Determination of soil–water sorption coefficients of volatile methylsiloxanes
Abstract
The sorption behaviors of 4 cyclic and linear volatile methyl siloxane (VMS) compounds between water and organic matter in 3 United Kingdom soils were studied by a batch equilibrium method using13C-enriched sorbates. Sorption and desorption kinetics and isotherms were determined for octamethylcyclotetrasiloxane (D4), decamethylcyclopentasiloxane (D5), octamethyltrisiloxane (L3), and decamethyltetrasiloxane (L4). Concentrations of [13C]-VMS in the soil and aqueous phases were measured directly by extraction and gas chromatography–mass spectrometry techniques. All VMS compounds were sorbed rapidly, reaching constant distributions in all soils by 24 h. Desorption kinetics were very rapid, with reattainment of equilibrium within 1 h. In the main, linear isotherms were observed for aqueous concentrations at or below 4% of the solubility limits. The average sorption organic carbon partition coefficient (log KOC) values across soils were 4.23 for D4, 5.17 for D5, 4.32 for L3, and 5.13 for L4, with standard deviations of 0.09 to 0.34. Desorption KOC values were systematically greater by 0.1 log units to 0.3 log units. The linear isotherms and low variation in KOC values across soils suggested partitioning-dominated sorption of the VMS. Compared with traditional hydrophobic organic compounds, KOC values for the VMS compounds were significantly lower than expected on the basis of their octanol–water partition coefficients. A linear free energy relationship analysis showed that these differences could be rationalized quantitatively in terms of the inherent characteristics of the VMS compounds, combined with the differences in solvation properties of organic matter and octanol. Environ Toxicol Chem 2014; 33:1937–1945. © 2014 The Authors. Environmental Toxicology and Chemistry published by Wiley Periodicals, Inc. on behalf of SETAC.
INTRODUCTION
Volatile methylsiloxanes (VMS) are a subset of oligomeric dimethyl silicones that are used widely in industrial, personal care, and household applications 1. These compounds are used because they exhibit unique physical–chemical properties, including low surface tension and odor, while having high thermal stability, hydrophobicity, and volatility. In addition, VMS are commercially available in large volumes and are not regulated as volatile organic compounds. In 2012, worldwide consumption of all silicone fluids, including VMS, was 639 metric tons, with approximately 29% being used in Western Europe, 25% in North America, and 39% in Asia 2. Siloxanes became a focus of attention because of their potential for persistence and bioaccumulation in the environment, based mainly on high predicted bioconcentration factors and long biodegradation half-lives 3.
The structure of VMS, containing 1 or more dimethylsilyloxy units (except for hexamethyldisiloxane), can be either linear or cyclic. An abbreviation of L is assigned to linear VMS having trimethylsilyl end groups, whereas cyclic VMS, comprised of only dimethylsilyloxy units, are denoted with a D. Because of relatively high vapor pressure 4, 5 and low water solubility 6, VMS tend to volatilize from aqueous solution and thus are expected to partition primarily to air during use 7 and after disposal to wastewater 8. However, based on high empirical values of the octanol–water partition coefficient (KOW) for these compounds 9, sorption by organic matter is expected to compete with volatilization as an additional intermedia transport mechanism. In surface waters, for example, VMS may sorb to particulate organic matter and deposit eventually to sediments 10, 11. Consequently, sorption by organic matter in soil or sediment will play an important role in determining the distribution, fate, and transport of VMS in the environment.
The organic carbon partition coefficient (KOC) is the single most important parameter for quantifying the sorption behavior of nonionic organic compounds in soil and sediment. For hydrophobic compounds, for which nonspecific partitioning is the predominant sorption mechanism, organic matter content is the key sorbent characteristic controlling KOC. Based on the assumption that 1-octanol can be used as a surrogate for organic carbon in solids/water partitioning in the environment, KOC values for neutral organic compounds are often estimated using empirical quantitative structure property relationships 12. In particular, single-parameter empirical correlations with KOW have been used extensively in environmental modeling, and generic relationships have been derived for traditional hydrophobic compounds, as reviewed by Doucette 12. It is well established that chemically diverse polar organics show substantial variation in these simple relationships 13, indicating that the sorption behaviors of these compounds cannot be completely replicated with 1-octanol 14, 15.
In recent years, a few measured KOC values for 2 VMS, octamethylcyclotetrasiloxane (D4) and decamethylcyclopentasiloxane (D5), have been found to deviate substantially from these predictions 16-18, although they are highly hydrophobic compounds. It is notable that the methods for measuring these KOC values included the static equilibrium method with isolated particulate organic carbon, and a dynamic method based on temporal decreases in VMS concentration during volatilization from river water. The reported values were not completely consistent by the 2 methods, although both were less than predicted from KOW. A reasonable question is: Do those deviations arise from measurement errors, or do they represent fundamental differences between the natural organic matter and 1-octanol, as sensed by VMS?
