Transcranial direct current stimulation treatment reduces, while repetitive transcranial magnetic stimulation treatment increases electroencephalography spike rates with refractory occipital lobe epilepsy: A case study
Abstract
Objective
Non-invasive brain stimulation has been suggested as an alternative/supplementary treatment for focal, refractory epilepsy. However, there are only a few studies and even fewer that directly compared transcranial direct current stimulation (tDCS) to repetitive transcranial magnetic stimulation (rTMS).
Methods
We report the case of a 20-year-old female patient with persistent epileptiform discharges in the left occipital region consistent with focal status epilepticus. The patient received 20 min sessions of tDCS (2 mA) for five consecutive days, with the cathode over the left occipital region and the anode over the contralateral prefrontal lobe. After initial improvement, the patient's condition worsened again, and thus it was decided to treat her with rTMS (1 Hz, 1800 pulses), also for five consecutive days. Before and after each treatment, spike frequency was recorded with electroencephalography (EEG).
Results
There was a significant decrease in spike frequency from pre- to post-tDCS treatment. Depending on the type of data analysis, there was either a near significant (p = 0.058, d = 0.51) or a significant (p < 0.001, d = 1.13) increase from pre- to post-rTMS treatment, and the patient reported a worsening of symptoms.
Significance
The study adds to a growing body of evidence on non-invasive brain stimulation treatments in focal refractory epilepsy. On the one hand, we corroborate its usefulness. On the other hand, we highlight that non-invasive brain stimulation might inadvertently worsen symptoms. Future research needs to determine which method, with which parameters, for which patient is beneficial (and detrimental).
Plain Language Summary
The study details the case of a patient suffering from epilepsy, located in the occipital region of the brain, who did not respond to several antiseizure medications. We treated with both transcranial direct current stimulation and repetitive transcranial magnetic stimulation. The transcranial direct current stimulation treatment resulted in a significant decrease in EEG spikes, yet the patient's condition worsened again after 2 weeks. We therefore used repetitive transcranial magnetic stimulation treatment, which, in fact, resulted in an increase in spike frequency. The results demonstrate both the potential benefits and risks of non-invasive brain stimulation as treatment in drug-resistant epilepsy.
Key points
- Multiple antiseizure medications failed to improve seizures in a patient with refractory focal occipital lobe status epilepticus.
- We administered both tDCS and rTMS targeting the left occipital lobe with the aim to reduce spike frequency and ultimately achieve cessation of seizures.
- tDCS treatment resulted in a significant decrease in EEG spikes, but 2 weeks later the patient's condition worsened again.
- We therefore used rTMS treatment, which resulted in an observable increase in spike frequency that, depending on the type of data analysis used, either approached significance or was statistically significant.
- The results demonstrate both the benefits and risks of non-invasive brain stimulation as a treatment in refractory epilepsy.
1 INTRODUCTION
Occipital lobe epilepsy represents a small subset of focal epilepsies (approx. 5%–10% of all cases1). The most common symptoms consist of visual hallucinations (e.g., seeing flashing lights or patterns), temporary blindness, eye movement disturbances, headaches, and postictal confusion.2 The epileptic condition encompasses a broad range of potential causes that range from metabolic and structural brain abnormalities to infectious agents. In some cases, the etiology of occipital lobe epilepsy remains unknown despite extensive investigation.3 To date, occipital lobe epilepsy remains relatively understudied compared to other epilepsy conditions, and approaches to both safe and effective treatment strategies can be a challenge.4 Even though some patients (63.7%) achieve seizure control with antiseizure medication, others (36.3%) face recurrent seizures that persist despite ongoing medical interventions.5 An alternative treatment option for cases of refractory occipital lobe epilepsy is focal resective surgery, where roughly two-thirds of patients achieve seizure freedom, defined as no disabling seizures for at least 1-year post-operation.4 However, post-surgical complications, particularly visual deficits, may occur.4, 6 Some patients are also not eligible for surgery due to multifocal seizure activity. Exploring alternative and less-invasive treatment options is therefore important.
