Multimodal non-invasive evaluation in MRI-negative epilepsy patients
Wei Wang and Qian Huang contributed equally to this work.
Abstract
Presurgical evaluation is still challenging for MRI-negative epilepsy patients. As non-invasive modalities are the easiest acceptable and economic methods in determining the epileptogenic zone, we analyzed the localization value of common non-invasive methods in MRI-negative epilepsy patients. In this study, we included epilepsy patients undergoing presurgical evaluation with presurgical negative MRI. MRI post-processing was performed using a Morphometric Analysis Program (MAP) on T1-weighted volumetric MRI. The relationship between MAP, magnetoencephalography (MEG), scalp electroencephalogram (EEG), and seizure outcomes was analyzed to figure out the localization value of different non-invasive methods. Eighty-six patients were included in this study. Complete resection of the MAP-positive regions or the MEG-positive regions was positively associated with seizure freedom (p = 0.028 and 0.007, respectively). When an area is co-localized by MAP and MEG, the resection of the area was significantly associated with seizure freedom (p = 0.006). However, neither the EEG lateralization nor the EEG localization showed statistical association with the surgical outcome (p = 0.683 and 0.505, respectively). In conclusion, scalp EEG had a limited role in presurgical localization and predicting seizure outcome, combining MAP and MEG results can significantly improve the localization of epileptogenic lesions and have a positive association with seizure-free outcome.
Plain Language Summary
Due to the lack of obvious structure abnormalities on neuroimaging examinations, the identification of epilepsy lesions in MRI-negative epilepsy patients can be difficult. In this study, we intended to use non-invasive examinations to explore the potential epileptic lesions in MRI-negative epilepsy patients and to determine the results accuracy by comparing the neuroimaging results with the epilepsy surgery outcomes. A total of 86 epilepsy patients without obvious structure lesions on MRI were included, and we found that the combinations of different non-invasive examinations and neuroimaging post-processing methods are significantly associated with the seizure freedom results of epilepsy surgery.
Key points
- In this study, a total of 86 MRI-negative epilepsy patients who received epileptogenic lesion resection surgery were included.
- Complete resection of the MAP-positive regions or the MEG-positive regions was positively associated with seizure freedom.
- When an area is co-localized by MAP and MEG, the resection of the area was significantly associated with seizure freedom.
- Scalp EEG abnormalities had very limited role in presurgical localization and in predicting the seizure outcome.
1 INTRODUCTION
In patients with drug-resistant focal epilepsy, complete surgical resection of the epileptogenic zone can be an effective treatment.1 Magnetic resonance imaging (MRI)-negative epilepsy still represents a major challenge for surgical management.2 Unfortunately, there is no single method that can always localize the epileptogenic zone. MRI and visual inspection of the interictal and ictal (video-) electroencephalogram (EEG) with the determination of the irritative zone and seizure onset zone, respectively, are cornerstone investigations in the presurgical evaluation protocol, as well as magnetoencephalography (MEG), which is a non-invasive modality that not only has temporal and spatial resolution approaching that of intracranial EEG, but also provides a whole-brain view of whole-head activities.3, 4
Recent advances in neuroimaging and image processing have shown that previous “negative MRI” reported by conventional visual analysis may have some subtle lesions after using MRI post-processing methods.5 A number of studies showed that a voxel-based MRI morphometric analysis program (MAP) is a useful tool for identifying subtle epileptic lesions.6-9 As non-invasive modalities are the easiest acceptable and most economic methods in determining the epileptogenic zone, we analyzed the localization value of MRI post-processing method, MEG, and scalp EEG in patients with MRI-negative epilepsy who received resection epilepsy surgeries in our epilepsy center.
2 PATIENTS AND METHODS
2.1 Patients
Patients were consecutively reviewed from our surgical database from January 2013 to March 2019. This study was approved by the Research Ethics Committee of Xuanwu Hospital, Capital Medical University. Written informed consent was obtained from each subject or their legal guardians. Patients were included in the study if they: (1) had epilepsy resective surgery; (2) had a preoperative 3 T MRI with T1-weighted (T1w) magnetization prepared rapid acquisition with gradient echo (MPRAGE) sequence; (3) had negative MRI by radiology report; (4) had preoperative scalp EEG and MEG; (5) had >12 months postsurgical follow-up. Patients were excluded if the MRI was of poor quality or the clinical data was incomplete.
