Feasibility of cardiac-based seizure detection and prediction: A systematic review of non-invasive wearable sensor-based studies
Abstract
A reliable seizure detection or prediction device can potentially reduce the morbidity and mortality associated with epileptic seizures. Previous findings indicating alterations in cardiac activity during seizures suggest the usefulness of cardiac parameters for seizure detection or prediction. This study aims to examine available studies on seizure detection and prediction based on cardiac parameters using non-invasive wearable devices. The Embase, PubMed, and Scopus databases were used to systematically search according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines. Human studies that evaluated seizure detection or prediction based on cardiac parameters collected using wearable devices were included. The QUADAS-2 tool and proposed standards for validation for seizure detection devices were used for quality assessment. Twenty-four articles were identified and included in the analysis. Twenty studies evaluated seizure detection algorithms, and four studies focused on seizure prediction. Most studies used either a wrist-worn or chest-worn device for data acquisition. Among the seizure detection studies, cardiac parameters utilized for the algorithms mainly included heart rate (HR) (n = 11) or a combination of HR and heart rate variability (HRV) (n = 6). HR-based seizure detection studies collectively reported a sensitivity range of 56%-100% and a false alarm rate (FAR) of 0.02-8/h, with most studies performing retrospective validation of the algorithms. Three of the seizure prediction studies retrospectively validated multimodal algorithms, combining cardiac features with other physiological signals. Only one study prospectively validated their seizure prediction algorithm using HRV extracted from ECG data collected from a custom wearable device. These studies have demonstrated the feasibility of using cardiac parameters for seizure detection and prediction with wearable devices, with varying algorithmic performance. Many studies are in the proof-of-principle stage, and evidence for real-time detection or prediction is currently limited. Future studies should prioritize further refinement of the algorithm performance with prospective validation using large-scale longitudinal data.
Plain Language Summary
This systematic review highlights the potential use of wearable devices, like wristbands, for detecting and predicting seizures via the measurement of heart activity. By reviewing 24 articles, it was found that most studies focused on using heart rate and changes in heart rate for seizure detection. There was a lack of studies looking at seizure prediction. The results were promising but most studies were not conducted in real-time. Therefore, more real-time studies are needed to verify the usage of heart activity-related wearable devices to detect seizures and even predict them, which will be beneficial to people with epilepsy.
Key Points
- There is promising evidence for seizure detection and prediction based on cardiac parameters using wearable devices.
- Most of the studies aimed to develop seizure detection algorithms, with only a few studies focusing on seizure prediction.
- Cardiac parameters used included heart rate, heart rate variability, or a combination of both, yielding diverse algorithm performance.
- Future studies should focus on prospectively validating algorithms with large-scale longitudinal data to enhance algorithm performance.
1 INTRODUCTION
Epileptic seizures are associated with an increased risk of depression, anxiety, seizure-related injuries, and premature death, known as sudden unexpected death in epilepsy (SUDEP).1, 2 The lifetime prevalence of epilepsy, including cases in remission, is 7.60 per 1000 people overall.3 A significant number of people with epilepsy (PWE) still experience inadequate seizure control, despite considerable progress in treatment and surgical interventions.4 Due to the unpredictability of seizures, physicians are reliant on patients and caregivers to document seizure events.5 However, self-reporting is often unreliable and inaccurate,6-8 posing challenges to timely and effective treatment, self-management, and the risk of seizure-related injuries and SUDEP.