In recent years, non-invasive neuromodulation techniques, such as transcranial Direct Current Stimulation (tDCS) and repetitive Transcranial Magnetic Stimulation (rTMS) have emerged as safe and tolerable methods for treating epileptic conditions. Despite positive outcomes, the therapeutic potential of these methods in epilepsy remains insufficiently explored.7-10 In a previous case report,11 three consecutive days of once daily tDCS sessions led to a notable reduction in epileptiform discharges and cessation of focal seizures. However, the patient experienced a worsening of symptoms a month later, prompting an extended treatment of another 14 consecutive days, which resulted in the sustained cessation of the focal seizures. Conversely, Marquardt et al.12 reported that a similar tDCS protocol had no significant impact on spike rates or seizure frequency in a patient with refractory, focal epilepsy. Building on these findings, a more recent study13 explored the long-term clinical efficacy of personalized, multichannel tDCS on three patients with drug-resistant epilepsy of diverse etiologies. This study differed significantly in its approach: each patient underwent twice daily tDCS sessions for five consecutive days, repeated across several cycles (11, 9, and 3, respectively), with a 2-month break between cycles. The results varied, with patients requiring different numbers of treatment cycles to achieve notable improvements in seizure frequency and severity. The discrepancies between these three studies can be due to differences in treatment protocols and/or patient characteristics. Specifically, variations in electrode placement, treatment duration, the patients' genotypes, or the severity of the conditions could have affected the outcome.
For rTMS, a case study by Starnes et al.9 reported that low frequency rTMS significantly reduced interictal epileptiform discharges and seizure frequency in a patient with refractory occipital lobe epilepsy, with sustained long-term benefits. By contrast, others showed only mild, temporary effects14 or no significant reduction in seizures15 after using low frequency rTMS in focal refractory patients.
In this study, we present the case of a patient who responded well initially to tDCS treatment, with similar parameters as Ng et al.,11 but deteriorated again 2 weeks after the treatment. We then applied rTMS treatment, allowing us to not only examine whether non-invasive brain stimulation methods in general can be used to reduce EEG spikes in focal, refractory epilepsy, but also to directly compare tDCS and rTMS for the first time.
2 CASE REPORT
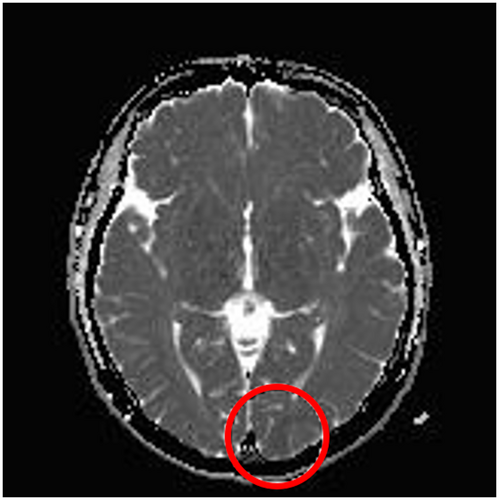
A 20-year-old female patient presented with elementary visual disturbances (described by the patient as flashes or lightning-like features perceived as bright spots), in addition to headaches and confusion, followed by a focal to bilateral tonic–clonic seizure, leading to hospital admission. A neurological examination revealed right hemianopsia and reduced facial sensitivity on the right side. Electroencephalogram (EEG) showed persistent, epileptiform discharges in the left occipital region that were consistent with focal, non-motor status epilepticus (Figure 1). Magnetic resonance imaging (MRI) showed pathological changes in the left occipital lobe (i.e., elevated signal on fluid-attenuated inversion recovery [FLAIR] sequences along with decreased diffusion in the left paramedian occipital lobe; Figure 2). She was admitted for status epilepticus treatment, and her ADHD medication (methylphenidate) was discontinued. Despite treatment with a combination of several antiseizure medications (diazepam, levetiracetam, phenytoin, topiramate, lacosamide, and clobazam), seizures did not improve. The patient underwent genetic, autoimmune, infectious, and metabolic tests that all failed to produce conclusive findings concerning the etiology of the occipital status epilepticus. When the patient had been stable for approximately a week, with no signs of seizure activity and some improvements in visual disturbances, she was discharged with ongoing antiseizure medication treatment.