The strategies for surgical resection of all patients were made during a multidisciplinary patient management conference, based on a combination of all the non-invasive data, including semiology, scalp EEG, positron emission tomography (PET), and MEG.
2.2 Scalp EEG data
A 64-channel video EEG acquisition was used and EEG recording and analysis was performed using Medicare Systems software. The scalp electrodes used were composed of disk-shaped electrode wire (Compumedics Ltd., Melbourne, Australia) and electrocardiogram (ECG) electrodes (CONMED Corp., Utica, NY, USA). The electrodes were sited according to the International 10–20 system. Epileptiform abnormalities included spikes, sharp waves, spike, and wave discharges. In order to avoid inaccurate recognition of the ictal onset time and location, data analysis and processing from the EEG were performed by two senior EEG technicians and by an epileptologist separately, depending on the first signs of ictal patterns in the EEG. The ictal patterns can be sudden onset rhythmic activities, repeated spikes/sharp waves, sudden appearance of low voltage activities, recurrent interictal discharges, et al., in concordance with clinical episodes, and should have a corresponding evolution. All the EEG readers (including EEG technicians and epileptologists) who participated in this study were experienced EEG readers. All of them had more than 2 years of training in EEG and epilepsy in the epilepsy center of Xuanwu Hospital of Capital Medical University which is one of the first batch of tertiary epilepsy centers in China and passed the national test for EEG technical accreditation of the China Association Against Epilepsy (CAAE). All the EEG technicians and epileptologists were blinded to the clinical data of the patients. The EEG localization value and its consistency with the resection region were assessed using common sublobar classification scheme (frontopolar, superior frontal, inferior frontal, mesial frontal, superior parietal, inferior parietal, mesial parietal, lateral occipital, mesial occipital, temporopolar, lateral temporal, and mesial temporal), as well as the analyzing of the MAP results, the MEG results and the concordance with the resection area.10, 11
2.3 MRI post-processing
MAP was carried out using SPM12 (Wellcome Department of Cognitive Neurology, London, UK) in MATLAB 2015a (MathWorks, Natick, MA) following previously established methods9, 12 and same as our previous procedures.13, 14 The clinical MRI protocol for epilepsy patients includes 3D T1w MPRAGE sequence, T2-weighted (T2w) turbo spin-echo (TSE) sequences and T2w fluid-attenuation inversion recovery (FLAIR) acquisition. MAP was performed on T1w MPRAGE images, using the z-score threshold of 4 to identify candidate MAP-positive regions on the junction file. The reviewers also examined whether there was an accompanying region on the extension file and the thickness file. Candidate MAP-positive regions were searched over the entire brain. High z-score areas due to artifacts and nonspecific white matter inhomogeneity were excluded. The postoperative CT of each patient was coregistered with the preoperative MRI using 3D Slicer 4.11 software (website: http://www.slicer.org), so that complete or incomplete resection of the MAP areas can be determined. All the MAP results were reviewed by two independent reviewers who were blinded to the clinical data and surgical information of the patients (WW and YL), and confirmed by a radiologist at the patient management conference.
2.4 MEG data
MEG data was recorded from a 306-channel whole-head MEG system (Elekta, Helsinki, Finland). The sampling rate of MEG data was 1000 Hz and a 0.1–300 Hz bandpass filter was applied. Each participant lay in a supine position and was instructed to remain still and keep both eyes closed but not to fall asleep during approximately 60 min of MEG data collection. The system comprised 102 locations at triplets, including one magnetometer and two orthogonal planar gradiometers. A three-dimensional digitizer, a Polhemus™ system (Colchester, NH, USA), was used to determine the location based on anatomical fiducial points for the following MRI-MEG coregistration. The MEG data with head movement more than 5 mm were discarded. A background MEG dataset was obtained to identify outside interferences such as environmental and system noise before the MEG data acquisition. Then the software Elekta Max-filter was applied to remove these interferences and complete the pre-processing of MEG data.
Single equivalent current dipole (SECD) model at the peak of the global field power of each interictal activity was used to calculate the location, orientation, and strength of dipole sources that best fit the measured magnetic fields.15 The interictal epileptiform spikes were typically identified by visual screening of the recorded data by an experienced neurologist (XZ), and their sources were localized by the SECD method using Neuromag software (Elekta, Stockholm, Sweden) and coregistered to the patient's MRIs.
