Variation in seizure risk increases from antiseizure medication withdrawal among patients with well-controlled epilepsy: A pooled analysis
Abstract
Objective
Guidelines suggest considering antiseizure medication (ASM) discontinuation in seizure-free patients with epilepsy. Past work has poorly explored how discontinuation effects vary between patients. We evaluated (1) what factors modify the influence of discontinuation on seizure risk; and (2) the range of seizure risk increase due to discontinuation across low- versus high-risk patients.
Methods
We pooled three datasets including seizure-free patients who did and did not discontinue ASMs. We conducted time-to-first-seizure analyses. First, we evaluated what individual patient factors modified the relative effect of ASM discontinuation on seizure risk via interaction terms. Then, we assessed the distribution of 2-year risk increase as predicted by our adjusted logistic regressions.
Results
We included 1626 patients, of whom 678 (42%) planned to discontinue all ASMs. The mean predicted 2-year seizure risk was 43% [95% confidence interval (CI) 39%–46%] for discontinuation versus 21% (95% CI 19%–24%) for continuation. The mean 2-year absolute seizure risk increase was 21% (95% CI 18%–26%). No individual interaction term was significant after correcting for multiple comparisons. The median [interquartile range (IQR)] risk increase across patients was 19% (IQR 14%–24%; range 7%–37%). Results were unchanged when restricting analyses to only the two RCTs.
Significance
No single patient factor significantly modified the influence of discontinuation on seizure risk, although we captured how absolute risk increases change for patients that are at low versus high risk. Patients should likely continue ASMs if even a 7% 2-year increase in the chance of any more seizures would be too much and should likely discontinue ASMs if even a 37% risk increase would be too little. In between these extremes, individualized risk calculation and a careful understanding of patient preferences are critical. Future work will further develop a two-armed individualized seizure risk calculator and contextualize seizure risk thresholds below which to consider discontinuation.
Plain Language Summary
Understanding how much antiseizure medications (ASMs) decrease seizure risk is an important part of determining which patients with epilepsy should be treated, especially for patients who have not had a seizure in a while. We found that there was a wide range in the amount that ASM discontinuation increases seizure risk—between 7% and 37%. We found that no single patient factor modified that amount. Understanding what a patient's seizure risk might be if they discontinued versus continued ASM treatment is critical to making informed decisions about whether the benefit of treatment outweighs the downsides.
Key points
- We pooled three datasets containing patients with well-controlled epilepsy who continued versus discontinued antiseizure medications.
- No individual patient characteristic significantly modified the odds ratio of having a seizure from discontinuation.
- The 2-year absolute seizure risk increase from discontinuation ranged from 7% (in the lowest-risk patients) to 37% (in the highest-risk patients), with most patients falling in between.
- Our work provides a basis for shared decision-making and will lead to future work developing an individualized calculator and contextualizing seizure risk according to patient preferences.
1 INTRODUCTION
Over 50 million people have epilepsy worldwide.1 Fortunately, two-thirds of patients become seizure free on antiseizure medications (ASMs).2 For this group, a central question is whether ASMs are necessary indefinitely. ASMs reduce morbidity and mortality by reducing seizures.3 However, ASMs may cause side effects4 that reduce quality of life.5 Seizure risk declines with increasing seizure-free intervals such that the absolute benefit of continued ASMs reduces over time.6 Accordingly, guidelines have endorsed considering ASM discontinuation for patients with acceptably low post-withdrawal seizure risk after detailed risk-benefit discussions.7-9
Two randomized trials have evaluated the average seizure risk increase from discontinuation.10-12 However, many patients will almost certainly derive greater or lesser than average benefit. Prior work has incompletely described the degree to which effects vary between patients. A “positive” trial (p < 0.05) suggests that, on average, ASMs reduce the chance of seizure recurrence.10 However, this does not mean that all individuals within any group benefit or benefit equally from treatment, or does a “negative” trial (group difference p-value > 0.0511) mean that no patients benefit. Calculating an individual's risk difference (comparing discontinuation vs. continuation) is needed to achieve precision medicine and personalize care, beyond what can be understood from group averages.
Additionally, an online calculator exists computing individualized post-discontinuation seizure relapse risks.13, 14 However, as above, a key piece is missing: what would the patients risk have been if they continued ASMs? Calculating only post-discontinuation risk without an explicit comparison to continuation fails to distinguish critically different scenarios, such as patients who would be high risk regardless of treatment (little benefit from treatment), versus patients who would be low risk on treatment but high risk off treatment (high benefit from treatment). Yet, prior work has largely paid attention to calculating only individualized post-discontinuation risks (“what factors suggest a higher or lower risk patient”, ie, risk factors) without comparison to individualized continuation risks (“what factors suggest a more or less effective treatment”, ie, effect modifiers).
