solutions. The standard curve for 15 different concentrations of 1-hydroxypyrene (0.05–200 ppb) had a correlation coefficient (r2) of 0.9994. Fluorescence sensitivities of glucuronide conjugate, sulfate conjugates, and 1-hydroxypyrene were different. The fluorescence peak area ratios for each metabolite were estimated. The conjugated metabolites were enzymatically deconjugated, and the fluorescence peak areas ratio between the conjugated pyrene and 1-hydroxypyrene were calculated. The estimated ratios of pyrene-1-glucuronide and pyrene-1-sulfate to 1-hydroxypyrene were 0.21 and 0.24, respectively.
Preparation of liver cytosolic fractions
Liver cytosolic fractions were prepared following the method of Omura and Sato 24. Liver samples were homogenized with potassium phosphate buffer (0.1 M, pH 7.4) on ice. Homogenates were transferred to a tube and centrifuged at 9000 g at 4 °C for 20 min. The supernatants, which contained the cytosolic fraction, were decanted to an ultracentrifugation tube, centrifuged at 105 000 g at 4 °C for 70 min, and then transferred to 1.5-mL tubes and stored at −80 °C. The protein concentration of cytosol fractions was analyzed by using the Lowry method 25.
Aryl SULT activity
The SULT activity for 1-hydroxypyrene was determined using a modification of the method of Ueda et al. 20. The concentration of the cytosolic fraction was adjusted to 250 µg protein/mL. The fraction (50 µL) was then mixed with 10 µL of 100 mM MgCl2, 10 µL of 50 mM Na2SO3, and 1 µL of 1-hydroxypyrene. Tris-HCl buffer (100 mM, pH 7.4) was added to make up 97.5 µL. The mixtures were preincubated at 37 °C for 5 min. The reaction was initiated by adding 2.5 µL of 1 mM 3′-phosphoadenosine 5′-phosphosulfate to produce a final volume of 100 µL. The reaction was run by incubating at 37 °C for 10 min and then stopped by adding 400 µL of ice-cold methanol. Reaction samples were placed on ice for 15 min prior to centrifugation at 750 g for 10 min. The supernatant was injected into the HPLC with the fluorescence detection system. The fluorescence intensity ratio between pyrene-1-sulfate and 1-hydroxypyrene was used for quantification.
Statistical analysis
All statistical analyses were performed using JMP 9.0 (SAS Institute). Data were subjected to cluster analysis, principal component analysis, and Spearman's ρ correlation. Kinetic parameters of SULT activities were determined using the Michaelis-Menten equation and Graph Pad Prism 5 (GraphPad Software), and tested for statistical significance using Student's t test (JMP 9.0). Results were considered statistically significant at p ≤ 0.05.
RESULTS
Identification of pyrene metabolites in rat urine (experimental exposure study)
We analyzed urine samples from rats, our mammalian model, to identify pyrene metabolites (Figure 1). After solid-phase extraction under these conditions, the retention time for the internal standard was 37.8 min, and the recovery rate (± standard deviation) was 106.4 ± 10.7%. The recovery rate at a retention time of 36.6 min was 105.1 ± 5.7% (fraction 1); 97.6 ± 10.2% at 24.7 min (fraction 2); and 108.3 ± 2.0%, 97.8 ± 8.0%, 98.8 ± 3.3%, 88.5 ± 1.5%, and 89.8 ± 3.9% at a retention time of 16.8 min, 17.4 min, 19.0 min, 20.1 min, and 26.8 min, respectively (fraction 3). Two peaks of fraction 1 (peak a and the peak of the internal standard) were noted (Figure 1A), whereas 1 peak (peak b) of fraction 2 (Figure 1B) and 5 peaks (peaks c–g) of fraction 3 (Figure 1C) were observed. On the basis of the standard solution of 1-hydroxypyrene, peak a (m/z 217) was identified as 1-hydroxypyrene. The ESI negative mass spectra of peak b (retention time, 24.4 min) had a parent ion at m/z 393 (MS) and a product ion at m/z 217 (MS2). Peak c (RT 17.4) corresponds to a major ion at m/z 393 (MS) with a product ion of m/z 313 and 233 (MS2). Peaks d–f had retention times of 17.4 min, 19.0 min, and 20.1 min that correspond to a major ion at m/z 313 (MS) with a product ion of m/z 233 (MS2). The metabolite in peak g (retention time, 26.8 min) contained a major ion at m/z 297 (MS) with a product ion of m/z 217 (MS2). These results indicate that peaks a and g are the conjugation products of 1-hydroxypyrene (m/z 217). Pyrenediol (retention time, 28.5 min) contained the conjugated metabolites of peaks c–f.