Dipole clusters were categorized into three types depending on their “tightness” information, consistent with previous studies.16 A tight cluster was defined by five or more dipoles located within a single sulcus and the two adjacent gyri bordering this sulcus. A loose cluster was defined by five or more dipoles located within one sublobar region. A scattered cluster was defined by five or more dipoles involving more than one sublobar region. If the patient had less than five dipoles, the MEG study was considered negative. The choice of five dipoles as the threshold is consistent with the American Clinical Magnetoencephalography Society Clinical Practice Guideline and previous studies.17 When assessing the concordance between MEG and MAP findings, if the positive regions located within the same sublobar, MEG and MAP findings were considered to be consistent. When determining the completeness of MEG resection area, if the positive region and the resection area located within the same sublobar, MEG-positive region will be considered to be completely resected. When MEG showed more than one positive region and one of them was located within the same sublobar with the resection area, the resection will be considered to be partially resected.
2.5 Seizure outcome and pathology
The results of seizure outcomes at 12 months were assessed using modified Engel's classification.18 Patients were considered completely seizure-free (Engel's class Ia) if they did not have any seizure or aura at the 12 month follow-up after surgery. Otherwise, the patients were considered not seizure-free (Engel's class Ib–IV). The classification of focal cortical dysplasia (FCD) was in accordance with the International League Against Epilepsy classification.19
2.6 Statistical analysis
Statistical analyses were performed using SPSS version 20.0. Independent Student t-test was used for continuous variables such as age and epilepsy duration. The relationships between seizure outcomes and gender, handedness, resection type, and other categorical variables were separately analyzed by Chi-square test. When the chi-square test included expected values <5, Fisher's exact test was used to calculate p-values. When counts of zero cells were recorded, odds ratios (ORs) were calculated using Haldane's modification, which adds 0.5 to all counts to accommodate possible zero counts.20 p-Values <0.05 (two-sided) were considered statistically significant.
3 RESULTS
3.1 Cohort summary
A total of 273 patients whose MPRAGE sequence was available received epileptogenic lesion resection surgery during the screened time in our epilepsy center. Among these patients, 177 had lesions on MRI, seven patients scalp EEG were unavailable and three patients had poor MRI quality. Therefore, 86 patients were included in this study (A detailed workflow of data collection and analysis process was shown in Figure S1). Forty-nine (57%) patients were male and the average age was 21.3 years (range: 1–49, standard deviation [SD] 9.8). All patients were right-handed. The mean duration of epilepsy was 11.1 years (range: 1.0–40.0, SD: 7.7). Age, epilepsy duration, and gender were not significantly associated with seizure freedom.
Meanwhile, there were 26 pediatric patients (<18 years of age) in this cohort, 16 of them (61.5%) were seizure-free. And there were 52 adult patients in this cohort, 31 of them (59.6%) were seizure-free. The seizure freedom rate between the pediatrics and adults in this cohort did not show a significant difference (p = 1.000).
3.2 Seizure outcomes and pathology
Seizure outcomes and pathology results are shown in Table 1. Eight patients (9.3%) were lost to follow-up. The mean follow-up duration was 32.2 months (range: 12.0–60.0, SD: 15.1). Forty-seven patients (60.3%) were completely seizure-free at one-year follow-up. Thirty-five patients had temporal lobe resection, and 43 patients received extratemporal lobe resection. Seizure freedom was not significantly associated with type of resection (p = 0.464), or temporal lobe vs. extratemporal lobe resection (p = 0.331).