The main objectives of the present study were 2-fold: first, to accurately determine the sorption behavior of 4 selected cyclic and linear methylsiloxanes in soils using a batch equilibrium method, with careful analytical measurements in both phases; and second, to reveal the differences between soil organic matter and 1-octanol as a partitioning medium for methylsiloxanes using the measured KOC values, which can explain their unique combination of partitioning behaviors in these media.
MATERIALS AND METHODS
Test sorbents and reagents
The 13C-enriched and natural isotopic abundance D4, D5, L3, and L4 compounds were obtained from Dow Corning; chemical purity for all siloxanes was 96% or greater by gas chromatography (GC). Both 1-octanol and 1-heptanol (≥99.5%) were supplied by Fluka, and N,N-dimethyl-formamide (DMF), acetonitrile (MeCN), and hexanes were obtained from Fisher Scientific. Calcium chloride (CaCl2) solution, 0.01 M, was used as the aqueous phase in the present study; the solutions were prepared from the dihydrate salt (Fisher Scientific) using deionized water from a Millipore Milli-Q Plus system and were filtered (0.2 µm) prior to use. Poly(dimethylsiloxane)-coated fused silica fibers (30-µm film) for solid phase microextraction were obtained from Supelco. All materials were used as supplied.
Soil characteristics
Three United Kingdom soils were selected for use in the present study that satisfied the recommendations in the Organization for Economic Cooperation and Development testing guideline 106 19 by covering a range of soil textures, organic carbon content, and pH. They included a silt loam (soil 164), a sandy loam (soil 248), and a sandy clay loam/sandy loam (soil 282) having organic carbon contents from 2% to 5.5% (% w/w). Soil pH values ranged from 5.5 to 8.3. Soil moisture contents of the test soils were determined concurrently with each experiment. Detailed information on each soil is given in the Supplemental Data (Table S1).
Experimental design
Sorption and desorption kinetics were determined at a single loading of each sorbate. For D4, the 3 soils were each spiked with 1000 ng [13C]-D4 per vessel at a soil:solution ratio of 1:20. For D5, samples were spiked with 200 ng [13C]-D5 at a 1:50 ratio. The L3/L4 experiments used the 1:50 soil:solution ratio, spiked with 90 ng each [13C]-L3 and [13C]-L4. The loadings and phase ratios were chosen to give accurately quantifiable concentrations in both phases for the kinetics experiments, and subsequent isotherm studies, taking care that initial aqueous concentrations not exceed solubility limits.
In the sorption (i.e., uptake from water) kinetics experiments, controls and spiked samples were mixed continuously and analyzed at time intervals between 0.5 h and 30 h. For desorption, samples were prepared in the same manner as for sorption and allowed to equilibrate for 24 h with continuous mixing. Subsequently, samples were centrifuged, and the aqueous phase was discarded and replaced with equivalent volumes of fresh 0.01 M CaCl2 solution. The soil pellets were resuspended and mixed continuously until sampled after 0.5 h to 30 h.
For sorption and desorption isotherm determinations, all 3 soils were evaluated at 6 different loadings that ranged from approximately 10 ng to 1000 ng [13C]-siloxane per vessel. Samples were prepared as described for the kinetic experiments and were equilibrated for 24 h (sorption and desorption) with continuous mixing. In the interest of efficiency, the 2 linear VMS were studied as a mixture after preliminary experiments were completed to confirm similar behavior of the individual sorbates over the range of loadings with soil 164.
Test conditions and sample preparation
Experiments were conducted in the dark inside an incubator. Temperature was recorded automatically once per hour using a HOBO digital temperature logger (Onset Computer). For the D4 experiments, temperature averaged 24.8 °C and ranged from 24.4 °C to 24.8 °C. For D5, temperature averaged 25.6 °C and ranged from 24.8 °C to 26.3 °C. For the combined L3/L4 experiments, the average temperature was 23.7 °C, with a range of 23.6 °C to 23.9 °C.
Because of low aqueous solubility 6 and propensity to volatilize from water 9, siloxane was added directly to individual test units by spiking a small volume of a solution in DMF into the soil–water slurry. The majority of the D4-spiked samples, and all of the D5-spiked or L3/L4-spiked samples, contained 0.1% v/v DMF. Tests with D4 showed no effect on measured distribution coefficient (Kd) values for cosolvent content up to 0.4% v/v.