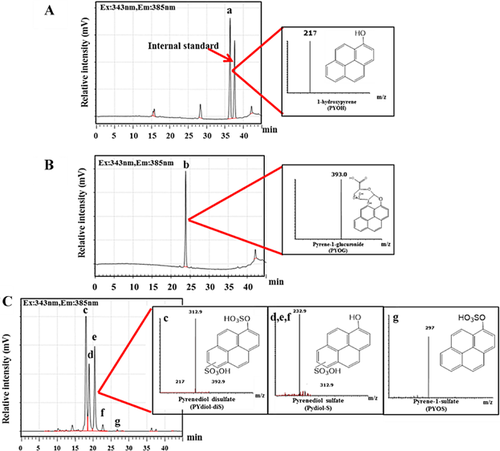
Deconjugation
Deconjugation was used to identify peaks for each pyrene metabolite. Peaks for rat urine were collected using a fraction collector connected to the HPLC system. Collected metabolites were treated with deconjugation enzymes (sulfatase, β-glucuronidase, and β-glycosidase) to identify each peak. With sulfatase treatment, peaks c–g disappeared while pyrenediol (peaks c–f) and 1-hydroxypyrene (peak g) emerged. With β-glucuronidase treatment, peak b disappeared and 1-hydroxypyrene appeared. However, no metabolites were deconjugated after β-glucosidase treatment. The MS spectra and the deconjugation study allowed identification of pyrene metabolites in rat urine as follows: peaks a, b, c, d–f, and g as 1-hydroxypyrene, pyrene-1-glucuronide, pyrenediol-disulfate, pyrenediol-sulfate, and pyrene-1-sulfate, respectively. Information based on retention time and MS spectra were used to identify and quantify pyrene metabolites from urine of various mammals (Table 2 and Figure 2).
| Animals (n) | Pyrene-1-sulfate (%) | Pyrene-1-glucuronide (%) | 1-Hydroxypyrene (%) |
|---|---|---|---|
| Deer (14) | 0.02–12.70 (10.2 ± 22.5) | 4.20–64.85 (77.8 ± 30.1) | 0.13–10.97 (15.0 ± 18.3) |
| Cattle (6) | 0.22–15.98 (13.3 ± 20.7) | 3.63–46.65 (81.2 ± 21.1) | 0.03–1.53 (5.5 ± 8.9) |
| Rabbit (2) | 5.15–13.07 (25.4 ± 14.9) | 2.19–6.98 (12.8 ± 9.1) | 16.26–27.29 (61.8 ± 24.1) |
| Horse (13) | 0.11–2.74 (12.9 ± 8.1) | 1.59–61.44 (78.9 ± 15.1) | 0.03–2.64 (8.2 ± 13.1) |
| Beef cattle (3) | 0.04–10.21 (37.3 ± 6.1) | 0.06–12.87 (54.0 ± 6.3) | 0.02–0.03 (8.8 ± 8.9) |
| Dog (2) | 0.43–1.53 (21.0 ± 0.1) | 0.93–5.09 (57.8 ± 16.7) | 0.67–0.69 (21.2 ± 16.5) |
| Guinea pig (2) | 1.62–2.79 (12.9 ± 7.3) | 1.12–1.14 (6.4 ± 1.4) | 11.54–18.09 (80.7 ± 8.7) |
| Hedgehog (2) | nd | 1.08–3.36 (44.9 ± 10.6) | 1.80–3.05 (55.1 ± 10.6) |
| Cat (2) | 0.69–1.35 (39.5 ± 29.5) | nd | 0.89–3.01 (60.5 ± 29.5) |
| Ferret (2) | 0.63–0.72 (31.1 ± 14.9) | nd | 0.88–2.79 (68.9 ± 14.9) |
| Pig (3) | 0.03–0.15 (19.3 ± 3.4) | 0.06–0.36 (46.8 ± 9.4) | 0.04–0.39 (33.9 ± 9.5) |
| Human (3) | 0.02–0.05 (16.3 ± 9.9) | 0.02–0.27 (66.8 ± 24.6) | 0.021–0.023 (16.9 ± 15.3) |
| Elephant (10) | 0.01–0.13 (24.7 ± 8.8) | 0.02–0.27 (56.8 ± 18.0) | 0.01–0.12 (18.6 ± 17.5) |
| Tapir (1) | 0.09 (51.7) | 0.04 (21.9) | 0.05 (26.4) |
| Chimpanzee (1) | 0.019 (22.1) | 0.016 (18.5) | 0.05 (59.4) |
| Bear (1) | 2.36 (29.6) | 1.88 (23.5) | 3.75 (46.9) |
- a Percentage of metabolites are shown in parentheses.
