Efficacy of phenobarbital is maintained after exposure to mild-to-moderate seizures in neonates
Abstract
To study the relationship between the delay in treatment and the efficacy of phenobarbital in neonates, we re-analyzed data from the NEOLEV2 study. Continuous video EEG (cEEG) from patients treated with phenobarbital was reviewed by neurophysiologists who marked each seizure. The time from seizure onset to phenobarbital, total seizure burden pre-phenobarbital, and maximum seizure density (summed seizure burden per hour) pre-phenobarbital were calculated and correlated with phenobarbital efficacy at 20 mg/kg and at 40 mg/kg. The time between seizure onset and phenobarbital treatment did not predict refractoriness to phenobarbital. However, the maximum seizure density per hour and total seizure burden before phenobarbital treatment were strongly correlated with efficacy. ROC curve analysis showed cut-offs of maximum seizure density pre-phenobarbital of 10 ½ min/h and total seizure burden pre-phenobarbital of 36 ¼ min had excellent discriminatory ability in separating patients in whom phenobarbital would be effective from patients in whom it would not be effective (AUC 0.84, p = 0.0002 and AUC 0.85, p = 0.0051). These data suggest that whereas neonates with high seizure density must be treated as an emergency, mild-to-moderate seizures remain responsive to phenobarbital if treated within a time frame of several hours.
Plain Language Summary
Phenobarbital is very effective at stopping seizures in newborns. But if phenobarbital is given after many hours of seizures, it becomes less effective. We do not know how quickly this happens. Our study found that it does not happen over the short term (<4 h). It is more difficult to stop seizures that cumulatively last more than 10 min/h.
Key points
- Neonatal seizure patients can be separated into two group based on whether their maximum pretreatment seizure burden is below or above 10 1/2 min/h.
- This cut-off has excellent discriminatory ability in predicting patients in whom phenobarbital will be effective.
- Over the short term (< 4h) phenobarbital remains effective in patients with mild to moderate seizure burden.
- Our data support revising and shortening our definition of neonatal status epilepticus. Formally defining t1 for neonatal status epilepticus as a single seizure lasting 5 min or a seizure density exceeding 8 min/h could be a powerful tool in expediting rapid treatment.
1 INTRODUCTION
Phenobarbital now has an FDA indication for the treatment of neonatal seizures and is the only antiseizure medication with this indication. Recent consensus ILAE guidelines1 recommend the use of phenobarbital for first-line treatment of neonatal seizures. It is also recommended that electrographic confirmation of seizures be obtained prior to treatment for most neonatal seizure types. There is a tension between the need to start antiseizure treatment as soon as possible and the need to establish cEEG monitoring, especially considering the need for transport of some patients to a NICU where cEEG is available and other clinical care priorities. This tension is made more acute by the fact that we do not know at what point seizures become harmful.
It is well documented that with increasing duration of status epilepticus, the efficacy of benzodiazepines and phenobarbital is reduced,2, 3 but the time frame over which this occurs is less clear and varies with the type of status epilepticus and patient population.2 Neonatal seizures are more pharmacoresistant.4, 5 In the neonate, immaturity of chloride co-transporters may cause GABA agonists to be less inhibitory and even cause depolarization in some neurons.6, 7 Ongoing high burden of seizure activity leads to endocytosis and reconfiguring of the GABA receptor, reducing its responsiveness to GABA agonists. Status epilepticus leads to increased intracellular chloride and increased extracellular potassium, with consequent hyperpolarization of neurons. Animal and in vitro models show changes in intracellular chloride commence almost immediately, and GABA receptor internalization can be seen as early as 10 min after onset of status epilepticus, becoming more established and pronounced by 30–60 min. Phenobarbital may remain effective after endocytosis and reconfiguring of the GABA receptor due to its actions on AMPA and kainate glutamatergic receptors.2
Valuable information as to what time frame the efficacy of phenobarbital decreases in neonates can also be found in clinical trial data. The NEOLEV2 trial was a multicenter-blinded randomized controlled trial of first-line efficacy of levetiracetam compared with that in neonates. In NEOLEV2, the efficacy of 20 mg/kg phenobarbital was 70%. In the contemporaneous neonatal seizure registry cohort observational study, only 35% of patients treated with 20 mg/kg phenobarbital were responsive to it.8 The NEST9 trial studied randomized treatment of electrographic seizures in a cohort with hypoxic ischemic encephalopathy. The median time to enrolment in the study was 26 h, due to delays in the transfer of outborn patients from remote geographical locations across Australia. In this cohort, only 22% of patients had a 50% or greater reduction of seizures in response to phenobarbital.10 We hypothesize that the higher efficacy in NEOLEV2 was due, at least in part, to the speed with which we detected seizures and initiated treatment. Recent analysis of cEEG data from the HEAL trial has shown that for every 5 min of cumulative seizure burden pretreatment, the likelihood of success of treatment dropped by 17%.11
Therefore, it is critically important that treatment progresses to the administration of phenobarbital before refractoriness to treatment develops and before a high-seizure burden likely to exacerbate brain injury is experienced.