Recent technological advancements have paved the way for improved treatment and management strategies through seizure detection and prediction. Seizure detection is the identification of a seizure upon onset, providing objective seizure quantification,9 while seizure prediction involves identifying physiological changes preceding a seizure and alerting patients and caregivers of a seizure risk at any given time. An online survey conducted by the Epilepsy Innovation Institute (Ei2) revealed that unpredictability was the most hindering aspect for PWE.10 A reliable seizure prediction system may reduce anxiety, improve quality of life, and potentially eliminate the risk of injuries and SUDEP. It can also be used in the context of treatment, as medications could be titrated according to periods of high or low seizure likelihood, further improving patient adherence and side effects.11, 12
Research on seizure detection or prediction based on non-cerebral signals has grown significantly due to the rising prevalence of wearable devices that can non-invasively measure signals such as accelerometer (ACC), electrocardiogram (ECG), electrodermal activity (EDA), and electromyography.13-16 By coupling these measurements with the application of machine learning tools, substantial progress has been made in generating new insights into seizure patterns (Figure 1). Dysfunction in the autonomic nervous system (ANS) has been particularly observed in focal seizures with a temporal lobe origin, as well as focal-to-bilateral and generalized tonic-clonic seizures.17 This systematic review mainly focuses on the cardiac changes associated with epileptic seizures as an indicator of seizure onset, given the compelling evidence for pre-ictal and ictal cardiac manifestations.17, 18 Alterations in heart rate (HR) are the most commonly observed ictal autonomic changes and could potentially serve as the earliest clinical sign of an impending seizure.17 This includes ictal tachycardia19, 20 or a decrease in heart rate variability (HRV),21 which is the variation in time intervals between successive heartbeats. HRV is a reflection of cardiac activity regulation by the ANS, suggesting its potential value in the identification of an upcoming seizure.21
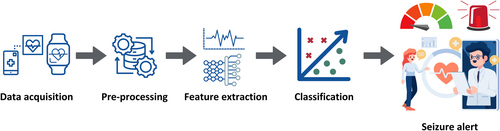
In this systematic review, we aim to examine currently available studies on seizure detection or prediction based on cardiac parameters using non-invasive wearable devices and to compare the performance between different cardiac parameters.
2 METHODS
2.1 Search strategy
The Scopus, PubMed, and Embase databases were used to conduct a systematic search in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. All available publications up to August 2022 were included, although it is worth noting that the utilization of sensor technology has only emerged in the last decade.22, 23 Keywords related to [“epilepsy” or “seizure”] were combined with terms related to [“detection” or “prediction”], [“heart rate” or “cardiac”] and [“wearable device”]. Searches in all databases were conducted based on title and abstract.
2.2 Study selection
All resulting articles were imported into Covidence software (Veritas Health Innovation), and duplicates were automatically removed. One reviewer screened titles and abstracts to identify relevant research articles. Two independent reviewers screened the full-text articles for inclusion, and conflicts were resolved by a third reviewer. The following inclusion criteria were used: (a) written in English; (b) observational studies (prospective or retrospective studies) involving human participants; (c) peer-reviewed original research articles related to seizure detection or prediction using wearable devices in people with epilepsy; (d) ECG or cardiovascular parameters were used as a basis for seizure detection or prediction, either alone or with other physiological signals, (e) provided at least one algorithm performance indicator as an outcome. We also included studies that analyzed blood volume pulse (BVP) obtained from photoplethysmography (PPG) signals, as it provides information about heart rate.24 Studies were excluded based on the following criteria: (a) articles identified as conference papers, reviews, book chapters, commentaries, editorials, and case reports; (b) ECG or cardiovascular data not collected using a wearable device; (c) studies that did not use cardiac parameters as a basis for seizure detection or prediction; (d) studies involving neonates.
2.3 Data extraction and synthesis of results
Data from the included studies were extracted electronically using Covidence. Data were extracted in the following categories: study identifiers (author, year of publication), study characteristics (study population, study setting, reference standard, total participants recruited, and number of patients analyzed), wearable device (wearable device used, device location, and physiological signal[s] collected), seizure detection or prediction algorithm (type of validation, detection or prediction, modality, cardiovascular parameter[s] used), and results (seizure type[s] and algorithm performance). Studies on seizure detection were analyzed separately from those on seizure prediction, which also included seizure forecasting.
2.4 Quality assessment
Two reviewers independently conducted the quality assessment for all included studies, and conflicts were resolved by discussion. The QUADAS-2 tool, a risk of bias tool that can be applied to primary diagnostic accuracy studies, was used to assess the quality of the studies included in the review using Review Manager version 5.4 (Cochrane Collaboration). The risk of bias was assessed based on each of these four domains: patient selection, index test, reference standard, flow, and timing. The patient selection domain assesses the method of patient recruitment and the patients included in the study. The index test and reference standard domains assess how they were conducted and interpreted, where interpretation of the index test results may be influenced by the knowledge of the reference standard and thus introduces the potential for bias. Concerns regarding applicability to the review question were also assessed for the first three domains. The flow and timing domain assesses the inclusion of all patients in the analysis and the interval between the index test and reference standard.25 All included studies were also evaluated based on proposed standards for clinical validation of seizure detection devices.26 The studies were categorized into five different phases based on key features, including subjects, recordings, analysis and alarms, and reference standard.