The 1991 MRC study did develop a two-armed individualized risk score under discontinuation versus continuation. However, that represented a single study, lacked potentially important variables (eg, syndrome, number of past seizures, and prior status epilepticus), and did not explicitly lay out the distribution of anticipated treatment effects.15 A better understanding of how much discontinuation may increase seizure risk across patients would foster more detailed counseling.
We asked: (1) Does discontinuation increase the odds of another seizure more so in some groups than others? (2) What is the possible range of seizure risk increases due to discontinuation, for patients at different levels of post-discontinuation risk? This latter question would inform the possible minimum and maximum effect of withdrawal on seizure risk from a population perspective. For example, how much might withdrawal increase for a low-risk patient? How much for a high-risk patient? And where do most patients fall along this continuum?
2 METHODS
2.1 Study design; datasets
- Medical Research Council (MRC) RCT10: Adults and children with epilepsy who were at least 2 years seizure free were randomized to discontinue (decrementing doses every ~4 weeks until off) versus continue ASMs, across 40 centers in the United Kingdom. Five percent of the withdrawal group did not withdraw and 35% of the continuation group did not continue, but this resulted in negligible bias.16
- The Akershus RCT (Lossius et al.11): Adults with epilepsy, excluding high-risk features (eg, juvenile myoclonic epilepsy), who were seizure free at least 2 years were randomized to discontinue (by approximately 20% every other week until off) versus continue ASMs, from one hospital's catchment area in Norway. Note that 146/149 (98%) of analyzed patients in the Lossius study followed their assigned protocol.
- Real-world retrospective multi-institution observational cohort17: All patients at least 1 years old at the time of their first outpatient visit when they were seizure free for at least 1 year, excluding specific high-risk features (eg, juvenile myoclonic epilepsy), were followed over maximally 7 years, from three health systems (University of Michigan; Stichting Epilepsie Instellingen Nederland; and Wilhelmina Childrens Hospital). Despite lack of randomization, this study provided a key methodological advantage over other withdrawal cohorts: this cohort followed all patients regardless of whether they withdrew ASMs and recorded the day on which it was decided to withdraw ASMs as documented in the electronic medical record. Capturing both “pre” versus “post” discontinuation periods provided within-subject data, while adjusting for seizure-free times, thus reducing confounding or selection bias otherwise inherent to cohorts following only post-discontinuation patients. Still, in sensitivity analyses, we restricted to only the above two RCTs.
2.2 Statistical analysis
2.2.1 Evaluating the average effect of discontinuation: “Base model”
We produced average cumulative seizure incidence curves for discontinuation versus continuation with a time-to-event discrete logistic regression model (with a cubic term for time) via the cumulative product method akin to Kaplan-Meier curve methodology (multiplying each months seizure-free probability times the previous months value).18, 19 This enabled us to avoid the proportional hazards assumption, which was violated in MRC.16 We standardized over confounders listed in Table S1. For RCTs, the main predictor was randomized arm throughout. For the observational cohort, the main predictor started out as “continuation,” which switched to “discontinuation” in the month when they decided to discontinue, if ever, to correctly attribute pre- versus post-discontinuation time. Multiple imputations addressed missing data.20-23
2.2.2 Evaluating what factors modify the effect of discontinuation: “Individual Interactions Model”
We evaluated what patient factors modified the odds ratio of discontinuation on seizure risk by adding interaction terms to the base model, between discontinuation and each variable listed in Table S1. Note that main effects for each risk factor in a model (ie, Table S3) evaluate what variables suggest a higher- or lower-risk patient. In contrast, interaction terms between risk factors and discontinuation in a model (ie, p-values in Table 1) evaluate for whom treatment is most effective.