Duplicate samples were prepared for each experimental endpoint (i.e., time point for kinetics or concentration for isotherms). For each experiment, a precise amount of moist test soil and approximately 25 g of 0.01 M CaCl2 were added gravimetrically to individual test vessels to obtain the desired soil:solution ratio and to minimize headspace volume. The test vessels were 20 mm × 125 mm borosilicate glass test tubes with threaded ends, fitted with Mininert valve screw caps. The soil–water mixtures were conditioned overnight on a rotational mixer prior to test substance addition. At test initiation, individual vessels were removed from the mixer; spiked with either [13C]-D4, [13C]-D5, or a mixture of [13C]-L3 and [13C]-L4 in DMF; and returned immediately to the mixer. The amount of siloxane added by spiking was determined analytically using the same GC–mass spectrometry (MS) method as described below for analysis of the soil; these data showed stability of the spiking solutions over the course of the present study and were used in mass balance calculations for samples.
Sample extractions and instrumental analysis
Just prior to analysis, each sample was centrifuged for 5 min at 1300 g to separate the soil solids from the aqueous phase. Experiments with D4 and soil 164, containing the highest combined fraction of silt and clay, showed no effect on the measured value of Kd when the centrifuge time to was increased to 30 min or the force was increased to 1800 g.
Isolation and concentration of [13C]-D4 and [13C]-D5 for the aqueous phase samples were accomplished by using a headspace solvent microextraction (HSME) method 20, whereas headspace solid phase microextraction (SPME) was used for determinations of [13C]-L3 and [13C]-L4. The SPME method was more convenient but could not be used for the D4 and D5 studies because of the excessive and persistent blank contribution from the polydimethylsiloxane fiber coating, which was superior to other commercially available SPME phases for extracting the siloxanes. Extraction of sorbed siloxanes from soil samples was performed by using a biphasic solvent extraction method with MeCN and hexane, which was previously developed for analysis of methylsiloxanes in sediment 21.
Quantification of [13C]-enriched siloxanes in HSME or SPME extracts of aqueous samples, and hexane extracts of soil samples, was performed by GC-MS using natural carbon (natC) abundance D4, D5, L3, and L4 as internal standards. Further details of the extraction procedures and GC-MS analyses are found in the Supplemental Data.
Calibration and quality control
The challenges associated with trace-level quantitative analysis of VMS are well documented and include adventitious sample contamination and matrix-related effects 21, 22. To minimize measurement errors in the present study, the 13C-enriched forms of VMS were used as sorbates, thereby reducing background signals in blanks and decreasing sensitivity to contamination by nonlabeled VMS. In addition, rigorous quality control (QC) procedures involving multipoint calibration, and analysis of blanks and independently formulated QC samples were instituted to support the validity of measurements on each sample set.
Calibration standards and QC samples were formulated to cover the concentration ranges used in the experiments. Using a variation of isotope dilution, calibrations were based on the relationship between the formulated amount ratio of [13C]- to [natC]-siloxane in the standards solutions and the ratio of measured peak areas from the resulting ion-specific chromatograms. Calibration of the HSME and SPME methods involved actual extractions of spiked aliquots of 0.01 M CaCl2. Analytical blanks and independently formulated QC samples (unspiked and spiked CaCl2 solution or soil extraction solvent, respectively) were analyzed during each batch of sample analyses to verify accuracy of the methods over the concentration range relevant to the experiment.
Duplicate control samples were analyzed during each experiment to confirm the absence of contamination or analytical artifacts. A control sample consisted of the soil and CaCl2 solution of the same weight and volume, respectively, as the actual test samples, but without test substance added. The control samples were otherwise treated in the same manner as the test samples.
Data analysis
Descriptive statistics and regression analyses were performed in Microsoft Excel 2003 or Minitab 15/16. Significant differences were defined as p ≤ 0.05. Percentage sorption (%At) and desorption (%Dt) were defined as the mass fraction of test substance sorbed by the soil or desorbed into the aqueous phase, respectively, relative to the total amount measured in the test vessel (soil and water phases) at time t during equilibration. Mass balance (% recovery) was defined as the fraction of test substance that was analytically recovered from the sample (soil and water phases) relative to the measured amount of substance applied.
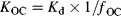 (1)
(1)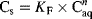 (2)
(2)Values of KF and n were obtained by linear regression of log Cs on log Caq.
RESULTS AND DISCUSSION
Calibration and QC performance
For the aqueous phase analysis methods, excellent calibration performance was observed for all analytes. Coefficients of determination (R2) exceeded 0.999, and the calibration models gave predictions for all standards that were accurate to within ± 13% and mostly better than 6% if the lowest level standard was not considered. A similar performance was observed for the soil analysis methods, although a slightly lower R2 value (0.993) was observed in the case of [13C]-L3. For all analytes, the errors for refit of the hexanes standards were almost exclusively within ± 10%.
