Impact of length of stay on diagnostic yield in the epilepsy monitoring unit: A multi-center retrospective 12-year Veterans Health Administration study
Abstract
Objective
Epilepsy Monitoring Units (EMUs) in Veterans Health Administration (VHA) Epilepsy Centers of Excellence (ECoE) are critical for the diagnosis and management of seizure disorders. Whether a shorter length of stay (LOS) in the EMU due to scheduling impacts diagnostic yield is unclear.
Methods
Data from 7074 EMU visits across 15 VHA EMUs (2012–2024) were analyzed. Based on usual admission schedules, EMUs were divided into “fixed” (typically Monday–Friday) or “flexible” subgroups. Diagnostic outcomes were classified as epileptic seizures (ES), psychogenic non-epileptic seizures (PNES), other non-epileptic events, and inconclusive. Diagnostic rates were compared between fixed and flexible sites using cumulative distribution functions and other statistical tests. Readmission data for initially inconclusive cases were also examined.
Results
Diagnostic outcomes showed the following distribution: 23% ES, 19% PNES, 11% other non-epileptic events, and 47% inconclusive. Similar distributions were seen between fixed and flexible sites, although a higher proportion of diagnostic admissions were completed earlier in fixed sites and over a longer average LOS at flexible sites. Admissions diagnostic of ES had longer LOS than all other outcomes (4.5 vs. 3.8 days, p < 0.001). Repeat EMU admissions were performed in 10% of patients and were more likely to be diagnostic of ES than PNES or other non-epileptic events.
Significance
About half of EMU admissions within VHA were non-diagnostic with respect to the patients' typical clinical events. ES and PNES were observed at approximately similar rates, although the diagnosis of ES required a longer LOS. Fixed sites did not appear inferior to flexible sites for reaching diagnostic conclusions in our analysis. The higher proportion of earlier diagnoses at fixed sites observed was likely a statistical effect of their predefined shorter admission lengths. Further investigations of EMU resource utilization based on individual goals of monitoring are necessary to better examine and improve efficiency.
Plain Language Summary
Epilepsy Monitoring Units (EMUs) are specialized hospital units used to diagnose and characterize seizures. This study looked at over 7000 admissions across 15 Veterans Health Administration EMUs to see whether length of stay affected diagnosis rates based on admission scheduling and seizure types. Regardless of whether patients were admitted on a fixed schedule (Monday–Friday) or a flexible schedule, about half of hospitalizations did not capture typical events. Diagnosis of epileptic seizures and psychogenic non-epileptic seizures occurred at similar rates, though diagnosing epileptic seizures took longer. Findings suggest fixed (shorter) hospital stays may be as effective as longer flexible hospitalizations.
Key points
- Fixed-length versus flexible duration EMU evaluations were compared across 7074 admissions spanning 12 years at 15 sites.
- Fixed admission schedules showed similar diagnostic yields to flexible schedules, demonstrating non-inferiority.
- Epileptic seizures required longer monitoring compared to all other diagnostic classifications.
- Among readmitted patients with prior inconclusive results, 68% remained inconclusive, while 18% were diagnosed with epileptic seizures.
1 INTRODUCTION
Epilepsy Monitoring Units (EMUs) are specialized inpatient units designed to provide continuous video EEG monitoring, enabling accurate characterization of seizure-like episodes and for presurgical evaluation of drug-resistant epilepsy. By characterizing seizures into epileptic seizures (ES), psychogenic non-epileptic seizures (PNES), mixed ES and PNES events, and other episodic non-epileptic events, EMUs facilitate the management of complex paroxysmal events. Presurgical evaluation consists of capturing and analyzing EEG during typical episodes, leading to seizure classification and treatment strategies, including anti-seizure medication (ASM) management, resective or ablative surgery, and implantation of devices for neuromodulation therapy.