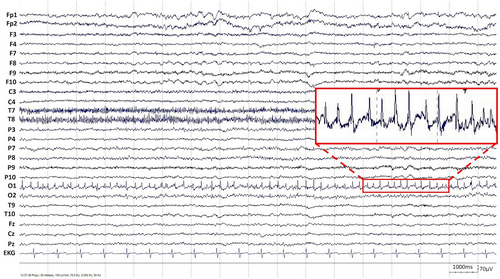
Over the next 4 months, she was readmitted four times with frequent focal seizures, primarily characterized by almost daily elementary visual disturbances (such as flashes and lightning-like features). The patient occasionally experienced unspecific twitches. However, these movements were not rhythmic and did not have consistent EEG correlates. Both EEG and MRI remained unchanged. She experienced several adverse effects from treatment. Particularly, there was a notable decline in her emotional and cognitive state that affected overall well-being. To provide further care for the patient, tDCS and rTMS were introduced as a form of supplementary, experimental treatment. With both tDCS and rTMS, we targeted the left occipital lobe with the aim to reduce spike frequency and ultimately achieve cessation of seizures. The patient was on constant doses of antiseizure medications (lamotrigine, lacosamide, clobazam, and perampanel) during both tDCS and rTMS treatments. After the rTMS treatment, the patient disclosed that she had been taking doses of ADHD medication that exceeded the amounts originally prescribed by her physician during both brain stimulation treatments, despite the physician's advice to discontinue. Moreover, another MRI examination found changes interpreted as focal cortical dysplasia in the left occipital lobe.
3 MATERIALS AND METHODS
The brain stimulation experiments were conducted at the Haukeland University Hospital, Bergen, Norway, and EK was the patient's main treating physician.
3.1 tDCS
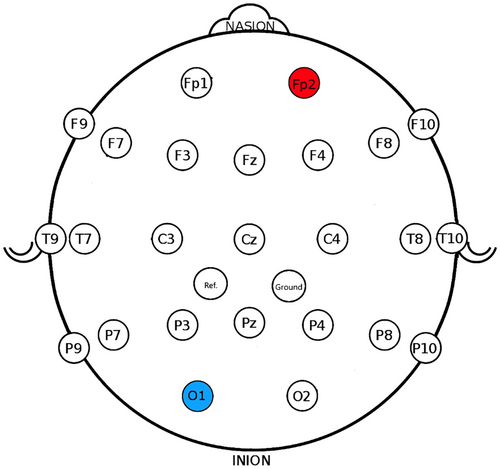
tDCS was administered using a battery-operated constant current stimulator (NeuroConn DC-stimulator) through a pair of rubber electrodes (7 × 5 = 35 cm2) in saline-soaked sponges. A hypoallergenic gel (SignaGel) was applied to the sponges to reduce discomfort. The international 10–20 system was used for electrode placement16: The cathode was positioned over the O1 region, corresponding to the left occipital lobe (Figure 3) to reduce spike frequency. This was based on findings demonstrating reduced excitability in the area underneath the cathode.17, 18 The anode was placed over the Fp2 region, corresponding to the right prefrontal lobe (Figure 3). In tDCS treatment for epilepsy, the anode is typically positioned contralaterally to the cathode to modulate neural activity across the brain, enhancing treatment efficacy.19 We chose the Fp2 region because the prefrontal lobe often serves as a control site in tDCS studies.19, 20 Additionally, this region was a non-epileptogenic area in our patient. After positioning both electrodes, an adjustable rubber strap was used to keep them securely in place.
tDCS was then administered once daily for 20 min (plus 30 s ramp-up and 30 s ramp-down) with a constant current of 2 mA for five consecutive days. The tDCS setup was in accordance with safety guidelines19, 21 and the patient completed all five tDCS sessions.
As described below, the patient's symptoms improved temporarily after the tDCS treatment. When they returned, it was decided to try rTMS instead. There was a 2-week break between the end of the tDCS and the beginning of the rTMS treatment.
3.2 rTMS
rTMS was administered with a neuronavigated stimulator (Nexstim NBS 5), targeting the left occipital region once a day for five consecutive days. MRI-based stereotaxis was used to achieve precise targeting of O1 and accurate stimulus delivery for the duration of the treatment. The rTMS protocol was chosen based on a previous successful treatment attempt in another case study.9 Each day, the patient received 1800 pulses at 1 Hz stimulation administered for 30 min. Stimulation intensity was set to 37% maximum stimulator output (MSO), which is 90% of the resting motor threshold (rMT22) measured on day 1 (41% MSO). The rMT was reassessed on day 2 (39% MSO), but the 37% intensity was kept for all sessions. The estimated electric field varied between 64 V/m and 93 V/m over the five treatment sessions. The rTMS sessions were administered according to safety guidelines22 and the patient completed all five rTMS sessions.