| Factor | Number of patients | Seizure-free | Not seizure-free | p | OR | 95% CI |
|---|---|---|---|---|---|---|
| Overall | 78 | 47 | 31 | |||
| Resection type | – | – | – | 0.464a | – | – |
| Temporal resection | 35 | 19 | 16 | 0.331b | 0.636 | 0.255–1.587 |
| Extratemporal resection | 43 | 28 | 15 | 0.401c | – | – |
| Frontal resection | 18 | 13 | 5 | |||
| Parietal resection | 5 | 3 | 2 | |||
| Opercular resection | 3 | 3 | 0 | |||
| Multilobar resection | 17 | 9 | 8 | |||
| Positive surgical pathology | 77 | 47 | 30 | 0.648d | – | – |
| FCD I | 43 | 23 | 20 | |||
| FCD IIa | 9 | 6 | 3 | |||
| FCD IIb | 5 | 4 | 1 | |||
| FCD III | 5 | 3 | 2 | |||
| HS | 2 | 1 | 1 | |||
| Others | 13 | 10 | 3 | |||
| MAP results | ||||||
| MAP+ fully resected | 30 | 24 | 6 | 0.028e | 3.692 | 1.123–12.136 |
| MAP+ not/partially resected | 25 | 13 | 12 | 0.555f | 1.408 | 0.451–4.395 |
| MAP− | 23 | 10 | 13 | |||
| MEG results | ||||||
| MEG+ fully resected | 30 | 25 | 5 | 0.007g | 4.706 | 1.449–15.286 |
| MEG+ not/partially resected | 33 | 17 | 16 | 0.241h | 0.471 | 0.132–1.679 |
| MEG− | 15 | 5 | 10 | 0.002i | 10.000 | 2.369–42.219 |
| Combine MAP results and MEG results | ||||||
| MAP+ MEG+ fully resected | 16 | 15 | 1 | 0.006j | 11.719 | 1.448–94.817 |
| MAP− and MEG− | 5 | 0 | 5 | 0.051k | 0.131 | 0.015–1.160 |
| Others | 57 | 32 | 25 | |||
| MAP+ not/partially resected and MEG+ not/partially resected | 11 | 4 | 7 | |||
| MAP+ not/partially resected and MEG+ fully resected | 7 | 5 | 2 | |||
| MAP+ fully resected and MEG+ not/partially resected | 11 | 8 | 3 | |||
| MAP− and MEG+ not/partially resected | 11 | 5 | 6 | |||
| MAP+ not/partially resected and MEG− | 7 | 4 | 3 | |||
| MAP+ fully resected and MEG− | 3 | 1 | 2 | |||
| MAP− and MEG+ fully resected | 7 | 5 | 2 | |||
| EEG results | ||||||
| EEG lateralization consistent with resection area | 47 | 30 | 17 | 0.683l | – | – |
| EEG lateralization not consistent with resection area | 1 | 1 | 0 | |||
| Unable to lateralize epileptogenic zone | 30 | 16 | 14 | |||
| EEG localization consistent with resection area | 33 | 21 | 12 | 0.505m | – | – |
| EEG localization not consistent with resection area | 5 | 4 | 1 | |||
| Unable to localize epileptogenic zone | 40 | 22 | 18 | |||
- Abbreviations: CI, confidence interval; EEG, electroencephalogram; FCD, focal cortical dysplasia; HS, hippocampal sclerosis; MAP, morphometric analysis program; MEG, magnetoencephalography; OR, odds ratio.
- a Multi-group analysis (all the resection type).
- b Chi-square test between two groups (temporal resection group vs. extratemporal resection group).
- c Multi-group analysis (all the extratemporal resection type).
- d Multi-group analysis (all the positive surgical pathology groups).
- e Chi-square test between two groups (MAP+ fully resected group vs. MAP+ not/partially resected group).
- f Chi-square test between two groups (MAP+ not/partially resected group vs. MAP− group).
- g Chi-square test between two groups (MEG+ fully resected group vs. MEG− positive not/partially resected group).
- h Chi-square test between two groups (MEG+ not/partially resected group vs. MEG− group).
- i Chi-square test between two groups (MEG+ fully resected group vs. MEG− group).
- j Chi-square test between two groups (MAP+ and MEG+ fully resected group vs. others group).
- k Chi-square test between two groups (MAP− and MEG− vs. others group).
- l Multi-groups analysis within three groups (EEG lateralization consistent with resection area, EEG lateralization did not consistent with resection area, and unable to lateralize epileptogenic zone).
- m Multi-groups analysis within three groups (EEG localization consistent with resection area, EEG localization did not consistent with resection area, and unable to localize epileptogenic zone).
Eighty-four patients had positive pathological results, including 48 FCD I (9 FCD Ia, 32 FCD Ib, 7 FCD Ic), 15 FCD II (9 FCD IIa, 6 FCD IIb), 5 FCD III, and 2 HS. Other positive pathological results included multiple cortical dysplasia (MCD), heterotopia, microglial cell proliferation, nonspecific neuronal loss, tuberous sclerosis complex (TSC), polymicrogyria, and ganglioglioma. Seizure freedom was not significantly associated with the type of surgical pathology (p = 0.648).