The duration of epilepsy monitoring can vary with the type of epilepsy and the purpose of monitoring. It has been shown that presurgical evaluation and characterization of symptomatic generalized epilepsy are associated with a longer EMU length of stay (LOS).1 However, the optimal duration of monitoring within the EMU has not been clearly defined. Prolonged LOS within the EMU has been linked to the need to capture typical events to reach a conclusive diagnosis.2 Presumed PNES is significantly more likely to have an inconclusive diagnostic outcome compared to other presumed diagnoses.3
Within the Veterans Health Administration (VHA), the Epilepsy Centers of Excellence (ECoE) have established EMUs nationally to improve care access and outcomes for Veterans with epilepsy.4 Despite their importance to the clinical mission of the ECoE, the operational efficiency and diagnostic yield of EMUs within the VHA remain underexplored. Two single-center studies have highlighted the diagnostic challenges of EMU evaluations within the VHA, with 42%–52% of the studies resulting in inconclusive outcomes.5, 6 It has been suggested that the sporadic nature of events and limited duration of monitoring may have led to a higher number of inconclusive outcomes.5 EEG technologist availability is a challenge within the VHA, which can lead to limited coverage over the weekends7 and a relatively “fixed” EMU schedule of (Monday–Friday) monitoring. The National Association of Epilepsy Centers (NAEC) guidelines recommend that all epilepsy centers should have 24/7, continuous, real-time video EEG monitoring, which allows for a more “flexible” EMU schedule where the LOS can be tailored according to clinical needs.8 However, there is a lack of comprehensive, long-term, multisite data comparing the performance of EMUs with limited monitoring capability versus those capable of more prolonged monitoring.
This longitudinal retrospective study aimed to address this gap by analyzing EMU data collected over 12 years from 15 VHA EMUs. The primary objective was to evaluate the diagnostic yield of EMUs and the factors leading to a conclusive diagnosis. Specifically, this study investigated the impact of different scheduling strategies—fixed versus flexible admissions—on diagnostic efficiency and length of stay (LOS).
2 METHODS
2.1 Study design and data sources
This retrospective analysis, approved by the VHA Neurology Clinical Programs as an operational project, with the goal of optimizing EMU care, utilized data from an EMU database maintained by the ECoE and supplemented by data obtained from the VHA Corporate Data Warehouse (CDW), a comprehensive database that includes patient encounters, clinical information, and diagnostic codes within the VHA. EMU admissions from 2012 to 2024 at 15 VHA-affiliated EMU sites were included (Supplementary Material 1). The primary data source was a legacy EMU database that included data submitted annually by individual ECoE site directors. As a secondary data source, EMU admissions were identified in CDW based on CPT billing codes (95720, 95951, 95956, 95953, 95700, 95726, 95722, 95718, 95724) and filtered for EMU based on criteria suggested by different sites such as admitting service (e.g., Neurology) and ward used for EMU admissions to distinguish them from continuous EEGs that use the same CPT code. Comparisons were made between these two data sources and discrepancies were resolved by manual chart review by multiple collaborators (KH, AS, ST, SK, MF). Incomplete or missing data (diagnostic classification) were also completed by these collaborators.
2.2 Patient population and classification
Patients were included if they underwent EMU monitoring during the study period. Diagnostic outcomes were classified by the patient's habitual episode, according to an established ECoE schema as follows: (1) epileptic seizures (ES) including focal and generalized seizures, (2) psychogenic non-epileptic seizures (PNES), (3) other events: these were non-epileptic events such as syncope, migraine, sleep disturbances, tremor, or other seizure mimics that were not PNES, and (4) inconclusive: when no definitive diagnosis was established after EMU. Details of the diagnostic classification used in the EMU database are given in Supplementary Material 2. Prior to analysis and LOS classification, outliers (LOS >3 standard deviations of the overall mean) were removed.