3.3 EEG
EEG recordings (25-channels with video monitoring following the 10/20 system, Figure 3) were conducted before and after each treatment phase. That is, before the initial tDCS session on day one and after the final tDCS session on day five, as well as before the initial rTMS session on day one and after the final rTMS session on day five. The EEG recordings were conducted at roughly the same time of day (between 3 PM and 5 PM), and the patient was awake during all recordings. EEG images are provided in the Appendix S1.
3.4 Data analysis
To assess the impact of tDCS and rTMS on EEG spike rates, five raters in total (three neurophysiologists, SE, KN, and EA and two tDCS/rTMS clinicians, LM and TT) assessed the frequency of spikes per second in the EEG recordings before and after treatments. To ensure the robustness of our results, we used two approaches. In both approaches, each 20-min EEG recording was segmented into 5-min intervals (i.e., 1–5 min, 6–10 min, 11–15 min, and 16–20 min). In the “random periods” approach, four raters (SE, KN, LM, and TT) independently selected ten 1-s, artifact-free periods to count spikes from each of the four 5-min intervals randomly. These ten spike counts were then averaged for each 5-min interval, resulting in average spike counts for each rater separately for these four conditions: pre-tDCS, post-tDCS, pre-rTMS, and post-rTMS. These averages constituted the four dependent variables. In the “same periods” approach, four raters (SE, EA, LM, and TT) counted spikes in the same artifact-free, 1-s periods within each 5-min segment. These periods were pre-determined. Specifically, for each 5-min segment, the 6th and 15th second windows were selected for spike counting. As in the primary approach, the spike counts were averaged for each 5-min interval for each rater across the same four conditions. Rater KN had to be replaced by EA for practical reasons.
In both the “random” and “same” approach, mean spike rates were subjected to a 2 × 2 repeated measures ANOVA with the within-subject variables Stimulation Type (tDCS/rTMS) and Time (Pre/Post-Treatment). Estimated marginal means and standard errors are reported for main effects. Significant interactions were followed up with post hoc, paired sample t-tests with Bonferroni correction. For post hoc tests, original means, standard errors, and t-values are reported, as well as Cohen's d effect sizes: small (d = 0.2), medium (d = 0.5), and large (d ≥ 0.823).
All statistical analyses were carried out using IBM SPSS Statistics version 29. The alpha level was set to p < 0.05; the assumption of sphericity (ε) was assessed by Mauchly's test of sphericity. Effect sizes are reported as partial eta squared values following the conventional threshold outlined by Maxwell et al.24: η2 = 0.01 (small effect); η2 = 0.06 (medium effect); η2 = 0.14 (large effect).
4 RESULTS
The “random periods” analysis revealed a significant main effect of Time, F(1, 15) = 39.53, p = 0.001, η2 = 0.73, with a decrease in spike rates from pre-treatment (M = 2.70, SE = 0.12) to post-treatment (M = 2.16, SE = 0.09). This main effect, however, was subject to a significant interaction between Time and Stimulation Type, F(1, 15) = 27.45, p < 0.001, η2 = 0.65. As can be seen in Figure 4A, there was a decrease in spike rates from pre- (M = 3.06, SE = 0.11) to post-tDCS treatment (M = 1.49, SE = 0.15). In contrast, there was an increase in spike rates from pre- (M = 2.33, SE = 0.23) to post-rTMS treatment (M = 2.83, SE = 0.16).
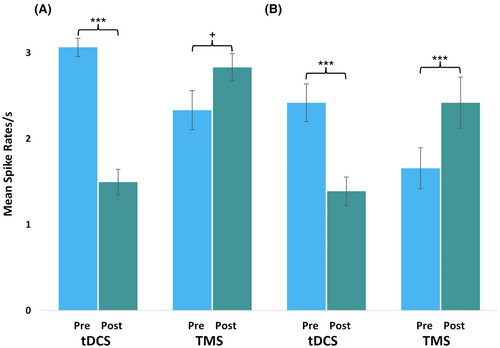
Post hoc tests revealed that the decrease in spike rates following tDCS treatment was significant (t(15) = 8.66, p < 0.001, d = 2.17), while the increase following rTMS treatment approached significance (t(15) = −2.05, p = 0.058, d = 0.51). The remaining main effect, Stimulation Type, did not become significant (p ≥ 0.081).