The QC samples were included with each analytical run to verify accuracy for each method over the operative range. For the aqueous phase QC samples, the relative error in predicted [13C]-D4 amounts at all levels ranged from −3% to 2% across all experiments. With [13C]-D5, aqueous QC samples gave errors in predicted amounts that were within ± 6% of expected for the isotherm experiments. In the case of [13C]-L3 and [13C]-L4, QC samples were consistently and uniformly underpredicted by 9% to 14% and 5% to 18%, respectively, suggesting a possible discrepancy in the preparation of the QC samples and calibration standards. Consequently, the aqueous sample concentrations could have been systematically over- or underdetermined.
For the QC samples associated with the soil method, the relative errors in predicted [13C]-D4 amounts were between −14% and 2%; excluding the results from a single experiment, the relative error ranged from −6% to 2%. For [13C]-D5, the relative error for soil QC samples ranged from −12% to −2%. Once again, QC sample results for the linear siloxanes showed slightly greater errors, ranging mainly from −14% to −7% for [13C]-L3, and from −17% to −7% for [13C]-L4. Together, the QC sample results demonstrated that in the situation where the measured aqueous and soil phase concentrations were in error by 12% each on average, but in opposite directions, the resulting bias in the calculated KOC values could be ± 0.10 log unit.
Control samples
The absolute values of the average apparent [13C]-D4 and [13C]-D5 concentrations in the aqueous and soil phases of the control samples for each experiment were 6% or less of the lowest measured concentrations in the isotherm studies and thus were even less at the higher loadings. Similar results were observed for [13C]-L3 and [13C]-L4, although the magnitude of the equivalent background concentrations in the soil phase ranged from 4% to 19% of the lowest isotherm concentrations. In the case of [13C]-L4, slightly greater equivalent concentrations were observed for the aqueous phase of the controls, up to 14% of the lowest sample concentration, which were also exclusively less than 0 (an artifact of the calibration blank). In general, these blank contributions were not a significant source of bias in the determination of sample concentrations, except possibly for the lowest loadings in the L3/L4 isotherm experiments, which is addressed below. By limiting background levels in blanks and controls, use of the 13C-enriched VMS allowed investigation of lower concentrations in the isotherm studies than would have been practically achievable otherwise.
Mass balance determination
Because of their high air/water partition coefficients 9 and susceptibility toward hydrolysis 16, 17, the VMS could be lost from the soil–water system. Consequently, mass balance was tracked during each experiment. Recoveries were calculated as described above. For [13C]-D4, [13C]-D5, and [13C]-L4, the average mass balance for samples within each sorption experiment ranged from 89% to 102%, with standard deviations of 7% or less (n = 12–14). Generally, recoveries decreased slightly with increasing equilibration time (kinetics experiments) and decreasing organic carbon content. Lower soil:water ratio and KOC value of the sorbate were also associated with lower recoveries within the stated range. Average recoveries were systematically lower for [13C]-L3, ranging from 66% to 87%, with standard deviations up to 9%. The experiments with L3/L4 were conducted at a lower soil:water ratio (1:50, vs 1:20 for D4) to accommodate the greater sorption of L4, which likely contributed to the lower recoveries of [13C]-L3. Because values of Kd were calculated from measured siloxane concentrations in each phase, the low recoveries were detrimental only if they interfered with the attainment of equilibrium. The results presented below indicated that this was likely not a concern.
Sorption/desorption equilibration times
The kinetics of equilibration were investigated for both sorption and desorption of each siloxane with all 3 soils, using a 1:20 or 1:50 soil:solution ratio. These ratios were optimized to ensure quantifiable aqueous concentrations for the lowest intended loadings in the subsequent isotherm experiments. The sorption behavior of each siloxane was characterized by rapid initial uptake from water, ranging from ≥85%A for [13C]-L3 up to ≥97%A for [13C]-D5 and [13C]-L4, within approximately 1 h of contact. This was followed by a period of slower uptake until a consistent distribution was demonstrated by 16 h to 24 h of contact, through the last time point at 30 h. Under the prevailing conditions, only minor differences in sorption kinetics were observed between soils, as shown in Figure 1 for [13C]-L4. Similar behavior was observed for the other sorbates (Supplemental Data, Figures S1–S3).