- nd = not detected
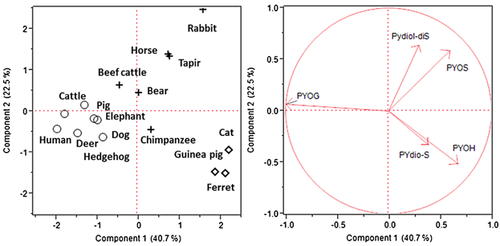
Cluster analysis and principal component analysis of pyrene metabolite profiles in mammalian urine
The profiles of pyrene metabolites in various mammalian urine samples were characterized by cluster analysis, and then the results were analyzed by principal component analysis (Figure 2). The result shows 3 major groupings: the pyrene-1-glucuronide group was strongly correlated with urine of cattle, deer, dogs, humans, hedgehogs, pigs, and elephants; the 1-hydroxypyrene and pyrenediol-sulfate groups include the urine of ferrets, cats, and guinea pigs; and the pyrene-1-sulfate and pyrenediol-disulfate groups were highly correlated with tapirs, rabbits, horses, beef cattle, bears, and chimpanzees.
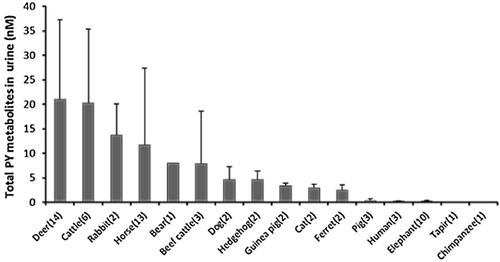
Quantification of pyrene metabolites in urine of various mammals
Pyrene metabolites in mammalian urine collected from animals in both rural and urban areas were determined, and the concentration ranges of each metabolite (pyrene-1-glucuronide, pyrene-1-sulfate, and 1-hydroxypyrene) are summarized in Table 2 and Figure 3. Pyrenediol-sulfate and pyrenediol-disulfate were not quantified in the present study because of the low fluorescence sensitivity for the product of deconjugation (pyrenediol). Higher concentrations of pyrene metabolites were detected in the urine of ungulates such as deer, cattle, and horses. Pyrene-1-glucuronide was readily detected in samples from several mammals (0.02–64.85 nM). The order of pyrene-1-glucuronide concentrations was deer > horses > cattle > beef cattle > rabbits > dogs > hedgehogs > bear > pigs > elephants > humans > guinea pigs > tapirs and chimpanzees. Pyrene-1-sulfate was found in almost all urine samples (0.01–15.98 nM) except those from hedgehogs. Concentrations followed the order cattle > deer > rabbits > beef cattle > horses > guinea pigs > dogs > cats > bear > elephants > pigs > ferrets > tapirs > humans and chimpanzees. Interestingly, pyrene-1-sulfate was present in pig urine, although pigs had been found to have poor SULT activity toward phenolic compounds. We found 1-hydroxypyrene in concentrations of 0.01 nM to 27.29 nM, with an order of rabbits > guinea pigs > deer > horses > cats > ferrets > bear > cattle > hedgehogs > pigs > dogs > elephants > chimpanzee > tapir and beef cattle.