2.3 Classifying lengths of stay: Clustering analysis and site director surveys
Several VHA EMUs admit patients for evaluation only for part of the week (typically Monday–Friday), while others have no particular predefined admission length constraints. To distinguish between these “fixed” and “flexible” sites, two approaches were used:
(1) Clustering analysis: Each patient's LOS was extracted based on admission and discharge dates in CDW. LOS was calculated by subtracting the admission date from the discharge date—a same day discharge would have an LOS of 0 days, while an admission from Monday to Friday would be 4 days. Four clustering algorithms were employed (K-means, Hierarchical, DBSCAN, Gaussian Mixture Models) with an aim to distinguish relatively fixed (4 days) versus flexible admission schedules based on features extracted from the distribution of patient LOS at each site as follows: (1) Short stays: which was the proportion of LOS ≤4 days, representing the Monday to Friday schedule; (2) Long stays: which captured the proportion of longer stays, LOS ≥5 days; (3) LOS ratio: which was the ratio of long stays versus short stays; (4) LOS 4 dominance: defined as the proportion of stays with LOS = 4 divided by the proportion of LOS = 3 days and LOS = 5 days combined to identify sites with a very high proportion of 4 days LOS, suggesting a fixed schedule, and (5) the continuity score: which measures the distribution's continuity, was calculated as the inverse of the number of gaps, defined as any LOS values >4 days where the count of patients was zero. A higher continuity score was presumed to indicate a more flexible schedule.
After feature definition and calculation, features were standardized using z-score normalization to ensure comparability across features. K-means clustering with k = 2 was applied to separate into two groups. The cluster with higher flexibility ratios and continuity scores was labeled as flexible, while the cluster with high LOS 4 dominance was labeled as fixed. Hierarchical clustering was performed using agglomerative clustering with Ward's linkage method. Density-Based Spatial Clustering of Applications with Noise (DBSCAN) was employed, with the epsilon and minimum points parameters tuned to identify core samples and account for potential noise points in the data. Finally, Gaussian Mixture Models (GMM) were used to fit a mixture of Gaussian distributions, with the optimal number of components determined via the Bayesian Information Criterion (BIC). The silhouette score was calculated to assess the quality of the clustering, measuring the cohesion within clusters and separation between clusters.9 To further validate the clustering robustness and stability, sensitivity analysis and bootstrapping were conducted for the K-means clustering. The sensitivity analysis systematically removed individual features to assess their impact on clustering results. For each feature subset, the silhouette score, the number of sites that changed classification, and the percentage of sites affected by feature exclusion were recorded. Resampling with replacement across 100 samples was performed for bootstrapping. A stability score was calculated for each site, representing the percentage of bootstrap samples in which the site retained its original cluster assignment.
(2) Survey of EMU Directors: The above division was further verified by surveying EMU site directors about their admission procedures. The medical directors at each included EMU were surveyed using a Microsoft form via email. Questions included the typical admission and discharge days of the week, the average LOS, a self-reported perception of their site's scheduling pattern being fixed or flexible (defined in the survey), and any other comments about scheduling patterns (Supplementary Material 1).
2.4 Statistical analysis
Cumulative distribution functions (CDFs) were used to quantify the cumulative proportion of outcomes at each LOS day in the whole cohort, as well as within the fixed and flexible groups. Days 4 and 7 were analyzed specifically to compare a Monday to Friday admission (LOS 4 days) with a full week admission (LOS 7 days). Kaplan–Meier estimates, with censoring of inconclusive evaluations at their calculated LOS, were performed to further compare the fixed and flexible groups. Nonparametric tests including Kolmogorov–Smirnov (KS) and Mann–Whitney U (MWU) tests were performed to compare the LOS between fixed and flexible LOS groups. Bonferroni correction was performed for all tests requiring multiple comparisons. Readmission rates, outcomes of inconclusive initial admissions, and outcomes and average LOS of the 2nd admission following an inconclusive diagnosis were also analyzed. All statistical analyses were conducted using Python 3.12.4 and libraries including Matplotlib 3.10, NumPy 2.2.2, Scikit-learn 1.6.1, and Statsmodels 0.14.4 in Jupyter Notebook 7.3. Significance was set at alpha of 0.05.