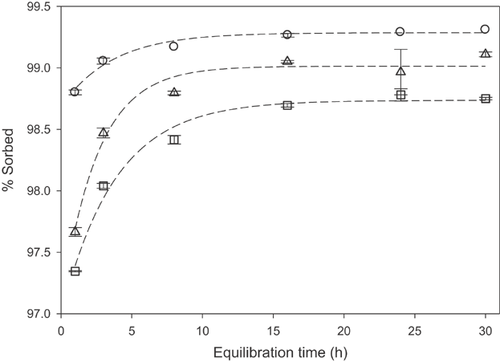
Based on the sorption results, a 24-h equilibration time was chosen for the studies of desorption kinetics. After the equilibrated aqueous phase was replaced with fresh CaCl2 solution at the end of the 24-h uptake period, desorption equilibrium was attained very rapidly for the siloxanes on all soils. As exemplified by [13C]-L3, showing the greatest extent of desorption in any experiment (up to 9%D on the low-OC soil), the fraction of siloxane desorbed was essentially constant from the first sampling time at 0.5 h or 1 h through the final desorption sampling at 30 h (Figure 2). The same behavior was observed for the other siloxanes (Supplemental Data, Figures S4–S6).

Hydrophobic sorbates can become increasingly desorption resistant as contact time with soil or sediment increases, and multisite sorption models are sometimes needed to characterize the different kinetic domains in the sorbent (i.e., rapidly desorbing, slowly desorbing, and so on) 23, 24. Given the shorter contact times used in the present studies, the fraction of siloxane associated with a slowly desorbing domain in the soil might have been limited, which, combined with the small mass of siloxane transferred to the aqueous phase, produced the observed desorption profiles. Additional experiments using longer sorption times could be performed in the future to elucidate this aspect of sorption behavior of the siloxanes. Based on the kinetic results and convenience with respect to timing of spiking and sampling events, 24 h was used as the equilibration time for both sorption and desorption in the subsequent isotherm experiments.
Sorption/desorption isotherms and KOC
Adsorption and desorption isotherms on all 3 soils were evaluated at 6 loadings covering a range of 2 orders of magnitude. At equilibrium, aqueous phase [13C]-siloxane concentrations (Caq) between 1 pg/g and 2000 pg/g were measured. This was equivalent to a range of 0.01% to 4% of the aqueous solubility limits 6 across all experiments. The corresponding measured concentrations in soil (Cs) were between 5 ng/g and 1400 ng/g (dry mass basis). The data from these experiments were interpreted using the Freundlich isotherm (Equation 2), with the results summarized in Table 1 for sorption (see Supplemental Data, Table S2, for desorption results). Coefficients of determination were ≥0.996 for all isotherm regressions, with most values exceeding 0.998. Excluding L4, values of the Freundlich exponent, n, ranged from 0.970 to 1.04. In only 1 of 19 isotherm experiments did the 95% confidence interval on n not include 1.00; the experiment that gave rise to the outlying value, 1.04, when repeated, gave a value of 1.00. These results indicated linear sorption and desorption isotherms for L3, D4, and D5 over the observed concentration range.
| Soil | R2a | n (±95% CI) | KF | log10 KF/%OC | log10 KOCb | |
|---|---|---|---|---|---|---|
| Cyclics | ||||||
| D4 | 164 | 0.9998 | 0.991 (0.981–1.00) | 496 | 4.16 | 4.17 |
| 248 | 0.9997 | 1.00 (0.988–1.01) | 335 | 4.22 | 4.22 | |
| 282 | 0.9988 | 1.02 (0.994–1.05) | 1080 | 4.29 | 4.27 | |
| Mean (SD) | 4.23 (0.06) | 4.22 (0.05) | ||||
| D5 | 164 | 0.9996 | 1.00 (0.982–1.01) | 5960 | 5.24 | 5.25 |
| 248 | 0.9983 | 0.977 (0.947–1.01) | 3580 | 5.25 | 5.29 | |
| 282 | 0.9989 | 1.04 (1.01–1.06) | 6060 | 5.04 | 4.98 | |
| 282c | 0.9994 | 1.00 (0.980–1.01) | 5140 | 4.97 | 4.98 | |
| Mean (SD) | 5.17 (0.14) | 5.17 (0.17) | ||||
| Linears | ||||||
| L3 | 164 | 0.9990 | 0.983 (0.961–1.00) | 625 | 4.26 | 4.28 |
| 248 | 0.9970 | 0.970 (0.932–1.01) | 439 | 4.34 | 4.37 | |
| 282 | 0.9985 | 0.980 (0.954–1.01) | 1240 | 4.35 | 4.38 | |
| Mean (SD) | 4.32 (0.05) | 4.34 (0.05) | ||||
| L4 | 164 | 0.9986 | 0.954 (0.929–0.980) | 5310 | 5.19 | 5.28 |
| 248 | 0.9983 | 1.01 (0.978–1.04) | 3740 | 5.27 | 5.26 | |
| 282 | 0.9968 | 0.984 (0.944–1.02) | 4645 | 4.93 | 4.95 | |
| Mean (SD) | 5.13 (0.18) | 5.16 (0.18) |
- a From regression of log Cs on log Caq, based on Equation 2.