| No./N (%) or median (IQR; N) | Adjusted p-value for interaction with discontinuation | ||
|---|---|---|---|
| Corrected for multiple comparisons?a | |||
| No | Yes | ||
| Study | |||
| MRC | 1013/1626 (62%) | Reference | Reference |
| Chart review | 464/1626 (29%) | 0.79 | 0.92 |
| Lossiusb | 149/1626 (9%) | 0.23 | 0.96 |
| Age at epilepsy diagnosis categoryc | |||
| <11 | 588/1614 (36%) | Reference | Reference |
| 11–17 | 395/1614 (24%) | 0.60 | 0.83 |
| 18+ | 631/1614 (39%) | 0.33 | >0.99 |
| Age at epilepsy diagnosisc | 14 (7–23; 1465) | ||
| Years seizure-free categoryc | |||
| <2 | 206/1625 (13%) | — | — |
| 2 to <3 | 523/1625 (32%) | — | — |
| 3 to <5 | 365/1625 (22%) | — | — |
| 5+ | 531/1625 (33%) | — | — |
| Number of ASMs | |||
| 1 | 1253/1626 (77%) | Reference | Reference |
| 2 | 307/1626 (19%) | 0.83 | 0.86 |
| 3+ | 66/1626 (4%) | 0.74 | 0.93 |
| Seizures impairing awareness | 857/1625 (53%) | 0.02 | 0.50 |
| Age at start of follow-up | 29 (18–45; 1626) | 0.05 | 0.63 |
| Epileptiform EEG | 454/1625 (28%) | 0.14 | 0.94 |
| Febrile seizures | 142/1473 (10%) | 0.15 | 0.94 |
| Prior status epilepticus | 105/451 (23%) | 0.22 | 0.96 |
| Female | 816/1626 (50%) | 0.35 | >0.99 |
| >9 lifetime seizures | 366/456 (80%) | 0.39 | >0.99 |
| Self-limited syndrome | 17/458 (4%) | 0.41 | 0.94 |
| Developmental delay | 235/1625 (14%) | 0.42 | 0.92 |
| Prior brain surgeryd | 49/1477 (3%) | 0.44 | 0.85 |
| Older-generation ASMe | 1411/1626 (87%) | 0.44 | 0.85 |
| Newer-generation ASM | 330/1626 (20%) | — | — |
| Motor seizures40 | 1420/1625 (87%) | 0.53 | 0.90 |
| Years of seizures before remission | 5 (1–14; 1465) | 0.54f | 0.88 |
| Years of seizure free | 3 (2–5; 1477) | 0.56 | 0.88 |
| Family history of seizures | 257/1475 (17%) | 0.60 | 0.83 |
| Abnormal neurological exam | 107/1162 (9%) | 0.71 | 0.93 |
| Prior discontinuation attempt | 262/1519 (17%) | 0.81 | 0.90 |
| Focal epilepsy | 793/1617 (49%) | 0.83 | 0.85 |
| Structural etiology | 298/1626 (18%) | 0.85 | 0.85 |
- Abbreviation: ASMs, antiseizure medications.
- a Uncorrected p-values represent interaction terms between each listed variable and discontinuation, from a discrete-time logistic regression including main effects and interactions for all listed variables. p-Values were corrected upward by setting the false discovery rate to 5%.24 Note that, per Table S1, three variables were present only in the retrospective cohort, thus displayed p-values for those variables (self-limited syndrome, more than nine seizures, status epilepticus) are from a model fit only on the retrospective cohort to avoid imputing such a large fraction. Conclusions were similar when assessing p-values for those variables imputing the datasets not containing those variables (self-limited syndrome: uncorrected p = 0.41, corrected p = 0.78; more than nine seizures: uncorrected p = 0.39, corrected p = 0.82; status epilepticus: uncorrected p = 0.06, corrected p = 0.48).
- b Lossius et al. randomized 160 patients but due to exclusions or protocol violations analyzed only 149.
- c Age at epilepsy diagnosis and years since last seizure were both entered into the model as categorical variables, given Lossius et al. recorded only categories rather than the underlying continuous value, and categorizing also reduced reliance upon linear assumptions.
- d Note that no patient in this dataset had brain surgery for drug refractory epilepsy. The listed surgeries were for other primary indications to treat the underlying condition. Also, note that we list prior brain surgery in this Table to describe our population. However, this variable was completely collinear with other variables (eg, >9 lifetime seizures) and thus this variable had to be removed from the base model to achieve convergence. Hence, prior brain surgery does not appear in Table S3.
- e Older generation: carbamazepine, diazepam, mogadon, phenobarbital, phenytoin, primidone, prominal, sulthiame, tridione, valproate, and ethosuximide. All others were considered newer generation. Given we entered older generation into our models, we did not also enter new generation.
- f The p-value displayed is for log (years of seizures before remission), given this skewed variable required transformation to achieve convergence in our imputation models.
Because exploratory subgroup analyses risk finding false positives, we used the Benjamini-Hochberg method setting the false discovery rate to 5%.24
2.2.3 Evaluating the distribution of seizure risk increases due to discontinuation across patients (“Risk Interaction Model”)
We then asked, “What was the absolute seizure risk increase due to discontinuation amongst the lowest-risk patients? What about for the highest- risk patients? Where do most patients fall along this spectrum?”