3 RESULTS
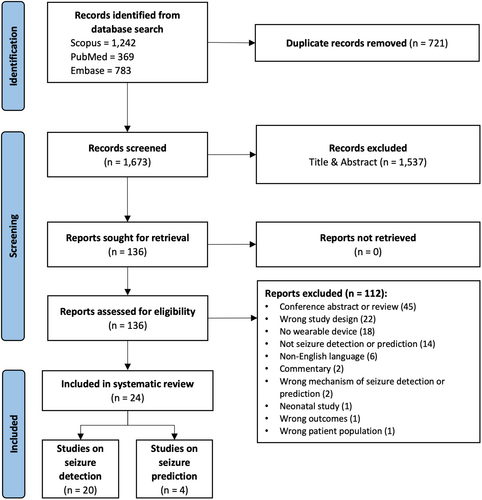
The database searches yielded a total of 2394 articles, out of which 1537 were screened based on title and abstract and 136 articles were selected for full-text review. After reviewing the full text based on eligibility criteria, 24 articles were included for analysis. Twenty studies evaluated seizure detection algorithms,27-46 whereas the remaining four studies focused on seizure prediction, including forecasting the likelihood of seizures (periods of high and low risk).24, 47-49 The screening stages and results are outlined in more detail in Figure 2. Articles were excluded mainly due to being a conference abstract or review, the study design not involving the validation of an algorithm on patients, or not using a wearable device to measure cardiovascular signals. Studies that recorded and analyzed cardiovascular signals before or during seizures but not in the context of validating a seizure detection or prediction algorithm were also excluded.
3.1 Studies on seizure detection
The characteristics of the 20 seizure detection studies included in the review are listed in Table 1. The number of patients included in the analysis for seizure detection ranged between 3 and 94 participants (median = 15.5 participants). Most studies took place in an inpatient setting (n = 17), where patients with a diagnosis of epilepsy admitted to an epilepsy monitoring unit (EMU) for presurgical evaluation, seizure assessment, or diagnostic purposes were recruited. Two studies were conducted in a residential setting, where they validated seizure detection devices for detecting nocturnal seizures.28, 40 In studies with inpatient monitoring, patients were allowed to move around freely and perform normal daily activities despite being confined to a hospital room.33, 42 Fifteen studies used video-electroencephalography (EEG) as a reference to validate seizure events,29-31, 33-39, 42-46 where clinical experts annotated the electrographic seizure onset and offset. The other studies used infrared-sensitive video cameras,28, 40 EEG without video recording,31 and video recording with EMFIT monitor41 as the reference standard. One study did not report on the reference standard used.27 Some of the studies also reported incomplete data and that not all patients that enrolled in the study were included in the analysis, mainly due to factors such as poor connection or signal quality,34, 44 withdrawal of participants,28 insufficient or unsuitable seizures,29, 30, 37, 39, 42, 46 unusable data,31, 32, 34 participant's non-compliance to study protocol.40
| Studies included (Author, year) | Study characteristics | Wearable devices | |||||
|---|---|---|---|---|---|---|---|
| Study population | Adults/children | Study setting | Reference standard | Wearable device to collect cardiac data | Location of device | Physiological signal(s) collected | |
| Heart rate (HR) | |||||||
| Ali and Alam 202027 | N/A | N/A | N/A | N/A | Prototype sensor | Wrist | ACC, PPG, TEMP |
| Arends et al 201828 | Adults with epilepsy ID with a history of >1 major nocturnal seizure per month and resided in a long-term facility of 1 of the participating epilepsy centers | Adults | Inpatient and outpatient | Infrared-sensitive video camera | Nightwatch | Arm | ACC, PPG |
| Cogan et al 201731 | Adults admitted to the EMU at Presbyterian Hospital of Dallas, Texas. | Adults | Inpatient | Video-EEG | Nonin WristOx2 | Wrist | EDA, PPG, SpO2 |
| Masse et al 201341 | Patients previously diagnosed with epileptic seizures, with some forms of HR changes | Adults | Inpatient | Video recording and EMFIT | Custom miniaturized ECG monitor | Chest | ECG |