- b Calculated from average value of Kd for all samples within the experiment (n = 12 typically; 6 concentrations × 2 replicates).
- c Repeat experiment.
- VMS = volatile methylsiloxanes; CI = confidence interval; KF = Freundlich coefficient; KOC = organic carbon partition coefficient; SD = standard deviation; D4 = octamethylcyclotetrasiloxane; D5 = decamethylcyclopentasiloxane; L3 = octamethyltrisiloxane; L4 = decamethyltetrasiloxane; Cs = test substance; concentration in the soil solids; Caq = test substance; concentration in the aqueous solution; Kd = distribution coefficient.
For L4, values of n were systematically lower, ranging from 0.932 to 1.01; 4 of 6 values, including all 3 of the desorption isotherms, were statistically less than 1.00. Excluding the lowest concentration from each desorption isotherm gave no or only a slight increase in n, as well as R2, but the upper 95% confidence limits remained less than 1.00. This suggested that error in low-level concentration measurements was not responsible for the observed deviations, which were reflected in KOC values that varied by 0.10 log units to 0.15 log units over the full concentration range. Presently, we have no alternative explanation for this phenomenon, which appeared to be unique to L4, and mainly desorption.
Values of the Freundlich coefficient, KF, ranged from approximately 340 g/g to 1300 g/g for D4 and from 3600 g/g to 9700 g/g for D5. For the linear siloxanes L3 and L4, values of KF were comparable to those of D4 and D5, respectively, ranging from 440 g/g to 1400 g/g for L3 and from 3700 g/g to 6700 g/g for L4. In all cases, values for desorption (KF,des) were greater than those for sorption (KF,ads) on the same soil; KF,des exceeded KF,ads by 12% to 23% for D4, 40% to 74% for D5, 2% to 12% for L3, and 8% to 37% for L4. For all sorbates, the greatest ratio of KF,des to KF,ads was observed for soil 282, which had the highest OC content of the 3 soils. The systematically greater values of KF,des could not be attributed to analytical error based on high reproducibility of the organic carbon results, and the shape and temporal overlap of the sorption and desorption kinetic profiles suggested that differences in contact time were also not responsible. The most likely explanation was the effect of dissolved or colloidal organic matter from the soil on the total aqueous phase siloxane concentrations in the sorption experiments, which was diminished when the equilibrated aqueous phase was exchanged with fresh CaCl2 solution for desorption. The extent of the discrepancy was generally greater for L4 and D5 because of their greater KOC values.
When the Freundlich exponent is equal to 1, KF is equivalent to Kd, and values of KF can be used to calculate the organic carbon normalized sorption coefficient, KOC. Values of log KOC were calculated from the values of KF for sorption (Table 1) and desorption (Supplemental Data, Table S2). Despite the slight apparent deviation from desorption isotherm linearity for L4, KOC values were calculated from KF,des values. In all cases, the variation in log KOC across soils was 0.3 log units or less and typically was less than 0.2 log units. The average values of log KOC for sorption were 4.23 and 4.32 for D4 and L3, respectively, whereas D5 and L4 gave values of 5.17 and 5.13, respectively. Desorption KOC values were greater by up to 0.2 log units.
The D5 sorption KOC value reported in the present study is approximately 1 log unit lower than values reported by Whelan et al. for volatilization experiments with filtered river water 18. However, direct comparison with the present study is complicated by methodological differences, including the assumption of equilibrium partitioning in the dynamic system. In addition, the form (i.e., particulate vs dissolved), source, and loading (≤5 mg C/L, vs ≥400 mg C/L in the present study) of the organic matter in the 2 studies could also contribute to the observed discrepancy.
The consistency of KOC values across soils, linearity of the isotherms, and reasonable agreement between sorption and desorption values indicated that absorption (i.e., partitioning) into soil organic matter was the predominate mechanism for interaction of the methylsiloxanes with soil under the conditions of these experiments. Furthermore, the results confirmed that the reduced mass balance observed in some of the experiments did not affect the determination of the equilibrium soil–water distributions of the pertinent sorbates.