One typical approach to evaluating whether treatment affects groups differently, like above in the individual interactions model, involves displaying odds ratios for groups “one-at-a-time” (eg, is treatment more effective for males vs. females, for normal EEG vs. abnormal EEG, etc.).25 However, in addition to possible false positives due to multiple comparisons,26 this conventional approach may also miss true differences—comparing groups based on only one factor at time does not represent the full range of differences between patients.26
Therefore, our primary analysis used an alternative approach called risk-based modeling (“Risk Interaction Model”).26-28 Risk-based modeling evaluates how the effect of treatment varies not according to just any single group at a time (individual interactions), but rather according to a patient's overall multivariable risk (risk interaction). In other words, the question is no longer, for example, “How does the effect of discontinuation on seizures differ for males versus females?” but rather the more encompassing question, “How does the effect of discontinuation on seizures differ for a high- versus low-risk patient?”29
To do so, we calculated each patient's predicted 2-year post-discontinuation seizure risk from the base model. To calculate this quantity, we set each patient's treatment variable to “discontinuation” throughout, regardless of their actual treatment, and then took the cumulative product of each patient's seizure-free probability each month up until 2 years.30 We then included an interaction between that quantity and whether they actually discontinued to allow for the possibility that the seizure odds ratio due to discontinuation may differ for higher- versus lower-risk patients.
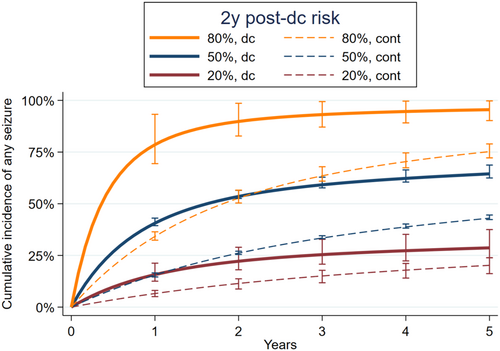
We displayed results in two complementary ways. First, to display detailed cumulative seizure risk information across time, we calculated predicted survival curves at three hypothetical levels of 2-year seizure risk, for discontinuation versus continuation. We chose levels to explore most of the range of our population's risk distribution: 20% 2-year post-discontinuation seizure risk to represent “low risk” (the 11th percentile of 2-year risks as predicted from the base model), 50% (the 76th percentile), and 80% (the 99th percentile). Second, we displayed discontinuation versus continuation seizure risks within 2 years, across the full range of seizure risks. We did so to display detailed seizure risks at a single point in time, across all possible risk levels.
We performed two sensitivity analyses. First, we repeated our main analysis except using 2-year discontinuation risks as calculated from the externally derived Lamberink model.13 Second, we repeated our main analyses limited to only randomized datasets.
Data were analyzed using SAS version 9.4 (Cary, NC) and Stata version 17.0 (College Station, TX).
3 RESULTS
3.1 Population description
We included 1626 patients [MRC: 1013 (62%); chart review: 464 (29%); Lossius: 149 (9%)], with a total of 4962 person-years of follow-up. Table 1 describes the overall population's characteristics. Table S2 describes each study's characteristics.
There were 678 (42%) patients who planned to discontinue at any point, either due to randomization [MRC: 510/1103 (50%); Lossius: 72/149 (48%)] or else as planned at office visits during follow-up [chart review: 96/464 (21%)].
For patients with any seizure during follow-up, the first seizure occurred at a median of 12 months (min: <1; IQR 5–25; max 83). The chance of having any seizure at any point during follow-up was 41% (281/678) for patients who planned to discontinue at any point versus 33% (313/948) for patients who planned to continue.
For patients without any seizure during follow-up, follow-up lasted a median of 49 months (min: <1; IQR 22–68; max 93). Median follow-up for patients without any seizure during follow-up was 49 months (IQR 25–66) for patients who planned to discontinue at any point versus 50 months (IQR 17–68) for patients who planned to continue throughout.
3.2 Evaluating the average effect of discontinuation on seizure risk (“Base Model”)
Table S3 displays the base model's coefficients. For example, discontinuation, shorter years of seizure free, more years of seizures before remission, and non-Lossius studies were correlated with greater odds of a seizure (p < 0.05).