3 RESULTS
3.1 EMU admission characteristics
Following LOS outlier removal, 7074 EMU admissions remained with a mean LOS of 3.9 ± 2.4 days (range 0–26 days). The median EMU admissions per year was 538 studies over the 12-year period. Among these, 23% captured ES, 19% captured PNES, 11% captured other non-epileptic events, and 47% were inconclusive. Table 1 shows the pairwise comparisons of mean LOS by outcome class—the LOS for ES (4.5 days) was longer than PNES (3.5 days, p < 0.001), other non-epileptic events (3.4 days, p < 0.001), and inconclusive admissions (3.9 days, p < 0.001).
| Group | Diagnostic outcome | ES | PNES | Other events | Inconclusive |
|---|---|---|---|---|---|
| Mean days (p value) | Mean days (p value) | Mean days (p value) | Mean days (p value) | ||
| All sites | ES | ES 4.5 | |||
| PNES | 3.5 vs. 4.5*** (<0.001) | PNES 3.5 | |||
| Other events | 3.4 vs. 4.5*** (<0.001) | 3.4 vs. 3.5 (1.0) | Other events 3.4 | ||
| Inconclusive | 3.9 vs. 4.5*** (<0.001) | 3.9 vs. 3.5*** (<0.001) | 3.9 vs. 3.4*** (<0.001) | Inconclusive 3.9 | |
| Fixed | ES | ES 4.3 | |||
| PNES | 3.5 vs. 4.3*** (<0.001) | PNES 3.5 | |||
| Other events | 3.3 vs. 4.3*** (<0.001) | 3.3 vs. 3.5** (0.008) | Other events 3.3 | ||
| Inconclusive | 3.7 vs. 4.3*** (<0.001) | 3.7 vs. 3.5 (1.0) | 3.7 vs. 3.3** (0.002) | Inconclusive 3.7 | |
| Flexible | ES | ES 4.9 | |||
| PNES | 3.5 vs. 4.9*** (<0.001) | PNES 3.5 | |||
| Other events | 3.7 vs. 4.9*** (<0.001) | 3.7 vs. 3.5 (1.0) | Other events 3.7 | ||
| Inconclusive | 4.3 vs. 4.9*** (<0.001) | 4.3 vs. 3.6*** (<0.001) | 4.3 vs. 3.7*** (<0.001) | Inconclusive 4.3 |
- Abbreviations: ES, epileptic seizures; LOS, length of stay; PNES, psychogenic non-epileptic seizures.
- * p < 0.05.
- ** p < 0.01.
- *** p < 0.001.
3.2 Determination of fixed and flexible groups
Clustering analysis: All four clustering algorithms successfully identified two distinct groups of EMU sites. K-means, Hierarchical, and GMM produced identical site classifications, while DBSCAN generated an inverse assignment pattern. Despite this difference in labeling, all methods achieved identical silhouette scores of 0.711.
Sensitivity analysis: Robustness of feature selection in the K-means clustering was demonstrated through sensitivity analysis. A total of 26 possible combinations were tested, with a minimum of two features in the analysis. The silhouette scores remained relatively stable, ranging from 0.43 to 0.71. Higher silhouette scores (≥0.70) included short stays, long stays, and LOS ratio as features and did not include LOS 4 dominance or continuity score. Correlation between the five features was also analyzed and demonstrated short stays, long stays, and LOS ratio to be highly correlated. Thus, the cluster separation using short stays, long stays, and LOS ratio was used to perform the site separation.
The bootstrap validation demonstrated the stability of the separation with an average stability score of 95.51%. All sites maintained a stability score greater than 80%. Five sites with relatively lower stability scores included: Baltimore (83.5%), Boston (84.5%), Durham (88.9%), Gainesville (91.4%), and Houston (91.2%). As a result of the clustering analysis and subsequent validation steps, 15 sites were separated into 10 “fixed” sites and 5 “flexible” sites. A detailed description of the clustering analysis is reported in Supplementary Material 3. The feature values and LOS distributions at different sites are reported in Supplementary Material 1.
Director survey: Self-reported fixed vs. flexible classification from the surveyed site directors confirmed the k-means classification of fixed and flexible sites (Supplementary Material 1). Most sites reported that their admission days are on Monday, except for Boston, which scheduled admissions on Sunday. Self-reported average LOS varied significantly among flexible sites, ranging from 3 days to more than 7 days.