To describe the time scale over which loss of phenobarbital efficacy develops, we conducted a ROC curve analysis of the relationship between time delay to treatment with phenobarbital, total cumulative seizure burden and seizure density, and response to treatment.
In NEOLEV2 60% of patients were randomized to first-line treatment with levetiracetam and only progressed to second-line treatment with phenobarbital if two loading doses of levetiracetam, (40 mg/kg + 20 mg/kg) given over a minimum period of 1 h proved ineffective. The other 40% of patients were randomized to first-line treatment with phenobarbital. This created a data set with a range of seizure density and a range of time delay to treatment with phenobarbital.
2 METHOD
Inclusion criteria for the NEOLEV2: Patients were infants with confirmed electrographic seizures of any etiology. Eligible patients had a corrected gestational age between 36 and 44 weeks (less than 2 weeks of age) with a weight of at least 2.2 kg. Patients were excluded if they had received any previous anticonvulsants (with the exception of short-acting benzodiazepines administered for sedation 24 h before enrollment), if the serum creatinine level was greater than 1.6 mg/dL, or if seizures were due to correctable metabolic abnormalities (such as hypoglycemia or hypocalcemia). Patients in whom death was imminent were excluded. Patients in whom EEG monitoring could not be commenced before the need to treat definite clinical seizures were not recruited.
Electrographic seizure was defined as a sudden, repetitive, stereotyped discharge of a minimum 10 s duration on one or more EEG channels with evolving frequency, amplitude, and morphology.
Total seizure burden before treatment with phenobarbital was calculated by summing the duration of each marked seizure prior to the administration of phenobarbital.
Seizure density was defined as the sum of marked seizures in minutes per hour.
Efficacy of phenobarbital was defined as the elimination of all seizures for at least 24 h. Infusions of phenobarbital 20 mg/kg were given over 15 min, and an additional 15 min was allowed for the medication to take effect before efficacy was assessed. Assessments of phenobarbital efficacy were verified by review of the EEG by 2 independent neurophysiologists. Neurophysiologists marked the beginning and end of each seizure. Annotations consisting of seizure start date, time, and duration were imported into an Excel database. The data were analyzed alongside data from our Redcap NEOLEV2 database, which captured whether phenobarbital was used as first or second-line treatment, the date and time of phenobarbital administration, the number of doses of phenobarbital administered, and the efficacy of phenobarbital in eliminating seizures.
Wilcoxon rank sum analysis was undertaken to investigate the relationship between time delay to treatment, maximum seizure density before treatment with phenobarbital, and total seizure burden before treatment with phenobarbital and the efficacy of phenobarbital. Efficacy of phenobarbital was graded as effective at 20 mg/kg in eliminating seizures; effective at 40 mg/kg in eliminating seizures; or ineffective.
Analyses were done within the first-line treatment with phenobarbital subgroup, the second-line treatment with phenobarbital subgroup, and in the group as a whole.
The association between time delay after seizure onset to phenobarbital, maximum seizure density pre-phenobarbital, total seizure burden pre-phenobarbital, and dichotomized phenobarbital efficacy was assessed using receiver operating characteristic (ROC) curve analysis to identify the cut-off points that would optimize sensitivity and specificity for separating patients in whom phenobarbital was ultimately effective and those in whom it was ineffective.