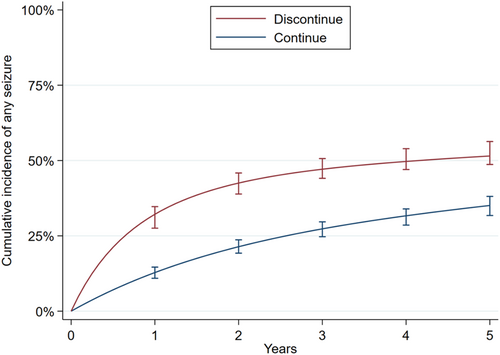
Figure 1 displays standardized cumulative incidence functions for discontinuation versus continuation. One-year seizure risk was 32% (95% CI 28%–35%) for discontinuation versus 13% (95% CI 11%–15%) for continuation. The absolute 1-year risk increase was thus 19% (95% CI 16%–23%). The 2-year seizure risk was 43% (95% CI 39%–46%) for discontinuation versus 21% (95% CI 19%–24%) for continuation. The absolute 2-year risk increase was thus 21% (95% CI 18%–26%).
3.3 Evaluating what patient factors modify the influence of discontinuation on seizure risk (“Individual Interactions Model”)
Age at start of follow-up (p = 0.05) and seizures impairing awareness (p = 0.02) significantly increased the odds ratio for the effect of discontinuation on seizures. Table 1 contains all interaction p-values. The odds ratio for a seizure in any given month comparing discontinuation versus continuation was 0.9 (95% CI 0.8–1.0) at age 30 years. This odds ratio increased by a factor of 1.2 (95% CI 1.0–1.4) for every decade, suggesting that discontinuation had a relatively larger effect for older patients. For example, this would imply a predicted odds ratio due to discontinuation of 0.9 × 1.22 = 1.3 at age of 50 years and an odds ratio of 0.9 × 1.24 = 1.8 at age of 70. The odds ratio for seizures in any given month due to discontinuation was 0.7 (95% CI 0.5–1.0) in the absence of seizures impairing awareness, versus 1.2 in the presence of seizures impairing awareness.
However, after setting the false discovery rate to 5%, no corrected, adjusted p-values remained significant. Also, there was little difference in discrimination (area under the curve: 0.72 for the base model and 0.73 for individual interactions model) or calibration (Figure S1) depending on whether interactions were included. Thus, we eliminated all individual interactions from the subsequent risk interaction model, below.
3.4 Evaluating the distribution of seizure risk increase due to discontinuation across patients (“Risk Interaction Model”)
The median (IQR) predicted risk of any seizure by 2 years was as follows (Figure S2): 39% (28%–50%) for discontinuation versus 19% (13%–26%) for continuation. Discontinuation increased the absolute 2-year seizure risk by a median of 19% (IQR 14%–24%).
We calculated survival curves for several hypothetical, representative seizure risks (Figure 2). For hypothetical patients with base model predicted 2-year discontinuation seizure risks of 20%, 50%, and 80%, the risk interaction model found discontinuation 2-year versus continuation risks of 22% versus 11% (risk difference = 11%), 54% versus 26% (risk difference = 28%), and 89% versus 53% (risk difference = 36%), respectively.
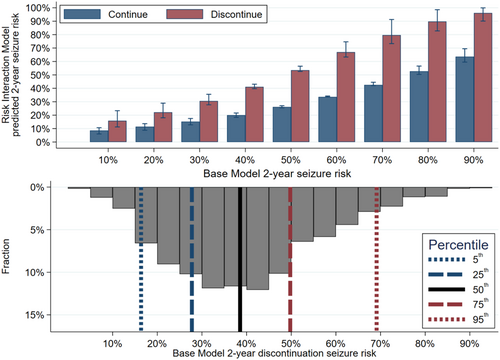
We then calculated 2-year seizure risks, across the entire risk spectrum. Figure 3 displays absolute risks across the risk spectrum (top) plus a superimposed histogram describing the risk distribution of our sample (bottom). Table 2A further describes these results, including that the absolute seizure risk increase ranged from 7% to 37%, and the odds ratio increased with increasing risk levels (p < 0.01).