Meanwhile, the follow-up duration of 49 patients (62.8%) is above 24 months, of which 28 patients (57.1%) achieved seizure-free at the last follow-up (14 patients had temporal lobe resection, 14 patients had extratemporal lobe resection; FCD I in 15 patients, FCD IIa in three patients, FCD IIb in three patients, FCD III in three patients, MCD in two patients, TSC in one patient, heterotopia in one patient). The follow-up duration of 29 patients (37.2%) is between 12 and 24 months, and 19 patients (65.5%) of them were achieved seizure-free at the last follow-up.
3.3 Scalp EEG results
All patients included had scalp EEG (results shown in Table 1), and 76 patients (76/78, 97.4%) had ictal EEG. The resection area of 47 patients (60.3%) is consistent with the lateralization of scalp EEG, in which 30 patients were seizure-free. The resection area of 33 patients (42.3%) is consistent with the localization of scalp EEG, and 21 patients of them were seizure-free. Eighteen patients (20.9%) showed ictal fast activities in a certain region which helps localization, and 57 patients (66.3%) showed ictal spike/ sharp activities. Neither the EEG lateralization nor the EEG localization showed statistical association with the seizure outcome (p = 0.683 and 0.505, respectively).
3.4 MAP findings
Because 8 patients were lost to follow-up, MAP results of 78 patients were analyzed (shown in Table 1). Fifty-five out of 78 patients (70.5%) had positive MAP findings, including 12 patients (15.4%) with multiple MAP-positive regions. Twenty-four out of the 30 patients (80%) who had MAP-positive regions fully resected became seizure-free; 13 out of 25 patients (52%) in the MAP-positive not/partially resected group were seizure-free. MAP had a sensitivity of 0.80, specificity of 0.56, positive predictive value (PPV) of 70.6%, and negative predictive value (NPV) of 68.4% in this cohort. The complete resection group had significantly better seizure outcomes than the no/partial resection group (p = 0.028), while the seizure outcome of the MAP-negative group did not show a significant difference with the MAP-positive not/partially resected group (p = 0.555). Of the 24 patients with MAP-positive regions fully resected who were seizure-free after a 1-year follow-up, nine patients were FCD I, four patients were FCD IIa, three patients were FCD IIb, two patients were FCD III, one patient was HS, and five patients were other pathology results.
3.5 MEG results
Sixty-three out of 78 patients (80.8%) had positive MEG results (including 20 patients had 1 single tight cluster, 19 had one single loose cluster, 12 had scatter, and 12 had multiple MEG clusters in different regions). Fifteen patients (19.2%) had negative MEG results. Twenty-five out of the 30 patients (83.3%) who had MEG-positive regions fully resected became seizure-free; 17 out of 33 patients (51.5%) in the MEG-positive not/partially resected group were seizure-free (shown in Table 1). The sensitivity and specificity of MEG in this cohort were 0.83 and 0.67, with a PPV of 83.3% and NPV of 66.7%. The complete resection group had significantly better seizure outcomes than the no/partial resection group (p = 0.007) or the MEG-negative group (p = 0.002). However, the seizure outcome of the MEG-negative group did not show a significant difference with the MEG-positive not/partially resected group (p = 0.241).
When analyzing the MEG clusters in detail (Table S1), 11 out of 13 (84.6%) patients with one single tight cluster fully resected became seizure-free, 10 out of 13 (76.9%) patients with one single loose cluster fully resected became seizure-free, all three patients with scatter fully resected became seizure-free, and one patient with two tight clusters (bilateral temporal lobe) became seizure-free when fully resected the tight cluster in the right temporal lobe. There is no significant difference between what kind of clusters fully resected and the seizure outcome (p = 1.000). And in the MEG-positive not/partially resected group, the cluster type (single tight cluster, single loose cluster, scatter, and multiple clusters) also showed no significant difference with the seizure outcome (p = 0.362).