3 RESULTS
cEEG data were analyzed from 66 patients; 27 received phenobarbital as first-line treatment and 39 received phenobarbital as second-line treatment after levetiracetam failed to control seizures. (2 patients with pyridoxine dependent epilepsy were excluded from this analysis since seizures in this condition are frequently refractory to all treatments other than pyridoxine). 35 patients had HIE as their underlying seizure etiology; 26 of these had Sarnat 2 HIE, and 9 had Sarnat 3 HIE. 23 received therapeutic hypothermia. Other etiologies included ischemic stroke (6), cortical laminar necrosis (1), viral meningitis (1), traumatic brain injury (2), intraventricular hemorrhage and hypoglycemia (1), intraventricular hemorrhage and meningitis (1), parenchymal hemorrhage (2), brain malformation (1), tuberous sclerosis (1), withdrawal from SSRIs (1) genetic epilepsy (4). Genetic investigations were not systematically done in all cryptogenic cases at the time of the study. The etiology of seizures was unknown in 9 patients. Detailed demographic data for these patients is shown in Table S1.
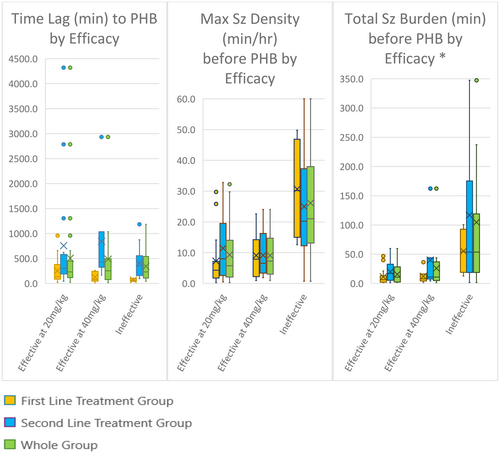
Median pre-phenobarbital maximum seizure density was 9.8 min/h (IQR 3.3–20.6 min/h). Ten patients had a maximum pre-phenobarbital seizure density of 30 min or greater.
For patients who received phenobarbital as first-line treatment, the median delay from the first onset of seizures to administration was 1 h 49 min (minimum 13 min, first quartile 75 min, 3rd quartile 3 h 37 min, maximum 16 h).
Time delay from onset of seizures to administration of phenobarbital was not strongly correlated with maximum seizure density (r = 0.05675, p = 0.65) nor with total seizure burden (r = 0.12, p = 0.33). Total pretreatment seizure burden was very highly correlated with pretreatment seizure density (r = 0.33, p < 0.001).
Time delay from onset of seizures to administration of phenobarbital was not discriminatory with respect to phenobarbital efficacy. Both maximum seizure density pre-phenobarbital and total seizure burden pre-phenobarbital were correlated strongly with phenobarbital efficacy (Figure 1). This was statistically significant when first-line and second-line phenobarbital patients were considered separately and was even more statistically significant in the whole patient group analysis (Table 1).
| Effective 20 mg/kg | Effective 40 mg/kg | Ineffective | p | |||||
|---|---|---|---|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | |||
| Time from seizure onset until PHB given (minutes) | First line | 16 | 134 (89–333) | 7 | 77 (48–243) | 4 | 63 (48–91) | 0.1326 |
| Second linea | 16 | 302 (203–540) | 7 | 430 (324–1036) | 16 | 352 (162–555) | 0.5114 | |
| Total | 32 | 233 (120–443) | 14 | 253 (77–430) | 20 | 232 (110–531) | 0.9940 | |
| Maximum seizure density before PHB given (minutes/hour) | First line | 16 | 4 (2–7) | 7 | 8 (2–14) | 4 | 30 (17–44) | 0.0245 |
| Second linea | 16 | 8 (3–19) | 7 | 7 (3–16) | 16 | 21 (13–37) | 0.0101 | |
| Total | 32 | 6 (2–14) | 14 | 7 (3–14) | 20 | 22 (14–38) | <.0001 | |
| Total seizure burden before PHB given (minutes) | First line | 16 | 6 (2–13) | 7 | 8 (4–16) | 4 | 54 (26–85) | 0.0429 |
| Second linea | 16 | 17 (6–33) | 7 | 11 (8–44) | 16 | 54 (20–159) | 0.0081 | |
| Total | 32 | 11 (2–27) | 14 | 11 (5–36) | 20 | 54 (20–113) | <0.0001 | |
- Note: ROC curve analysis found the optimal cut-off point for maximal seizure density pre-phenobarbital was 10 ½ min/h. The optimal cut-off point for total seizure burden pre-phenobarbital was 36 ¼ min. Both cut-off points had excellent discriminatory ability12 AUC 0.84, p = 0.0002 and AUC 0.85, p = 0.0051. A maximal seizure density pre-phenobarbital of more than 10 ½ min/h and a total seizure burden pre-phenobarbital of more than 36 ¼ min significantly increased the risk that phenobarbital was ineffective in seizure cessation, OR 22.8(4.63–112.6), p = 0.0001 and OR 15.6 (4.30–56.2), p < .0001, respectively (Table 2).