| Base model hypothetical 2-year discontinuation risk (%) | Risk interaction model 2-year discontinuation risk (%) | Risk interaction model 2-year continuation risk (%) | Absolute risk increase (%) | Odds ratio | Number needed to harm |
|---|---|---|---|---|---|
| (A) All three studies | |||||
| 10 | 16 (11–23) | 9 (6–11) | 7 (3–14) | 2.0 (1.3–3.6) | 13.7 (6.1–37.7) |
| 20 | 22 (18–29) | 11 (9–14) | 11 (6–17) | 2.2 (1.6–3.5) | 9.3 (5.8–15.4) |
| 30 | 31 (28–36) | 15 (13–18) | 15 (12–21) | 2.5 (1.9–3.5) | 6.5 (4.7–8.4) |
| 40 | 41 (40–43) | 20 (18–22) | 21 (18–26) | 2.8 (2.3–3.6) | 4.7 (3.9–5.4) |
| 50 | 54 (53–56) | 26 (25–27) | 27 (23–33) | 3.3 (2.6–4.3) | 3.6 (3.0–4.3) |
| 60 | 67 (63–75) | 34 (34–34) | 33 (26–44) | 4.0 (2.9–6.7) | 3.0 (2.3–3.9) |
| 70 | 80 (73–91) | 43 (42–45) | 37 (26–50) | 5.3 (3.2–13.1) | 2.7 (2.0–3.9) |
| 80 | 90 (83–99) | 53 (50–57) | 37 (21–48) | 7.9 (3.7–40.7) | 2.7 (2.1–4.7) |
| 90 | 96 (90–100) | 64 (60–69) | 33 (13–43) | 14.2 (4.5–279) | 3.1 (2.4–8.0) |
| (B) Only the two RCTs | |||||
| 10 | 15 (9–21) | 8 (4–11) | 7 (2–16) | 2.0 (1.2–4.3) | 14.1 (6.2–56.5) |
| 20 | 22 (16–29) | 11 (7–14) | 10 (5–18) | 2.2 (1.5–3.8) | 9.6 (5.4–18.7) |
| 30 | 30 (27–37) | 16 (13–19) | 15 (11–22) | 2.4 (1.9–3.5) | 6.7 (4.6–8.9) |
| 40 | 42 (41–44) | 21 (19–24) | 20 (17–25) | 2.6 (2.1–3.3) | 4.9 (4.0–5.7) |
| 50 | 55 (55–57) | 29 (27–33) | 26 (22–35) | 3.0 (2.4–4.7) | 3.8 (2.8–4.6) |
| 60 | 69 (67–75) | 38 (35–47) | 31 (22–48) | 3.6 (2.5–8.1) | 3.2 (2.1–4.5) |
| 70 | 82 (80–92) | 50 (44–62) | 33 (17–53) | 4.8 (2.7–17.2) | 3.0 (1.9–6.0) |
| 80 | 92 (88–99) | 62 (53–79) | 30 (6–49) | 7.1 (2.9–56.8) | 3.3 (2.0–14.5) |
| 90 | 97 (95–100) | 74 (63–92) | 23 (−4 to 40) | 13.3 (3.6–356) | 4.3 (2.5–334) |
- Note: We display absolute risk increases in discontinuation versus continuation, odds ratios, and number needed to harm (to produce at least one seizure relapse within 2 years due to discontinuation in one additional patient) for patients at various levels of risk. The left column is the base model‘s hypothetical discontinuation risk. We entered that quantity into the risk interaction model, in which we allowed the relative effect of discontinuation to vary across patients with different base model risks, hence the base model hypothetical discontinuation risk is not identical to the risk interaction model‘s predicted discontinuation risk.
We conducted several sensitivity analyses. Base model 2-year discontinuation predictions correlated moderately with Lamberink model predictions (Spearman's ρ = 0.61; 95% CI 0.55–0.67; Figure S4). Two-year discontinuation predicted probabilities from the Lamberink model had a median of 66% (IQR 49%–81%; Figure S2). Survival curves using Lamberink model predictions instead of base model predictions (Figure S3, left; n = 464) or when using RCT data only (Figure S3, right; n = 1161) were similar to the main analyses. Sensitivity analyses restricted to only RCT data further demonstrated little qualitative change compared with our main results (Table 2B and Figure S5).
4 DISCUSSION
We quantified variation in seizure risk increase due to ASM discontinuation, after a period of seizure freedom. The effect of discontinuation did not strongly depend on any single risk factor. Instead, as expected, a patient's overall risk, after accounting for all risk factors, did strongly determine the absolute risk increase associated with ASM discontinuation. While it can be intuited qualitatively that patients with higher absolute risk likely experience greater absolute risk reduction from treatment, our study's key contribution was to quantify the distribution of absolute risk increases across the risk spectrum. This sort of quantitative understanding of an individual patients treatment effect is needed when deciding whether the absolute benefit outweighs the downsides of treatment. Another insight from this study is that not only did absolute discontinuation seizure risk increase for patients at higher risk but also relative seizure risks increased with increasing risk. This suggests that higher-risk patients may derive greater both absolute and relative benefit from ASMs and identifies a low-risk population for whom discontinuation may be particularly considered given treatment effects even smaller than might have been predicted by group average treatment effects alone.