3.6 Combine MAP results and MEG results
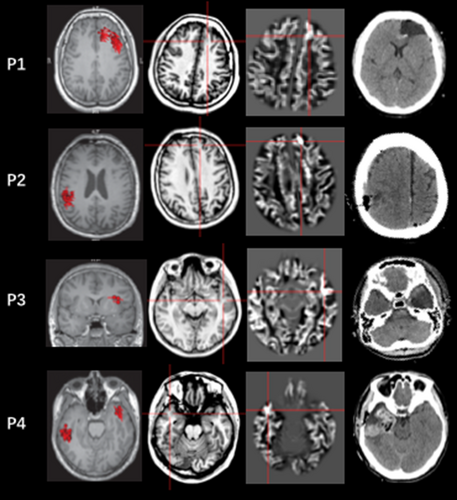
When combining MAP results and MEG results (shown in Table 1), MAP-positive and MEG-positive fully resected group had a higher percentage of patients who were seizure-free after surgery than other groups (p = 0.006, shown in Table 1), with a sensitivity of 0.94, specificity of 1, PPV of 100% and NPV of 83.3%. The seizure outcome of MAP-negative and MEG-negative group did not show any significant difference from other groups listed above (p = 0.051). The details of the seizure-free patients' number in MAP-positive and/or MEG-positive patients in different resection types and different pathology results are shown in Table S2. Figure 1 shows different MAP and MEG results in four patients. Patient 1 had both MAP-positive regions and MEG-positive regions in left frontal lobe, when resected this region, this patient became seizure-free. The MAP-positive regions and MEG-positive regions were inconsistent in Patient 2 and Patient 3, when the MEG-positive region (Patient 2) or the MAP-positive region (Patient 3) was resected, both of these two patients reached seizure-free. When there are multiple MEG-positive regions (Patient 4), the MAP result also helped distinguish one, the resection of which led to the eventual seizure freedom of this patient.
3.7 Other results (semiology, PET and stereo-EEG)
As for the semiology, 70 out of the 78 patients (89.7%) presented focal epilepsy (67 of them also presented secondary generalized tonic–clonic seizure after the initial focal semiology), and eight patients (10.3%) only had generalized tonic–clonic seizures. These eight patients all had MAP-positive and MEG-positive results in the same regions, and 7 out of them (87.5%) were seizure-free (shown in Table S2). In the 70 patients with focal epilepsy or focal secondary generalized epilepsy, 40 patients (57.1%) were seizure-free. Of the eight patients with generalized tonic–clonic seizure, 7 patients (87.5%) were seizure-free. Seizure freedom was not significantly associated with epilepsy type (p = 0.136).
Fifty-one patients in this cohort did PET before epilepsy surgery, and 32 of them (62.7%) had positive results consistent with the surgery resection area. Twenty-one out of the 32 patients (65.6%) were seizure-free after a 1-year follow-up. The PET results of 19 patients were inconsistent with the surgery resection area, and 7 (36.8%) out of them were seizure-free. Seizure freedom was significantly associated with positive PET results (p = 0.046). PET results were concordant with MEG-positive results in 25 patients, of which 18 patients (72.0%) were seizure-free. Twenty patients had PET results consistent with both MAP-positive and MEG-positive regions, of which 16 patients (80.0%) were seizure-free.
Although we mainly focused on the evaluation value of non-invasive methods, we showed some invasive results here to give a better information of this cohort. Fifty-six patients (56/78, 71.8%) in this cohort had stereo-EEG (SEEG) implanted before resection surgery. Of these 56 patients who had SEEG, 43 patients had positive MEG results and the MEG results of 25 patients (58.1%) were consistent with the invasive findings. Thirty-nine patients had MAP-positive results, and 19 of them (48.7%) had MAP results consistent with the invasive results.
4 DISCUSSION
In the presurgical evaluation of drug-refractory MRI-negative epilepsies, non-invasive localization is of paramount importance and can directly affect invasive evaluation and surgical resection. However, accurate localization of MRI-negative epilepsy presents significant challenges. In the present study, we examined the localization values of the common non-invasive method for MRI-negative epilepsy patients.
Scalp EEG is routinely used in clinical for the diagnosis and evaluation of epilepsy, and it is one of the basic examinations for the localization of epileptogenic zone. However, in this study, we found limited localization value of scalp EEG. Whether scalp EEG can predict seizure outcome is still controversial. Greiner et al. reviewed 54 patients who underwent hemispherectomy and found that scalp EEG does not predict hemispherectomy outcome.21 Conversely, Smith et al. found that five of 25 children had bilateral independent epileptogenic foci, and three of these five (60%) were seizure-free postoperatively, considered a poor outcome in the overall sample.22 Burkholder et al. found that unilateral-only interictal epileptiform discharges (IEDs) on preoperative scalp EEG and complete resection of interictal epileptiform discharges on baseline intraoperative electrocorticography are associated with better outcomes following standard amygdalohippocampectomy in magnetic resonance imaging–negative temporal lobe epilepsy.23 In this study, the lateralization and localization of the scalp EEG were determined in the presurgical multidisciplinary patient management conference based on ictal EEG pattern and interictal epileptiform discharges, and we used a mixed cohort of MRI-negative focal epilepsy patients instead of a certain epilepsy syndrome, which may be the reason that lead to different results with the previous studies.