- a Where PHB was given as second-line treatment, LEV 60 mg/kg was the first-line treatment.
| OR (95% CI) per minute increase | p | AUC | Optimal Cut point | ||
|---|---|---|---|---|---|
| ROC curve analysis | |||||
| Time from seizure onset until PHB given (minutes) | Total | 1.00 (0.999–1.001) | 0.4285 | 0.4967 | 623.4 |
| First Line | 1.02 (0.99–1.04) | 0.2109 | 0.7717 | 76.4 | |
| Second Line | 1.00 (0.999–1.002) | 0.2410 | 0.5625 | 370.2 | |
| Maximum seizure density before PHB given (minutes/hour) | Total | 0.90 (0.85–0.95) | 0.0002 | 0.8359 | 10.5 |
| First Line | 0.86 (0.76–0.98) | 0.0218 | 0.9130 | 10.5 | |
| Second Line | 0.92 (0.86–0.98) | 0.0073 | 0.7880 | 19.1 | |
| Total seizure burden before PHB given (minutes) | Total | 0.97 (0.95–0.99) | 0.0051 | 0.8457 | 36.3 |
| First Line | 0.92 (0.86–0.99) | 0.0294 | 0.8913 | 36.3 | |
| Second Line | 0.98 (0.96–1.00) | 0.0455 | 0.7908 | 39.3 | |
| Effective N (%) | Ineffective N (%) | OR (95% CI) | p | |||
|---|---|---|---|---|---|---|
| Odds ratios using the optimal cut points | ||||||
| Maximum seizure density before PHB given (minutes/hour) | Total | ≤10.5 | 33 (71.74) | 2 (10.0) | 22.8 (4.63–112.6) 1.00 | 0.0001 |
| >10.5 | 13 (28.26) | 18 (90.0) | ||||
| First line | ≤10.5 | 18 (78.26) | 0 (0) | - | - | |
| >10.5 | 5 (21.74) | 4 (100) | ||||
| Second line | ≤19.1 | 18 (78.26) | 7 (43.75) | 4.63 (1.14–18.8) 1.00 | 0.0318 | |
| >19.1 | 5 (21.74) | 9 (56.25) | ||||
| Total seizure burden before PHB given (minutes) | Total | ≤36.3 | 40 (86.96) | 6 (30.0) | 15.6 (4.30–56.2) 1.00 | <0.0001 |
| >36.3 | 6 (13.04) | 14 (70.0) | ||||
| First line | ≤36.3 | 21 (91.3) | 1 (25.0) | 31.5 (2.14–463.1) 1.00 | 0.0119 | |
| >36.3 | 2 (8.7) | 3 (75.0) | ||||
| Second line | ≤39.3 | 20 (86.96) | 5 (31.25) | 14.7 (2.93–73.3) 1.00 | 0.0011 | |
| >39.3 | 3 (13.04) | 11 (68.75) | ||||
4 DISCUSSION AND CONCLUSIONS
Painter et al.13 showed that higher neonatal seizure severity predicted reduced drug efficacy in 1999. Our study is one of only two since that study that has been able to investigate this relationship further with quantified cEEG data. Our data align well with the other recently reported study by Numis et al.11 Their analysis of the cEEG data from 39 patients in the HEAL trial showed strong correlations between pretreatment seizure density and phenobarbital efficacy as we have. In their study, the probability that phenobarbital was effective reduced by 17% with every 5-min increase in maximum seizure density. Numis et al. did not find a correlation between total seizure burden and efficacy but postulated that a larger study might; indeed, our study in 66 patients does show this. However, the total seizure burden cut-off did not identify any patients in whom phenobarbital was not effective that were not also identified by high pre-treatment seizure density.