| Lazeron et al 202240 | Children (3-18 years old) with refractory epilepsy and an ID living at home or in a specialized institutional residential care setting | Children | Inpatient and outpatient | Infrared-sensitive video camera | Nightwatch | Arm | ACC, PPG |
| Bottcher et al 202229 | Patients (7-80 years old) with a diagnosis of epilepsy recruited as part of their standard clinical epilepsy care | Adults and children | Inpatient | Video-EEG | Empatica E4 | Wrist | ACC, EDA, PPG |
| Bruno et al 202130 | People with focal epilepsy admitted to the EMU at King's College Hospital, London UK for diagnostic reasons or presurgical evaluation | Adults and children | Inpatient | Video-EEG | Bespoke upper arm band | Arm | ECG, ACC, EDA, sEMG |
| van Andel et al 201744 | Patients (above 2 years old) with a history of nocturnal seizure frequency >1 seizure/wk, admitted to one of the centers for long term (>24 h) video-EEG monitoring | Adults and children | Inpatient | Video-EEG | Shimmer sensor | Arm | ACC, ECG |
| Cogan et al 201532 | Patients admitted to the EMU | N/A | Inpatient | EEG | Nonin WristOx2 | Wrist | EDA, PPG, SpO2 |
| Henze et al 202135 | Epilepsy patients under video-EEG monitoring | N/A | Inpatient | Video-EEG | cosinuss° In-Ear sensor | Ear | ACC, PPG |
| Zsom et al 201946 | Patients undergoing inpatient video EEG long-term monitoring | N/A | Inpatient | Video-EEG | Empatica E4 | Wrist | EDA, PPG |
| Heart rate variability (HRV) | |||||||
| Munch Nielsen et al 202242 | Patients admitted to the EMU at Zealand University Hospital for diagnostic evaluation | Adults and children | Inpatient | Video-EEG | Bittium Faros 180° | Chest | ACC, ECG, EEG |
| Forooghifar et al 201933 | Patients with epilepsy who underwent in-hospital recording of their seizures for diagnostic purposes | N/A | Inpatient | Video-EEG | SmartCardia INYU wearable sensor | Chest | ECG |
| Blood volume pulse | |||||||
| Tang et al 202143 | Patients admitted to the Boston Children's Hospital EMU | Adults and children | Inpatient | Video-EEG | Empatica E4 | Wrist or ankle | ACC, EDA, PPG, TEMP |
| Heart rate and heart rate variability | |||||||
| Jahanbekam et al 202136 | Adult patients aged 18 years or older with refractory epilepsy who underwent video-EEG monitoring | Adults | Inpatient | Video-EEG (non-invasive scalp-EEG recordings) | EcgMove | Chest | ECG, ACC, EDA |
| Vandecasteele et al 201745 | Patients with refractory epilepsy who underwent presurgical evaluation at UZ Leuven Gasthuisberg | Adults | Inpatient | Video-EEG | Bittium Faros 180° (ECG) and Empatica E4 (PPG) | Chest and wrist | ECG |
| Hegarty-Craver et al 202134 | Children (2-17 y) undergoing video-electroencephalogram monitoring for clinical care | Children | Inpatient | Video-EEG | Zephyr Biopatch and Bittium Faros 180° | Chest | ACC, ECG |
| Jeppesen et al 201738 | People (5-76 years old) with a diagnosis of probable focal or generalized epilepsy, enrolled for a long-term video-EEG monitoring in Aarhus University Hospital | Adults and children | Inpatient | Video-EEG | ePatch device | Chest | ECG |
| Jeppesen et al 201939 | Patients who were enrolled for diagnostic reasons or presurgical evaluation in the EMU | Adults and children | Inpatient | Video-EEG | ePatch device | Chest | ECG |
| Jeppesen et al 202037 | Patients who were enrolled for diagnostic reasons or presurgical evaluation in the EMU | Adults and children | Inpatient | Video-EEG | ePatch device | Chest | ECG |
- Abbreviations: ACC, accelerometry; ECG, electrocardiogram; EDA, electrodermal activity; EEG, electroencephalogram; EMU, epilepsy monitoring unit; HR, heart rate; ID, intellectual disability; iEEG, intracranial electroencephalography; N/A, information not available; PPG, photoplethysmography; sEMG, surface electromyography; SpO2, blood oxygen saturation; TEMP, temperature.