3.3 LOS comparison of fixed vs. flexible sites
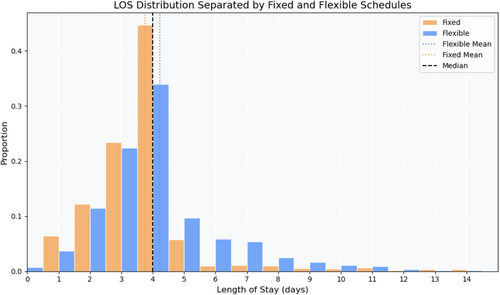
A histogram showing fixed and flexible site LOS distributions is shown in Figure 1. A sharp drop-off is seen in the fixed sites after Day 4 while there is a longer taper for the flexible group. A longer mean LOS in the flexible group was seen compared to the fixed group (4.2 vs. 3.8 days, p < 0.0001, Figure 1); the median LOS was the same between both groups (4 days).
EMU diagnostic yields between fixed versus flexible sites were compared and had broadly similar distributions for ES (23.7% for fixed vs. 22.1% for flexible), PNES (18.9% vs. 19.1%), other events (12.3% vs. 9.2%), and inconclusive outcomes (45.1% vs. 49.5%).
3.4 LOS distributions for fixed vs. flexible sites
In fixed sites, the LOS was significantly higher for ES (4.3 days) compared to PNES (3.5 days), other events (3.3 days), and inconclusive outcomes (3.7 days). Similarly, flexible sites had a higher LOS for ES (4.9 days) compared to PNES (3.5 days), other events (3.7 days), and inconclusive outcomes (4.3 days). Between fixed and flexible sites, flexible sites had a significantly longer LOS for ES (4.9 vs. 4.3 days, p < 0.001) and inconclusive outcomes (4.3 vs. 3.7 days, p < 0.001), but no differences for PNES or other events. These analyses are shown in Table 1 along with the all-site distributions.
3.5 Diagnostic yield by LOS day
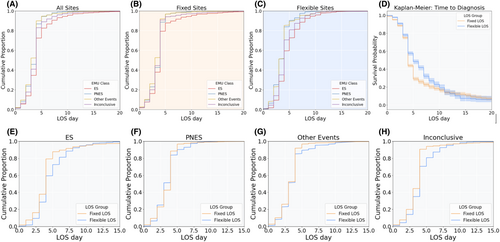
All sites: The relationship between EMU diagnostic outcomes for each LOS day is shown as CDFs in Figure 2 and statistically compared in Table 2. Figure 2D shows the results of Kaplan–Meier analysis, which revealed similar results. By 4 days, 72% of ES were diagnosed, which was significantly lower than PNES (87%, p < 0.001), other non-epileptic events (90%, p < 0.001), and inconclusive outcomes (83%, p < 0.001). Similarly, by 7 days, 91% of ES were diagnosed, which was lower compared to PNES (98%, p < 0.001), other non-epileptic events (97%, p < 0.001), and inconclusive evaluations (94%, p < 0.001).
| Group | Diagnostic outcome | ES | PNES | Other events | Inconclusive |
|---|---|---|---|---|---|
| % diagnosed (p value) | % diagnosed (p value) | % diagnosed (p value) | % diagnosed (p value) | ||
| LOS 4 days | |||||
| All sites | ES | ES 72% | |||
| PNES | 87% vs. 72%*** (<0.001) | PNES 87% | |||
| Other events | 90% vs. 72%*** (<0.001) | 90% vs. 87% (0.20) | Other events 90% | ||
| Inconclusive | 83% vs. 72%*** (<0.001) | 83% vs. 87%** (0.007) | 83% vs. 90%*** (<0.001) | Inconclusive 83% | |
| Fixed | ES | ES 79% | |||
| PNES | 89% vs. 79%*** (<0.001) | PNES 89% | |||
| Other events | 92% vs. 79%*** (<0.001) | 92% vs. 89%* (0.04) | Other events 92% | ||
| Inconclusive | 91% vs. 79%*** (<0.001) | 91% vs. 89% (0.07) | 91% vs. 92% (0.39) | Inconclusive 91% | |
| Flexible | ES | ES 59% | |||
| PNES | 84% vs. 59%*** (<0.001) | PNES 84% | |||