Identifying patients with both low absolute and low relative treatment effects would enable clinicians to target patients for safest discontinuation. Our work represents one step along this larger goal—to develop an enhanced individualized seizure risk calculator including both discontinuation and continuation estimates. We will use this study's findings (ie, absence of strong interactions between discontinuation and individual factors, but interaction with post-discontinuation risk) to inform our future modeling strategies. We are planning future studies to hopefully improve upon post-discontinuation seizure prediction, which would be useful given the existing model13, 14 demonstrated moderate discrimination (area under the curve 0.65) during internal-external validation, followed by moderate-to-poor discrimination (areas under the curve ranging from 0.51 to 0.71) and some overprediction during external validation31-33 in addition to overprediction in our datasets. Then, we will be able to apply the discontinuation effects observed in the current study to derive a two-armed calculator (not just one armed, as is the current calculator14) making the crucial comparison for a particular individual regarding what their seizure risk might be if they continued versus discontinued ASMs.
Even if one could estimate counterfactual risks perfectly, however, we acknowledge that determining what constitutes an acceptably “low” risk increase remains a challenge. Risk is never zero, regardless of whether the patient is treated, it is always possible that a patient may have difficulty regaining seizure control after a relapse, and all patients and clinicians will have a different level of risk tolerance. We recently surveyed American Academy of Neurology Members who indicated that they would tolerate a median (IQR) post-discontinuation risk of 15% (IQR 9%–29%) for convulsive seizures and 24% (IQR 11%–35%) for nonconvulsive seizures in adults.34 In our sample, only 18% had a post-discontinuation risk per the base model below 24%. Still, epilepsy is diagnosed when 10-year seizure risk rises to at least 60%. Although available datasets generally do not contain such long-term follow-up, as a rough estimation, given survival curves substantially level off after the first several years, we note that 76% of our population had a 2-year post-discontinuation predicted seizure rate of less than 50%, thus there may be a sizeable population who could be discontinuation candidates.
Regardless, we have learned from this study that, given 2-year absolute treatment effects ranged from 7% to 37%, whenever the downsides of ASMs are felt to not be worth a substantial seizure reduction (absolute risk reduction over 37%, or number needed to harm less than 3), the patient should probably discontinue. However, if the downsides of ASMs are felt to be minimal and the patient would accept remaining on ASMs even if they provided only minimal seizure reduction (absolute risk reduction less than 7%, or number needed to harm at least 14), it would seem justified to continue in most cases. Many patients' values and preferences will fall between these two extremes, in which case having an accurate individualized seizure risk prediction for such patients could directly inform decision-making. Of course, the above statistics are only estimates and it is quite possible that patients may fall outside of these bounds depending on an expert clinician's knowledge of the patient based on factors that were not available as variables in our datasets. However, our work provides the best data-driven estimates to date of variation in discontinuation effects. In our future work, we will use what we have learned in the current study to develop our results into an individualized risk prediction calculator to assist with decision-making for patients in whom intermediate seizure probabilities would matter, and we hope to conduct future work improving our knowledge of how low seizure risk may need to be to confer benefit to discontinuation across different patient phenotypes.
Increasing age predicted an increasing relative effect of discontinuation. We had hypothesized that increasing age might predict decreasing effects from discontinuation, given some literature suggesting that older patients might be able to be treated with lower doses, thus “easier to treat.”35-38 A relationship between older age and relative effects of seizure discontinuation would be critical information, if true, given older adults have the highest incidence of epilepsy compared with any other time in life.39 However, interpreting this significant result requires caution. This was one of many exploratory analyses and was no longer significant after accounting for multiple comparisons. Also, we entered age as a linear interaction term—the true biological effects of age may be more complex. Future work should seek to disentangle age versus factors associated with age, such as differential baseline adherence. Additionally, if older patients in our sample were treated with similar doses as younger patients, they could have seen effectively greater serum concentrations due to impaired hepatorenal metabolism, which would be yet one more reason explaining the apparent increased efficacy of ASMs. Our work has begun exploring these important yet complex possible interactions and will require future validation.