3.1.1 Wearable devices
Information on wearable devices used in the seizure detection studies is listed in Table 1. A wide range of non-invasive wearable devices were used to collect the ECG or HR data. Four studies used Empatica E4,29, 43, 45, 46 three studies used the ePatch device,37-39 and three studies used Bittium Faros 180°.34, 42, 45 Other wearable devices that were noted include Nightwatch,28, 40 Nonin WristOx2,31, 32 SmartCardia INYU wearable sensor,33 Zephyr Biopatch,34 cosinuss° In-Ear sensor,35 EcgMove,36 and Shimmer sensor.44 Interestingly, two studies developed custom wearable devices to collect the ECG or PPG data.30, 41 The devices were primarily either worn on the chest (n = 8)33, 34, 36-39, 41, 42 or wrist (n = 5).27, 29, 31, 32, 46 In the remaining studies, the wearable devices were worn on the arm (n = 4)28, 30, 40, 44 and ear (n = 1).35 One study used two wearable devices, where one device was worn on the chest and the other on the wrist,45 while another study allowed their participants to wear the device either on their wrists or ankles.43
3.1.2 Seizure detection algorithms
Information on the seizure detection algorithms and their performance are listed in Table 2. Eleven studies extracted HR features for the seizure detection algorithms, with a combined sensitivity range of 56%-100% and a false alarm rate (FAR) of 0.02-8/h. Most of the studies that used HR as a basis for their seizure detection algorithm (eight out of 11 studies) validated their algorithm retrospectively using an existing dataset.27, 29-32, 35, 41, 46 For example, data analysis and algorithm testing were performed after the recording of physiological signals from patients (offline). Of the 11 seizure detection studies based on HR, four studies involved adult participants,27, 28, 31, 41 three studies included both adult and child participants29, 30, 44 and one study involved children only.40 The remaining three studies did not report information on the participants' ages.32, 35, 46
| Studies included (Author, year) | Type of validation | Total participants recruited | No. of patients analyzed | Adults/children | Modality | Seizure type | Results of algorithm performance |
|---|---|---|---|---|---|---|---|
| Heart rate (HR) | |||||||
| Ali and Alam 202027 | Retrospective | 3 | 3 | Adults | Multimodal | Convulsive seizures |
Sensitivity: 85% FAR: 26.09% |
| Arends et al 201828 | Prospective | 34 | 28 | Adults | Multimodal | Nocturnal seizures: TCS, GT, HK, OM |
Sensitivity: 86% PPV: 49% FNAR: 0.03 /night FPAR: 0.25 /night |
| Cogan et al 201731 | Retrospective | 20 | 10 | Adults | Multimodal | FOIA, FBTCS, GTCS |
3 Sensors (n = 6): Sensitivity: 100%, 100% (P) PPV: 86%, 100% (P) FAR: 0.015/h, 0.000/h (P) |
| Masse et al 201341 | Retrospective | 3 | 3 | Adults | Unimodal | TCS, generalized tonic and hypermotor |
Sensitivity: 75% PPV: 70.4% |
| Lazeron et al 202240 | Prospective | 25 | 23 | Children | Multimodal | Nocturnal seizures: TCS, GT, HK, OM |
Sensitivity: 93.2% (mean 85.9%, range 47.4%-100% [95% CI 59.9%-100%] FNAR: 0.02/h (range 0.003-0.12) Median PPV: 58.1% (mean 55.5%, range 1.2%-86.6%) |
| Bottcher et al 202229 | Retrospective | 243 | 6 | Adults and children | Multimodal | FBTCS, GTCS |
Sensitivity: 75% FAR24: 13.4 PPV: 2.1% |
| Bruno et al 202130 | Retrospective | 51 | 12 | Adults and children | Multimodal | Focal motor seizures with impaired awareness and focal motor aware seizures |
|