| Other Events | 85% vs. 59%*** (<0.001) | 85% vs. 84% (0.62) | Other events 85% | ||
| Inconclusive | 71% vs. 59%*** (<0.001) | 71% vs. 84%*** (<0.001) | 71% vs. 85%*** (<0.001) | Inconclusive 71% | |
| LOS 7 days | |||||
| All sites | ES | ES 91% | |||
| PNES | 98% vs. 91%*** (<0.001) | PNES 98% | |||
| Other events | 97% vs. 91%*** (<0.001) | 97% vs. 98% (1.00) | Other events 97% | ||
| Inconclusive | 94% vs. 91%*** (<0.001) | 94% vs. 98%*** (<0.001) | 94% vs. 97%** (0.003) | Inconclusive 94% | |
| Fixed | ES | ES 92% | |||
| PNES | 98% vs. 92%*** (<0.001) | PNES 98% | |||
| Other events | 98% vs. 92%*** (<0.001) | 98% vs. 98% (0.82) | Other Events 98% | ||
| Inconclusive | 95% vs. 92%*** (<0.001) | 95% vs. 98%*** (<0.001) | 95% vs. 98%** (0.003) | Inconclusive 95% | |
| Flexible | ES | ES 88% | |||
| PNES | 97% vs. 88%*** (<0.001) | PNES 97% | |||
| Other events | 95% vs. 88%** (0.002) | 95% vs. 97% (0.15) | Other events 95% | ||
| Inconclusive | 92% vs. 88%** (0.004) | 92% vs. 97%*** (<0.001) | 92% vs. 95% (0.12) | Inconclusive 92% | |
- Abbreviations: ES, epileptic seizures; LOS, length of stay; PNES, psychogenic non-epileptic seizures.
- * p < 0.05.
- ** p < 0.01.
- *** p < 0.001.
Fixed versus flexible sites: Diagnostic outcomes between fixed and flexible sites at 4 and 7 days are compared in Table 3 and visualized in Figure 2D–G. At 4 days, diagnoses were completed at greater rates in the fixed sites compared to flexible sites across all diagnostic classifications, including ES (79% vs. 59%, p < 0.0001), PNES (89% vs. 84%, p = 0.01), other non-epileptic events (92% vs. 85%, p = 0.004), and inconclusive outcomes (91% vs. 71%, p < 0.0001). The differences persisted at 7 days; however, the absolute difference between the fixed and flexible groups completed admissions was converging: ES (92% vs. 88%, p = 0.046), PNES (98% vs. 97%, p = 0.29), other non-epileptic events (98% vs. 95%, p = 0.041), and inconclusive outcomes (95% vs. 92%, p < 0.001). Log-rank tests assessing differences in fixed and flexible sites confirmed a difference between the two admissions schedules (p < 0.0001).
| EMU classification | Fixed | Flexible | Chi-squared test | p value | ||
|---|---|---|---|---|---|---|
| Median LOS (days) | % diagnosed | Median LOS (days) | % diagnosed | |||
| LOS 4 days | ||||||
| ES | 4 | 79 | 4 | 59 | 72.4 | <0.001*** |
| PNES | 4 | 89 | 4 | 84 | 5.8 | 0.016** |
| Other events | 4 | 92 | 4 | 85 | 7.5 | 0.006** |
| Inconclusive | 4 | 91 | 4 | 71 | 222.8 | <0.001*** |
| LOS 7 days | ||||||
| ES | 7 | 92 | 7 | 88 | 3.9 | 0.046* |
| PNES | 7 | 98 | 7 | 97 | 1.1 | 0.29 |
| Other events | 7 | 98 | 7 | 95 | 4.2 | 0.041* |
| Inconclusive | 7 | 95 | 7 | 92 | 12.0 | <0.001*** |
| End of study | ||||||
| ES | 26 | 100 | 22 | 100 | - | - |
| PNES | 24 | 100 | 11 | 100 | - | - |
| Other events | 25 | 100 | 17 | 100 | - | - |
| Inconclusive | 25 | 100 | 22 | 100 | - | - |
- * p < 0.05.
- ** p < 0.01.
- *** p < 0.001.