4.1 Our work has limitations
First, intention to discontinue (eg, being randomized, or deciding at a clinic visit) is not identical to actually discontinuing and thus our findings could understate the influence of discontinuation. However, we previously demonstrated that the effect of nonadherence to randomized arm in MRC was trivial,16 and adherence to randomized arms was nearly perfect in Lossius data.11
Second, each interaction must be interpreted in the setting of included confounders and modeling assumptions, it is not possible to test for all possible higher-order effects or interactions, and no dataset in existence has measured all possibly relevant variables. For example, datasets did not contain lifestyle factors (eg, sleep, substance use, and alcohol) or baseline ASM adherence. We see this as an opportunity for future prospective data collection and emphasize that we included among the strongest datasets currently in existence for this question. Also, heterogeneity is a strength in risk prediction work to the extent that it has been captured—we measured many important dimensions such as demographics, semiology, focality, prior intracranial surgery, EEG results, ASM characteristics, epilepsy and seizure-free durations, and more. Although we acknowledge that future work more completely specifying a patient's particular self-limited epilepsy syndrome would be helpful, as this was not specified in the randomized data.
Third, in real clinical practice, discontinuation versus continuation are not the only options. Clinicians can also switch ASMs or partially reduce treatment. Our prior work produced the first-ever dose-response curves regarding ASM discontinuation to explore how seizure risk changes across partial dose reductions.16
Fourth, each dataset has limitations. For example, while clinical trials are not confounded, patients enrolled in clinical trials may not exactly represent its intended source population due to the nature of recruitment and structured observation, and the Lossius et al trial enrolled an unusually low-risk population. However, the MRC study was conducted across 40 sites in the United Kingdom, supporting its generalizability to its enrolled population, and the Lossius study provides the highest quality evidence to date given it was double blinded. In the observational chart review, unmeasured factors related to seizure risk may have influenced the decision to withdraw. Still, our observational chart review provides real-world multicenter data, we specifically sought to reduce selection bias by studying all patients (not just discontinuers) including a variable for the time of discontinuation if any, and ultimately results were unchanged when excluding this observational cohort. However, we acknowledge these data were collected from academic centers that may not reflect patients and practices in community centers.
5 CONCLUSIONS
Many patients with epilepsy attaining a seizure-free period may have acceptably low risk to eventually attempt ASM discontinuation, and our study highlights marked variation in discontinuation effects as a step toward identifying those patients. No individual variable robustly predicted relative differences in the influence of discontinuation on seizure risk. Increasing age and seizures impairing awareness predicted a greater relative influence of discontinuation on seizure relapses in uncorrected analysis, but not after correcting for multiple comparisons. Our results will be useful to clinicians by demonstrating the range of effects that might be expected from discontinuation in the population. Our future work will seek to contextualize optimal seizure risk thresholds below which discontinuation may be considered and develop a two-armed point-of-care seizure risk calculator applying what we have learned from this study.
AUTHOR CONTRIBUTIONS
S. W. T. conceived of and designed the study, collected data, executed the statistical analysis, and wrote the manuscript. G. S. designed the study and collected and interpreted the data. A. K., J. S., and M. V. S. collected data. All authors edited the manuscript. J. F. B., R. D. T., and K. P. J. B. provided study supervision.
FUNDING INFORMATION
These funding sources had no role in the planning or execution of this project. Dr Terman was recently supported by the Susan S Spencer Clinical Research Training Scholarship and the Michigan Institute for Clinical and Health Research J Award UL1TR002240. Dr Terman is now supported by an American Epilepsy Society Research and Training Fellowship for Clinicians. Dr Terman was a member of the Junior Investigator Intensive Program of the US Deprescribing Research Network, which is funded by the National Institute on Aging (R24AG064025). Dr Slinger is supported by the friends UMC Utrecht/MING Fund. Dr Springer is supported by the National Institutes of Health K01 NS117555. Dr Ziobro is supported by the PCHD19 Alliance/American Epilepsy Society Research Training Fellowship for Clinicians and the National Institutes of Health K08 NS124937. Dr Otte is supported by the Dutch Epilepsy Fund and the friends UMC Utrecht/MING Fund. Dr Thijs reports lecture and consultancy fees from Medtronic, UCB, Theravarance, Zogenix, Novartis, and Arvelle, and grants from EpilepsieNL, Medtronic, Michael J Fox Foundation, NewLife Wearables and Health-Holland, Top Sector Life Sciences & Health Netherlands Organization for Health Research and Development (ZonMW) [Brain@home, Project number: 114025101], and the Christelijke Vereniging voor de Verpleging van Lijders aan Epilepsie. Dr Skvarce, Dr Koek, Dr Burke, Dr Lossius, Prof Marson, Dr Bonnett and Dr Braun reports no relevant funding.
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
Statistical code for this project may be obtained from the authors. Data can only be shared with agreement from primary investigators and data use agreements where applicable.