SULT activity in pigs
Enzyme kinetics experiments were performed to confirm SULT activity in pigs. Kinetic analysis results of SULT-dependent activity toward 1-hydroxypyrene are shown in Table 3. Sulfation activity of 1-hydroxypyrene was detected in pigs. Kinetic parameters of SULT-dependent activities toward 1-hydroxypyrene in pigs were compared with those in rats using liver cytosol fractions. Maximum velocity (Vmax) in rats was significantly greater than that in pigs (563.0 ± 45.6 pmol/min/mg protein and 58.5 ± 10.6 pmol/min/mg protein, respectively). However, the Michaelis constant (Km) value in pigs was significantly smaller than that in rats (9.4 ± 1.8 nM and 258.7 ± 21.5 nM, respectively). It is notable that the Vmax/Km of pigs indicated higher efficiency than that in rats.
| Vmax (pmol/min/mg protein) | Km (nM) | Vmax/Km (mL/min/mg protein) | |
|---|---|---|---|
| Pigs | 58.5 ± 10.6 b | 9.4 ± 1.8 b | 6.2 ± 0.7 a |
| Rats | 563.0 ± 45.6 a | 258.7 ± 21.5 a | 2.2 ± 0.2 b |
- a Data represent the mean ± standard deviation for each species (males only). Values in a column with different letters have significant differences (p < 0.05).
- Vmax = maximum velocity; Km = Michaelis constant.
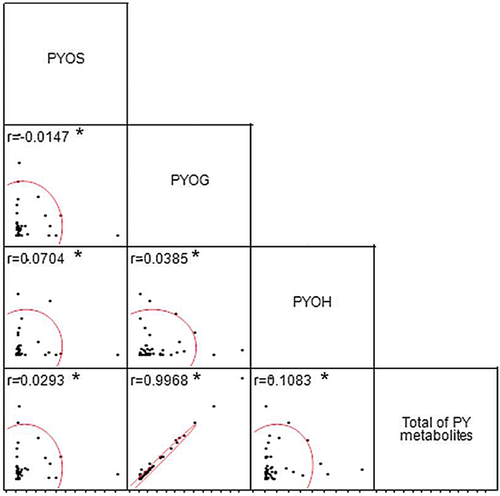
Correlations of pyrene metabolites in mammalian urine
No correlations were observed between certain pyrene metabolites (Figure 4) such as pyrene-1-sulfate and pyrene-1-glucuronide (r = 0.0147), pyrene-1-sulfate and 1-hydroxypyrene (r = 0.0704), pyrene-1-sulfate and total pyrene metabolites (r = 0.0293), pyrene-1-glucuronide and 1-hydroxypyrene (r = 0.0385), and 1-hydroxypyrene and total pyrene metabolites (r = 0.1083). A strong positive correlation was found between pyrene-1-glucuronide and total pyrene metabolites (r = 0.9968; Spearman's ρ < 0.05).
DISCUSSION
Polycyclic aromatic hydrocarbons are found to universally contaminate food and the environment. Therefore, animals may be exposed to pyrene from various sources such as consumption of contaminated food, soil, or water or inhalation of contaminated air. Conjugated metabolites were detected in the urine of 16 mammal species with daily exposure to pyrene. Our results show that urinary metabolite composition in various mammals can clearly characterize interspecific differences in phase II reactions, especially in SULT and UGT activities.
Concentration of pyrene metabolites in urine from various mammals
High levels of pyrene metabolites were observed particularly in ungulate species such as deer and cattle. Though gender was not of concern in the present study, species differences are generally greater than sex differences in the metabolism of xenobiotics including pyrene. High levels of pyrene metabolites observed in ungulates may correlate with a high consumption level of contaminated materials. Surprisingly, pyrene metabolites were also detected in urine from companion animals such as cats, dogs, guinea pigs, hedgehogs, and ferrets. Although the sample size of some species (tapir, chimpanzee, bear, and ferrets) was small, we report our findings as useful baseline information for these species.
Interspecific differences in pyrene sulfation
Different patterns of pyrene metabolites were found in the mammalian species that were studied. In principal component analysis, pyrene sulfate conjugates were detected in nearly all domesticated livestock, companion animals, and captive and free-ranging wildlife. Not only pyrene-1-sulfate but also pyrenediol-sulfate and pyrenediol-disulfate were detected in the urine from these mammals. These metabolites were also detected in previous studies using rats and lower vertebrates (amphibians and fish) 13, 20. Although sulfation occurs in various mammals, pigs have been reported to have limited aryl SULT activity 26. However, pyrene sulfate conjugates including pyrene-1-sulfate and pyrenediol-sulfate were detectable in pig urine. To investigate aryl sulfation activity in pigs, 1-hydroxypyrene was used as a substrate, and kinetics parameters (Vmax, Km, and Vmax/Km) were compared with those of rats. Kinetic analysis results of SULT dependent activity toward 1-hydroxypyrene showed clear differences in SULT-dependent activity between pigs and rats. The low Vmax value in pigs corresponded with those in previous reports 27. However, pigs had lower Km values compared with rats, indicating that pig SULT had greater substrate affinity than rat SULT. Interestingly, Vmax/Km values were significantly greater in pigs compared with rats, indicating that pigs had higher SULT enzyme efficiency.