Comparison of diagnostic outcomes: Within each group, there were significant differences in LOS between ES and other diagnostic categories (p < 0.01) in both fixed and flexible groups (Table 2).
3.6 Diagnostic yield of subsequent EMU admissions
There were 697 patients (9.8% of total cohort) who had multiple EMU visits, of whom 311 (45%) had an inconclusive first admission. On subsequent readmission, 68% of patients with an initial inconclusive admission had repeat inconclusive evaluations, while 18% were diagnosed with ES, 8% with PNES, and 6% with other non-epileptic events. Indications for readmissions were not available in this administrative-based dataset.
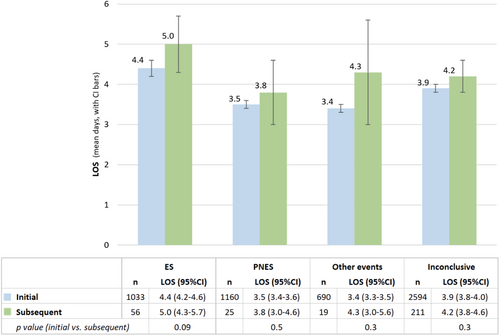
The mean LOS for the first inconclusive visit was 3.9 days. On the subsequent visit, the mean LOS was 4.2 days for a repeat inconclusive diagnosis, 5.0 days for ES, 3.8 days for PNES, and 4.3 days for other non-epileptic events (Figure 3). Repeat readmission durations were not significantly different from initial admission LOS. A minimal trend toward longer subsequent admission was seen for those capturing ES (mean 5.0 days, 95% CI 4.3–5.7 days) compared to the initial admission (4.4 days, 95% CI 4.2–4.6 days, p = 0.0937), but not PNES or other non-epileptic events. Among the 697 patients who had multiple EMU visits, there were 546 (78.3%) with one readmission, 114 (16.4%) with two, 27 (3.9%) with three, and 10 patients (6.9%) with four or more readmissions (Supplementary Material 4).
4 DISCUSSION
This longitudinal multisite analysis of EMU diagnostic outcomes in the VHA revealed clinically relevant and real-life variability in admission patterns and diagnostic yield based on site-specific and patient-specific factors. LOS in the EMU was found to be a function of both underlying diagnosis and admission schedules. While EMU admission volumes varied substantially between sites, the distribution of diagnostic outcomes was relatively consistent, with approximately half of evaluations being non-diagnostic and ES or PNES each captured in about a fifth of cases. Repeat EMU admissions were performed in 10% of patients and were more likely to be diagnostic of ES than PNES or other non-epileptic events.
There was a consistent correlation between the diagnostic classification and LOS. Evaluations capturing ES were associated with longer hospitalizations as in prior reports,2 regardless of site-specific admission procedures (fixed vs. flexible durations). Among diagnostic admissions, CDF curves estimated that 4 days of continuous video EEG recording were sufficient to capture ES in 72% of admissions, compared to 87% for PNES and 90% for other non-epileptic events. However, these rates overestimate efficiency due to the large number of non-diagnostic evaluations. Repeat EMU evaluations after a first inconclusive admission were diagnostic in about a third of cases, predominantly representing patients with ES, and required longer durations of monitoring. Prior single-site studies evaluating EMU characteristics report average durations between 1.5 and 7.2 days, aligning with LOS in this VHA study, with variability in diagnostic yields based on institutional characteristics and referral indications.2, 10-13 In one of the larger studies of 1000 pediatric EMU admissions, 47% were inconclusive or uneventful, similar to the rate here.13
The comparison between fixed and flexible admission schedules demonstrated a difference in time to diagnosis, as indicated by the Kaplan–Meier analysis, with fixed schedules showing a faster diagnostic rate. However, by Day 12, the rate of diagnosis converged, suggesting the cumulative proportion of diagnosed patients becomes similar. There was a similar median LOS of 4 days in both groups, although average LOS was higher at sites employing flexible schedules, driven by a skewed distribution with a minority of prolonged admissions and a bimodal peak at 7 days. However, this analysis did not account for cases in which the goals of EMU admission were EEG background characterization for interictal and/or subclinical epileptiform abnormalities or ASM adjustment, in which a lack of spell capture with an “inconclusive” diagnostic outcome is expected, and thus, the goals may bias toward a shorter or longer LOS at sites with flexible schedules. Additionally, based on the goals of monitoring, interictal epileptiform activity alone may be sufficient to guide management in certain cases and was observed in 17% of non-diagnostic evaluations. Future investigation into site and patient-specific factors, including differences in patient referral patterns, medication tapering, and patient demographics, should be performed to further delineate differences in fixed vs. flexible admission schedules.