Neither our study nor Numis et al. showed that a longer time delay to treatment was associated with lower efficacy of phenobarbital. This was somewhat unexpected. As outlined in the introduction, when the efficacy of phenobarbital is studied over a long time frame, as we do if we compare efficacy in the NEOLEV2 study, the Neonatal Seizure Registry, and the NEST trial, the efficacy of phenobarbital does reduce over time. However, within the shorter time frame most patients in the NEOLEV2 and HEAL trial were treated in (mostly less than 4-h delay), absolute time delay to treatment is not significantly correlated with the efficacy of phenobarbital. What predicts phenobarbital efficacy is seizure density and cumulative seizure burden. The observations made comparing studies with different times to treatment can be explained, since seizure density in acute symptomatic seizures escalates within the first 24 h of life.14, 15
The ROC curve analyses conducted in this study are useful in defining seizure density and cumulative seizure burden cut points. Phenobarbital is very likely to be effective if pretreatment seizure density is less than 10 min/h. The receiver operator characteristic curve analyses had excellent discriminatory ability to separate patients in whom PHB was effective from those in whom it was ineffective. This provides grounds to separate neonates with seizures into two categories, those with high seizure burden and those with mild-to-moderate seizures.
Our data support revising our definition of neonatal status epilepticus. Definitions of status epilepticus for other age groups have been revised and shortened over time based on animal experiments and clinical research.16 Current definitions have two components: t1, which is the length of seizure beyond which mechanisms responsible for seizure termination fail, and t2, which is the time point at which long-term consequences can occur. In neonates, there is no agreement on the definition of status epilepticus,17, 18; however, a seizure burden of 30 min/h continues to be a widely accepted definition of status epilepticus.19 The data presented here support revising our definition of neonatal status epilepticus since we see dramatically reduced odds that phenobarbital will be effective if pretreatment seizure density exceeds 10.5 min/h. Many clinicians already apply a looser definition of neonatal status epilepticus, treating with urgency any seizure that exceeds 5 min.20 Because neonatal seizures are so frequently subclinical, there is no visual cue to the neonatal team signaling the urgency to treat. Formally defining t1 for neonatal status epilepticus as a single seizure lasting 5 min or a seizure density exceeding 8 min/h could be a powerful tool in expediting rapid treatment. (Seizure density of 8 min/h is suggested to provide a safety margin from the cut-off point identified at 10.5 min/h).
The seizure density and total seizure burden cut-off points we have found for maintained phenobarbital efficacy happen to align quite well with cut-off points found to separate patients based on neurodevelopmental outcome.21 Kharoshankaya et al. explored the relationship between seizure burden and neurodevelopmental outcome in their HIE cohort and found that cut-off points for maximum seizure density of 13 min/h and 40 min total seizure burden separated out patients with good neurodevelopmental outcome from those with poor neurodevelopmental outcome with good sensitivity and specificity. This held after correction for HIE grade and hypothermia treatment. In the group of patients with low seizure burden, outcomes were not worse than in patients without seizures.22 Alharbi et al. suggest that while a high seizure burden of 60 min or more was associated significantly with lowered neurodevelopmental scores, “a total seizure burden below 60 min may not have a significant effect on outcomes, in which case these values may represent a meaningful and perhaps even achievable target for seizure burden control”. This means that patients with neonatal seizures separate into two groups based on seizure density, and the grouping predicts both efficacy of phenobarbital and risk for neurodevelopmental outcome.
Sixty percent of neonatal seizures last 90 s or less.23 In expert reviews, it is acknowledged that many clinicians, guided by a primum non-nocere principle, seek an alternative strategy for brief and self-limited neonatal seizures.11, 20, 24 Since the publication of the current ILAE guidelines, it has become more controversial to recommend anything other than rapid administration of phenobarbital for all neonatal seizures. We must acknowledge that there are numerous uncertainties in our current management of neonatal seizures. Phenobarbital has been approved based on its efficacy in seizure cessation, but we lack definitive evidence that aggressive monitoring and treatment of electrographic neonatal seizures improve neurodevelopmental outcome.9, 20, 24-29 There are concerns regarding the side effects and neurotoxicity of phenobarbital.30-39 The rationale for treatment with phenobarbital is strongest in patients with high seizure burden.21, 22 This study strengthens the idea that neonates with seizures can be divided into two groups. The group with mild-to-moderate seizures remains responsive to phenobarbital (at least over a short time frame) and has a good prognosis neurodevelopmentally. More research is needed for all aspects of neonatal seizures, but particularly in neonates with mild-to-moderate seizures, it must be acknowledged that the best treatment strategy remains unclear.