This study showed that completely resecting the MAP-positive regions and MEG-positive regions in MRI-negative epilepsy patients had significantly better seizure outcomes than the no/partial resection group. This is consistent with previous studies.9, 13, 14, 24, 25 Furthermore, combining MAP results and MEG results can achieve a better seizure outcome. MEG is a clinical neurophysiology tool, like EEG, MEG has excellent temporal resolution. Compared to EEG, MEG has superior spatial resolution, because the recorded magnetic signals are not attenuated or influenced by the intervening layers of CSF, bone, or scalp.26 MAP is purely a structural image processing technique which does not provide any electrical or functional information. Besides, both MAP and MEG may have false-positive results.9, 13 Therefore, combining multiple non-invasive methods could achieve complementary value, and linking MAP and MEG can provide a strong anatomo-electrical indication of an underlying epileptogenic focus, and may contribute to improve seizure outcomes.
When multiple MAP regions were present, a MEG correlate provides excellent electrophysiological confirmation of the epileptogenic relevance. In the 12 patients who had multiple MAP-positive regions, 4 patients had one MAP-positive region consistent with MEG-positive regions, and 3 of these 4 patients had MAP-positive regions fully resected, all of the 3 patients became seizure-free. Conversely, when multiple MEG-positive regions are presented, MAP can also help identify potential structural lesions. There were 18 patients had multiple MEG-positive regions in this cohort, and 3 of them had one MEG-positive regions consistent with MAP-positive regions which were completely resected, and all of these 3 patients became seizure-free after resection. The proportion of localizing studies for combined tests was higher than that of the individual test. This is in line with previous studies,24, 25 including other presurgical evaluations, such as FDG-PET and subtraction ictal SPECT coregistered to MRI (SISCOM).
Because of the retrospective nature of our study, selection bias may exist. Patients with relatively focal or restricted epileptic pathologies were more likely to receive surgery. The positive rate of MAP is higher than previous reports, some positive results may be “over-reading” and false-positive regions may also exist. Due to the extended timespan of the inclusion, MR techniques, pathological diagnostic criteria, and presurgical evaluation have evolved substantially during this period and, therefore, potential heterogeneity may occur. The seizure outcome in the present study is better than in previous studies.27-29 As previous studies reported, the seizure freedom rate of FCD may decrease with the extension of the follow-up time. Some patients in this study have only been followed up for 1 year, and cannot obtain follow-up results for 2 years or even longer, which may lead to bias in determining the seizure freedom rate.
5 CONCLUSIONS
Presurgical evaluation is still challenging for MRI-negative epilepsy patients. Although scalp EEG abnormalities had a very limited role in presurgical localization and in predicting the seizure outcome, combining MAP and MEG results can significantly improve the localization of epileptogenic lesions and have a positive association with seizure-free outcome. Other anatomo-electroclinical information should always be considered for the decision making of surgical regime.
AUTHOR CONTRIBUTIONS
WW drafted the manuscript and participated in MRI post-processing as well as data analysis. QH, QZ, and JH helped collecting the clinical data, EEG data, neuroimaging data and followed up with the patients. XZ did MEG data analysis. LL reviewed the EEG results. YL is responsible for the study design, overall arrangement, MRI post-processing, and neuroimaging data coregistration. YW provided consultation and guidance in experiment design and data analysis.
ACKNOWLEDGMENTS
This study was supported by the Talent Promotion Project of Xuanwu Hospital of Capital Medical University (YC20220205), the National Natural Science Foundation of China (No. 81801285), National Support Provincial Major Disease Medical Services and Social Capability Enhancement Project, and Beijing Natural Science Foundation(Z200024).
CONFLICT OF INTEREST STATEMENT
None of the authors have any conflict of interest. We confirm that we have read the Journal's position on issues involved in ethical publications and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.