Analysis of KOC and comparison with KOW
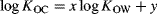 (3)
(3)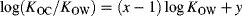 (4)
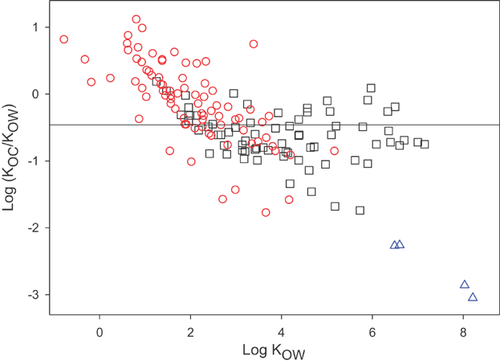
(4)A value of 1 for x is sometimes assumed 28, implying that the ratio KOC/KOW is constant for the compounds to which Equation 4 is applied. Representative values for the ratio are 0.41 29 and 0.35 28; that is, y = −0.39 or −0.46, respectively. This ratio is approximately 2 to 3 orders of magnitude lower for the methylsiloxanes, as shown in Figure 3, which includes data for 155 polar and hydrophobic organic compounds from Nguyen et al. 14 and Poole and Poole 30. Why do the VMS, which are often assumed to belong to the generic hydrophobic class, exhibit such different behavior? In other words, what are the fundamental differences between chemicals, including VMS, and partition media, which explain their KOC/KOW ratios?
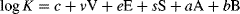 (5)
(5)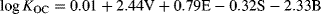 (6)
(6)Equation 6 was only slightly different from that obtained without the siloxanes, and the statistics (SE = 0.27, R2 = 0.967, F = 1139) were comparable to those reported for modeling of the individual datasets. The model coefficients showed a blend of contributions from V, E, and S that reflected the fact that 2/3 of the 75 compounds in the Nguyen et al. dataset were common to the data set used by Poole and Poole, as well as inclusion of the methylsiloxanes, which have unique combinations of these descriptors (Supplemental Data, Figure S8). The deviation between calculated (Equation 6) and experimental KOC values was 0.03 log units for both D4 and D5, whereas calculated values for L3 and L4 were both too low by 0.40 log units to 0.44 log units. Very recently, Endo and Goss 39 reported reduced KOC fitting errors for D4, D5, and (dodecamethylcyclohexasiloxane (D6; estimated log KOC = 6.03), based on adjustment of their B descriptor values, as well as optimization of the KOC LFER model coefficients, using a different training set 15. However, in the present study's results, the log KOC values of the 4 siloxanes were well represented by the original descriptors and nonoptimized LFERs.
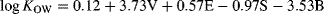 (7)
(7)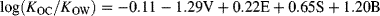 (8)
(8)The coefficients of Equation 8 reflect the differences in properties of (wet) soil/sediment organic matter and octanol. The negative v coefficient indicates that wet organic matter is a more cohesive solvent relative to wet octanol, whereas the positive e and s values indicate that wet organic matter engages solutes more effectively through interactions involving permanent or induced dipoles. Wet organic matter is also a better hydrogen bond donor than wet octanol (positive b), but they have equivalent hydrogen bond acceptor capacity (a ≈ 0).
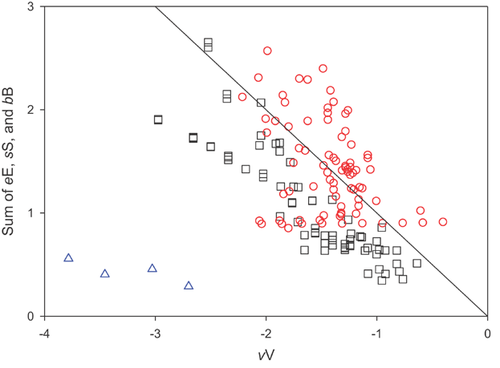
The value of log (KOC/KOW) can be constant for a series of compounds only when variation in the vV product term across compounds is offset by compensatory variation in their eE, sS, and bB product terms. What are the differences in the properties of the hydrophobic organic and VMS compounds that can explain the differences in behavior depicted in Figure 3? Their descriptor values show that these classes differ in several aspects (Supplemental Data, Figure S8). First, the VMS are generally larger molecules, giving rise to larger contributions to log (KOC/KOW) from the cavity formation term, thereby reducing KOC relative to KOW. Second, traditional hydrophobics are much more polarizable (E) than VMS, and polarizability often increases with size (V) for the former, while decreasing for VMS. This trend reinforces the effect of increasing molecular size on the reduction in KOC/KOW for VMS, while the opposite trend partially offsets the cavity effect for the traditional hydrophobics. Dipolarity/polarizability (S) exhibits a similar trend with respect to the comparison between VMS and traditional hydrophobics. For VMS, values of S are close to 0 regardless of size, having practically no effect on their KOC/KOW ratios, whereas for traditional hydrophobics, the influence of S is similar to that of E. Finally, VMS are more hydrogen bond basic (larger B) than traditional hydrophobic chemicals but, relative to their size, are less basic than small polar compounds. The contribution of bB to increasing KOC/KOW is slightly greater for VMS compared with traditional hydrophobics; however, the discrepancy is modest compared with the contributions from differences in polarity and polarizability.