We suggest mild-to-moderate seizures could be pragmatically defined as seizures with a maximum seizure density of less than 8 min/h. In addition, all individual seizures should be less than 3 min in duration, in light of data showing seizure duration predicts the duration of subsequent seizures.24 Any definition will be arbitrary to a degree. Mehta et al. chose to use a cut-off of 7 or more seizures to define high seizure burden, without further description of the duration of seizures or monitoring.40 However, Numis et al. conclude that maximal seizure burden is a more useful descriptor. A seizure burden density measure is simple to generate within existing EEG analysis tools, providing a rolling sum of seizures in the past hour as marked by the neurologist or automated seizure detector. The cut-offs suggested here are at a safety margin of 2/12 min/h below the cut-off point identified in our analysis as predicting phenobarbital efficacy and 5 min/h below the cut-off point identified by Kharoshankaya et al. Using this definition, 56% of the NEOLEV2 cohort had mild-to-moderate seizures prior to treatment.
Treatment within the NEOLEV2 study was not instantaneous; the median time to first-line treatment was 1 h 49 min, despite all that was done to try to optimize treatment: Continuous EEG monitoring was started in patients with HIE as soon as they were stabilized, and this was often done after hours. The Persyst neonatal seizure detector ran during recording. In the first 24 h, EEG was continuously reviewed via the internet using TeamViewer software by EEG technicians. Study neurologists at each site reviewed the record regularly and whenever contacted by EEG technicians or the neonatal team for suspected seizures. Delays to treatment reflect the time taken for the study neurologist to be contacted, to confirm electrographic seizures, to prescribe the study medication, to obtain investigational pharmacy medication, and to begin infusions. In addition, the seizure markings on which these data are based were made not by the study neurologist at the bedside, but later by an independent neurophysiologist scrutinizing the records and therefore able to detect brief and low voltage seizures, which may have been missed by the study neurologist at the bedside until they evolved into something more clear-cut. We should continue to try to reduce the time to treatment; however, publishing these data on realistically achievable time frames for treatment may avoid a council of perfection, which can be the enemy of the good.
Preparation of sterile phenobarbital for infusion via central lines frequently takes 20 min to achieve after it is prescribed. This will be too slow to prevent patients with high seizure density from exposure to cumulative seizure burdens that have been associated with poor neurodevelopmental outcomes. Consideration should be given to ways to reduce time to treatment. Ensuring peripheral line access as well as central line access can reduce the time for preparation of phenobarbital. Alternatively, preparation of sterile phenobarbital ahead of the onset of seizures in patients at high risk of neonatal seizures can help expedite treatment.
There are many limitations to this study. Although this is the largest comparative trial on neonatal seizure therapy using cEEG data, the patient sample size remains relatively small. Additionally, the duration of seizures before the initiation of cEEG monitoring is unknown. In patients with recognized HIE who receive therapeutic hypothermia and cEEG monitoring shortly after birth, we are likely to have captured the onset of seizures. However, only a third of our patients fall into this category. Other patients are placed on cEEG monitoring because of suspected seizures that may have been ongoing for hours or days. In these patients, our calculated total seizure burden before phenobarbital is inaccurate. In these cases, however, we still have the maximum seizure density to guide treatment decisions. We recognize that, in this study, patients were treated in an environment with intensive monitoring resources, which is often not feasible outside of a research setting.
FUNDING INFORMATION
The NEOLEV2 study was funded by the FDA 1 RO1FD004147. The REDCap database is supported by NIH Cooperative Agreement UL1TR001442. The Persyst EEG software company worked closely with the authors on the NEOLEV2 study and provided their software to the researchers free of charge but has had no input into this manuscript. Dug Yeo Han's work on this paper was funded by the Starship Foundation.
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
DISCLOSURE
CS, GER, and RHH consulted for SPARC pharmaceuticals leading up to their application for FDA license for phenobarbital as a treatment for neonatal seizures. The authors have no other financial relationships relevant to this article to disclose.
ETHICS STATEMENT
IRB or Ethics Board approval was in place at each study site, and written parental consent was obtained for each participant.
CLINICAL TRIAL REGISTRATION
NEOLEV2 was registered with ClinicalTrials.gov (Trial registration number NCT01720667). NEOLEV3 is registered with ClinicalTrials.gov (Trial registration number NCT05610085).
Open Research
DATA AVAILABILITY STATEMENT
Data will be shared with any researcher who contacts the corresponding author with a research question.