The “same periods” analysis confirmed the significant interaction between Time and Stimulation Type, F(1, 15) = 33.97, p < 0.001, η2 = 0.69. As can be seen in Figure 4B, here, too, was a significant decrease in spike rates from pre- (M = 2.42, SE = 0.22) to post-tDCS treatment (M = 1.39, SE = 0.17, t(15) = 4.51, p < 0.001, d = 1.13). The increase in spike rates from pre- (M = 1.67, SE = 0.24) to post-rTMS treatment (M = 2.42, SE = 0.30) now became significant, (t(15) = −5.56, p < 0.001, d = 1.39), while the remaining main effects of Time (p = 0.236) and Stimulation Type (p = 0.369) did not become significant.
4.1 Tolerability of tDCS and rTMS treatments
All five tDCS sessions were well-tolerated, with no reported side effects. The absence of side effects was confirmed by direct questioning after each session. For the rTMS treatment, prior to the session on day one, the patient described symptoms of headache and visual hallucinations, and the technician observed right-hand twitching during the EEG recording. At the end of the rTMS session, the patient reported sensations of being tired. On day two, the rTMS session was well-tolerated, and the patient did not report any adverse side effects. After the rTMS session on day three, the patient reported periods of twitches in her right upper arm that persisted for approximately 24 h, along with an increase in visual hallucinations. Despite this, the patient expressed a strong desire to continue with the rTMS treatment on day four. Notably, the frequency and intensity of these twitches and hallucinations did not surpass the fluctuations experienced by the patient in the 2 months leading up to the rTMS treatment. During the rTMS treatment on day five, the rTMS technician observed increased twitches in the right upper arm and shoulder area, as well as twitching in both legs.
5 DISCUSSION
The aim of this study was to use tDCS and rTMS as supplementary, experimental treatments in a patient with refractory, occipital lobe epilepsy. We found a significant reduction in EEG spikes after five consecutive days of tDCS treatment, corroborating Ng et al.11 and suggesting an acute effect of cathodal tDCS on reducing cortical excitability. Contrary to Ng et al.,11 however, the tDCS effect was not sustained over time as the patient's condition worsened after 2 weeks. There might be several reasons as to why our study did not achieve the same long-term results as in Ng et al.,11 including differences in patient-specific characteristics, epileptic conditions, tDCS electrode placement, and treatment length. For example, similar to our findings here, Ng et al.11 also observed a deterioration of the patient's symptoms after initial improvements from a 3-day tDCS treatment. Only when Ng et al.11 implemented a 14-day treatment duration did interictal epileptiform discharges disappear and clinical seizures cease with long-term beneficial effects. Given the success of a more personalized approach as seen in the Daoud et al.13 study, which tailored tDCS protocols over extended periods with variable cycles for individual patients, future studies should explore if extending tDCS treatment beyond five consecutive days, in a manner that is customized to the patient's specific condition, might yield a more robust long-term effect in treating refractory occipital lobe epilepsy.
We considered placing the anode on the contralateral shoulder, as in other studies.25, 26 This extracephalic placement can be beneficial because it can enhance spatial focality of cathodal stimulation, minimize current shunting (i.e., ensure that the current passes through the brain rather than directly between the electrodes), enhance electrical field distribution in deeper brain regions, and improve participant comfort.27 Nevertheless, there is a concern that extracephalic positioning might alter current flow unpredictably and affect other critical areas such as the respiratory system, heart, and brainstem.28 Therefore, we refrained from an extracephalic setup. Future studies should explore extracephalic electrode placement to enhance treatment efficacy while carefully monitoring for potential risks to critical areas.
The switch from tDCS to rTMS was made because the patient's symptoms worsened before we had sufficient time to evaluate the EEG for any improvements. Given that rTMS has a stronger effect on the targeted brain areas, we opted to proceed with rTMS. This decision was made to improve symptoms in a severely ill patient, rather than as part of a planned comparison between tDCS and rTMS. A proper comparison between the two methods would require a randomized trial, ideally with a crossover design. However, such a trial does not exist to our knowledge so far. Thus, while our tDCS/TMS comparison is far from ideal, it remains the only one in the literature at this point.