Given the findings of this study and the resources and costs involved in hospital admissions for continuous video EEG monitoring, efforts should be made to facilitate actionable diagnostic outcomes. EMU evaluations diagnostic of PNES commonly change management and are cost effective,12, 14-16 as well as presurgical evaluations to guide epilepsy surgery. Broadly applicable interventions may include pre-admission counseling of patients in regard to the expectations for prolonged admissions, pre-admission tapering of ASMs with long half-lives,17-19 standardizing more rapid inpatient ASM tapering, and/or allocation of resources to support longer monitoring. There has also been an interest in strategies to improve the diagnostic yield of EMUs by pro-ictal scheduling at times of increased seizure likelihood,20 personalized seizure forecasting based on self-reported seizure diaries,21 and the use of automated seizure detection algorithms to enhance diagnostic efficacy.22, 23 Thus, patient-specific strategies may involve scheduling based on the timing of prior seizures and typical seizure frequency, considering patient-specific susceptibility (e.g., women with catamenial epilepsy), and determining risks of longer monitoring with regard to tolerability. Current sites that operate with a fixed schedule can have intermittent long stay weekends. The ability to predict long stays or seizure likelihood is an active area of further investigation. These strategies rely on improved understanding of patient-specific factors and administrative coordination to implement EMU admissions at the opportune times.
4.1 Limitations
This study has limitations. Outcomes were focused only on capture of clinical events without defining the subset of EMU admissions for presurgical evaluation, seizure quantification, or other goals that may bias both outcome and LOS metrics.24 The analysis was also not designed to specify on which day of admission the first diagnostic event occurred, or how many events were captured in total. LOS metrics were based on day of admission and day of discharge, which is likely a modest overestimate of EEG recording hours. The readmission data were limited to administrative data only, without specific patient chart review. Further studies should analyze reasons for readmission and explore patient-specific characteristics to elucidate predictive factors for repeat admissions.
Site-specific variations in clinical resources, including the availability of EEG technologists, EEG readers, and hospital beds, may have impacted scheduling strategies and outcomes. There is potential bias in EMU utilization based on varying individual institutional policies; however, given the similar distribution of diagnostic outcomes across both lower and higher volume sites and similar patient populations between VA sites, this is likely not a significant confounder. This analysis was restricted to EMU sites within VHA, which have different patient populations and constraints compared to hospitals in the private sector. In comparison with the general population, the VHA has more patients with PNES and diagnoses of post-traumatic stress disorder (PTSD). Another uncertainty in generalizing these findings outside the VA includes denials or limitations to hospital admissions by private insurers, which is not relevant within the VA. However, these findings may aid other centers in informing their EMU admission policies. Additional studies comparing healthcare systems are necessary to determine the generalizability of these results.
5 CONCLUSION
Fixed admission schedules appeared to be non-inferior to flexible schedules; however, further investigation of EMU resource utilization based on individual goals of monitoring is necessary to better examine and improve efficiency. This, however, provides a justification for smaller community hospitals that are resource limited to employ a fixed admission schedule to optimize staffing and allocation of resources and expand EMU services to a wider population. Future efforts should focus on developing targeted strategies for patient selection and monitoring duration based on the suspected diagnosis and institutional constraints.
ACKNOWLEDGMENTS
We acknowledge Martin Salinsky and Linda Benson for maintaining a legacy version of the database used in the current analysis.
ETHICS STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
DISCLOSURES
None of the authors has any conflict of interest to disclose. All authors are employees or trainees at the VHA.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from Veterans Health Administration. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the author(s) with the permission of Veterans Health Administration.