Figure 4 shows that the differing relationship between KOC and KOW for VMS compared with polar and hydrophobic organic compounds can be rationalized in terms of combined weaker van der Waals and hydrogen bonding interactions between VMS and organic matter, which fail to compensate for the thermodynamic penalty associated with their large size. In summary, the KOC/KOW behavior of VMS compared with the hydrophobic chemicals is an expression of their unique properties, combined with the differences in solvation characteristics of organic matter relative to octanol. This analysis demonstrates that although the partitioning behaviors of VMS follow the same chemical principles as those of other nonionic compounds, they might not obey simplified 1-parameter relationships developed for other classes of chemicals having generally similar baseline properties (e.g., low aqueous solubility, high KOW).
Implications for estimation of KOC from KOW
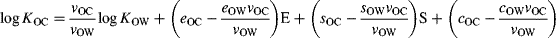 (9)
(9)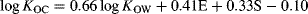 (10)
(10)As expected, A and B were not included in any of the models at the selected significance level, and the coefficients of the remaining parameters were very close to the expected values based on Equation 6 and Equation 7. Not surprisingly, the value of 0.66 for the coefficient of log KOW lies within the range of values observed for the single-parameter correlations with log KOW discussed above 13, and typical values of E and S could yield values of the sum of these terms that are consistent with the range of regression constants from these various correlations. The coefficients of E and S compensate for the incorrect balance of contributions to log KOC from dipole and induction interactions encoded by 0.66 · log KOW. This reinforces that the success of Equation 3 for predicting log KOC of diverse chemicals, including VMS, is limited by the direction and magnitude of variation in hydrogen bond basicity, dipolarity, and polarizability within the training set.
Finally, the predictive ability of Equation 10 was characterized by external validation, using the Kennard Stone algorithm 43 to divide the data into separate training (n = 47) and test (n = 109) sets based on their normalized values of E, S, log KOW, and log KOC. Based on the model derived for the validation training set, having coefficients comparable to Equation 10, the resulting root mean square error in predicted log KOC values for the test set was 0.26, with an average error of 0.02 and an absolute average error of 0.20 (Supplemental Data, Figure S10). No difference in performance was observed between the hydrophobic (n = 55) and polar (n = 52) chemicals, whereas KOC values for the 2 VMS in the test set were both underpredicted by 0.3 log units. Estimated KOC values by Equation 6 and Equation 10 for additional organosilicon compounds having measured KOW values and solute descriptors are given Table 2. Obviously, Equation 10 will be most useful for estimating accurate KOC values of compounds having more extreme character with respect to dipolarity and polarizability, which do not lie within the domain of an established class-specific model.
| Compound | log KOW | log KOW Ref. | E | S | B | V | Predicted log KOC | |
|---|---|---|---|---|---|---|---|---|
| Eqn. 6 | Eqn. 10 | |||||||
| Hexamethyldisiloxane | 5.20 | 27 | −0.270 | −0.187 | 0.299 | 1.5041 | 2.84 | 3.16 |
| Dodecamethylpentasiloxane | 9.41 | 27 | −0.936 | −0.175 | 0.717 | 3.2627 | 5.63 | 5.68 |
| Dodecamethylcyclohexasiloxane | 8.87 | 9 | −0.880 | −0.119 | 0.802 | 3.5172 | 6.08 | 5.37 |
| Tetramethylsilane | 3.24 | 44 | −0.054 | −0.096 | 0.142 | 0.9179 | 1.91 | 1.99 |
| Dimethylsilanediol | −0.41 | 9 | 0.275 | 0.354 | 0.7535 | −0.15 | ||
- a Solute descriptors taken from Poole et al. 36 and Ahmed et al. 37; B value not available for dimethylsilanediol.
- E = the excess molar refraction describing the polarizability of a molecule relative to the hypothetical n-alkane of the same size; S = solute dipolarity; B = solute hydrogen bond basicity; V = McGowan's characteristic volume; KOC = organic carbon partition coefficient; KOW = octanol-water partition coefficient.
SUPPLEMENTAL DATA
Tables S1–S4.
Figures S1–S10. (492 KB PDF).
Acknowledgment
The research conducted in the present study was funded by CES–Silicones Europe and the Silicones Environmental, Health, and Safety Center.