Depending on the type of analysis, our study further revealed a near significant or significant increase in spike rates after five consecutive days of low frequency rTMS treatment. This corresponds to a medium or large effect size and is in line with the patient's subjective report of adverse side effects and the rTMS technician's observations. These results are inconsistent with those of Starnes et al.9 who reported that low frequency rTMS significantly reduced interictal epileptiform discharges and seizure frequency in a patient with refractory occipital lobe epilepsy, with sustained long-term benefits. To our knowledge, no other study reported an increase in spike frequency after rTMS treatment in focal refractory epilepsy. There are, however, instances where low frequency rTMS did not produce beneficial effects above control14, 15 or where some patients (2 out of 11) had a significant increase in seizure frequency (not spike frequency as reported here) either during or after rTMS treatments.29 A possible explanation for these contradictory findings can be patient-specific characteristics or the influence of concurrent medication use. Fregni et al.,30 for example, reported that rTMS outcomes were dependent on plasma valproate levels, suggesting that different antiseizure medications could potentially modulate the inhibitory or excitatory effects of rTMS. Similarly, the higher than prescribed dosage of methylphenidate taken by our patient during both brain stimulation treatments could have affected this study's outcome. Although methylphenidate is generally considered safe for children with epilepsy, its effects in adults are less well studied.31 Additionally, the impact of high dosages of methylphenidate is also not well documented to our knowledge. Therefore, its impact on our study's outcome remains elusive. We also cannot rule out that the tDCS treatment impacted the subsequent rTMS treatment despite the 2-week break between treatments. The interaction between these two neurostimulation methods remains an intriguing area for future research.
As a single case report with no placebo control, it is difficult to draw firm conclusions from our study. Electromyogram measures to assess muscle activity in addition to EEG would have been preferable but were not possible due to limited time/resources. The fact that rTMS did not show a reduction might have also been due to the relatively short duration of 1 week. In depression, for example, rTMS treatment often spans several weeks.32 Finally, although the rTMS target was localized based on structural imaging and although the patient rested in a chair specifically designed for rTMS, we cannot rule out that subtle movements of the patient might have led to slight shifts of the coil position.
The findings in this case report, however, are meaningful in two ways. Firstly, according to guidelines on the therapeutic use of tDCS, it is not yet a well-established therapeutic tool in any kind of epilepsy condition, despite there being some promising results.8 The findings in our study therefore add valuable data to this evolving field, showing that tDCS may have the potential to reduce spike frequency in focal, refractory epilepsy. This is especially relevant for less common conditions like occipital lobe epilepsy, where conducting large-scale randomized controlled trials represents a challenge. Secondly, we find it important to report that neurostimulation with rTMS may, potentially, worsen epilepsy in individual cases. Since this is a case study, we cannot conclude with certainty that the rTMS treatment triggered the deterioration of the patient's state, but determining whether rTMS treatment can, in fact, have detrimental effects and under which conditions rTMS might become detrimental (or beneficial) clearly warrants further investigation.
AUTHOR CONTRIBUTIONS
Conception and design of the study: Tine Tronrud, Marco Hirnstein, Eivind Kolstad, Tom Eichele, and Lynn Marquardt. Acquisition of data: Tine Tronrud and Lynn Marquardt. Analysis and interpretation of data: Tine Tronrud, Marco Hirnstein, Eivind Kolstad, and Lynn Marquardt. Drafting the manuscript or figures: Tine Tronrud and Marco Hirnstein. Critical review and revision: All authors.
ACKNOWLEDGMENTS
We would like to thank Sindre Eng, Kristoffer Noszka, and Eivind Aanestad for their assistance in assessing spike frequency on the EEG recordings. We are particularly grateful to the patient who made this study possible. This research was funded by Haukeland University Hospital.
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflict of interest to disclose. We confirm that we have read the journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ETHICS STATEMENT
The responsible regional ethics committee ruled that the tDCS and rTMS treatments fell under the category of additional, experimental treatments, intended to benefit the patient. Consequently, the committee recognized that the attending physician had the competence to supervise the application of tDCS and rTMS without the need for explicit authorization from the committee.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors due to privacy/ethical restrictions.