It has been reported that SULT1A subfamily enzymes are responsible for the sulfation of small phenolic substrates such as p-nitrophenol, 1-naphthol, acetaminophen, and dopamine 28. Additionally, pyrene has been found to induce SULT1A1 mRNA in mice 29. Thus, SULT1A1 is thought to be an important enzyme involved in the sulfation of pyrene. Phylogenetic assessment of SULT1A genes has been performed in a large range of species 30. Mice, rats, dogs, cattle, rabbits, and pigs all have genomes containing a single SULT1A1 gene 31, 32. Hominoid SULT1A loci were found to be orthologous to rodent SULT1A1. Although human SULT1A has 4 forms, human SULT1A1 most closely resembles rodent SULT1A1 in sequence and it functions as an ortholog 30. We may therefore assume that 1-hydroxypyrene is catalyzed by an enzyme encoded in the SULT1A1 gene and that sulfation of pyrene metabolites may occur at normal levels in mammals, including pigs 31, 32. However, sulfation may be limited in some mammalian species such as hedgehogs as we were unable to find pyrene sulfate metabolites in hedgehog urine. Currently, there is limited information on xenobiotic metabolism in hedgehogs.
Interspecific differences in glucuronidation
Glucuronide was the dominant metabolite in various species, as demonstrated in the principal component analysis. The human subjects were healthy and nonsmoking, and 77% of total pyrene metabolites were excreted as glucuronide conjugate. It has been reported that human UGT1A6, -1A7, and -1A9 enzymes are mainly involved in glucuronidation of 1-hydroxypyrene, similar to findings in mice 29, 33-35. Glucuronide levels have been reported to account for more than 80% of total pyrene metabolites in human urine 34—similar to results in the present study—as the majority of 1-hydroxypyrene was observed to be conjugated with glucuronide, and a strong correlation between pyrene-1-glucuronide and total pyrene metabolites was found. These levels might be caused by high activity of UGT enzymes, which have been shown to be similar in studies using human urine 10, 36. In particular, we identify a close relationship between pyrene-1-glucuronide and total pyrene metabolites in ungulates, where glucuronidation was the main conjugation route of pyrene. The UGT activities may be higher than those of SULT in ungulates. Several other substrates have also been reported to be highly conjugated by UGT in ungulates 37, 38.
Glucuronide conjugates were detected in urine from most species. However, glucuronide conjugates could not be detected in cat or ferret urine in the present study. It has been reported that feline species and ferrets have no or low UGT activity toward phenolic substances 6.
CONCLUSION
The present study demonstrates that mammalian species differ in their abilities to process phenol metabolites, especially in phase II reactions. However, there is limited information on species differences in levels of phase II conjugation in vivo. Clear interspecific differences in urinary pyrene metabolites were derived using our novel screening method. We strongly suggest considering use of not only genomic and in vitro studies but also screening of urinary metabolites of phase II reactions in domesticated livestock, companion animals, and captive and free-ranging wildlife as important methods to characterize interspecific differences. Our findings indicate that the pyrene metabolites can be used as effective biomarkers to screen species exposure to high levels of environmental contaminants as PAH. They can be especially of use in screening high-risk species after accidental contamination, such as after oil spills.
Acknowledgment
A. Saengtienchai and Y. Ikenaka contributed equally to the present study. We are grateful to the Kasetsart University Veterinary Teaching Hospital (Kampangsan and Bangkhan Campuses), the Faculty of Veterinary Medicine, the Kasetsart University (Thailand), and the Laboratory of Wildlife Biology and Medicine, Graduate School of Veterinary Medicine, Hokkaido University (Japan), for the provision of urine samples. We are grateful to L. Thompson for proofreading the text. The chemical analyses were technically supported by T. Ichise. The present study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan awarded to M. Ishizuka (numbers 24248056 and 24405004) and Y. Ikenaka (number 26304043).