| van Andel et al 201744 | Retrospective | 95 | 23 | Adults and children | Multimodal | Nocturnal seizures: GTC, hypermotor, GT, cluster seizures |
HR only: Sensitivity = 56%-71%; FAR = 2.3 /night HR + Mvt: Sensitivity = 71%-87%; FAR = 5.9-6.3/night |
| Cogan et al 201532 | Retrospective | 5 | 3 | N/A | Multimodal | Secondarily generalized and complex partial seizures |
Sensitivity: 100%, 100% (P) Specificity: 83%, 100% (P) Accuracy: 92%, 100% (P) |
| Henze et al 202135 | Retrospective | N/A | 17 | N/A | Multimodal | TCS |
Mean detection latency: 13 s FAR: 192/24 h Sensitivity: 0.856-0.91 PPV: 0.016 |
| Zsom et al 201946 | Retrospective | 30 | 18 | N/A | Multimodal | Epileptic seizures, PNES, convulsive and non-convulsive | Accuracy: 78% |
| Heart rate variability (HRV) | |||||||
| Munch Nielsen et al 202242 | Retrospective | 30 | 3 | Adults and children | Multimodal | Focal tonic and focal nonmotor seizures |
Focal tonic: Sensitivity = 84%, FAR = 8/24 h Focal nonmotor seizures: Sensitivity = 100%, FAR = 13/24 h and 5/24 |
| Forooghifar et al 201933 | Retrospective | N/A | 18 | N/A | Unimodal | Focal seizures |
Sensitivity: 88.7% Specificity: 85.7% |
| Blood volume pulse | |||||||
| Tang et al 202143 | Retrospective | N/A | 94 | Adults and children | Multimodal | FBTCSs, GTCSs, focal tonic seizures, focal subclinical seizures, focal automatisms, focal behavior arrest, focal clonic seizures, generalized tonic seizures, and generalized epileptic spasms |
|
| Heart rate and heart rate variability | |||||||
| Jahanbekam et al 202136 | Retrospective | N/A | 35 | Adults | Multimodal | GTCS, FANMS, FOIA, FBTCS |
Sensitivity: 67% FP rate: 0.03/h |
| Vandecasteele et al 201745 | Retrospective | N/A | 11 | Adults | Unimodal | FOIA |
Sensitivity: 64% (overall: 70%) PPV: 2.03% (overall: 2.15%) FAR: 2.35/h (overall: 2.11/h) |
| Hegarty-Craver et al 202134 | Retrospective | 62 | 18 | Children | Multimodal | TCS and focal seizures |
Sensitivity: 72% FP rate: 0.04/h |
| Jeppesen et al 201738 | Retrospective | 14 | 14 | Adults and children | Unimodal | N/A |
Sensitivity: 99.979% PPV: 99.976% |
| Jeppesen et al 201939 | Retrospective | 100 | 43 | Adults and children | Unimodal | FBTCS, GTCS, FOIA, FAS |
Sensitivity: 93.1% FP rate: 0.04/h |
| Jeppesen et al 202037 | Retrospective | 47 | 11 | Adults and children | Unimodal | FBTCS, GTCS, FOIA, FAS |
Sensitivity: 87.0% FP rate: 0.38/h |
- Abbreviations: ACC, accelerometry; AUC-ROC, area under the receiver operating characteristic curve; BVP, blood volume pulse; CI, confidence interval; ECG, electrocardiogram; EDA, electrodermal activity; FAR, false alarm rate; FAS, focal aware seizures; FANMS, focal aware non-motor seizures; FBTCS, focal to bilateral tonic–clonic seizures; FNAR, false negative alarm rate; FOIA, focal onset impaired awareness seizures; FP, false positive; FPAR, false positive alarm rate; GT, generalized tonic; GTCS, generalized tonic-clonic seizures; HK, hyperkinetic seizures; HR, heart rate; HRV, heart rate variability; IoC, improvement over chance; Mvt, movement; N/A, information not available; OM, other major seizures; P, personalized algorithm; PNES, psychogenic non-epileptic seizures; PPV, positive predictive value; sEMG, surface electromyography; TCS, tonic clonic seizures; TiW, time in warning.
Studies with adult participants achieved a combined sensitivity range of 85%-100%. The first study used a unimodal approach, where a custom miniaturized wearable ECG monitor was developed and integrated with a beat-detection algorithm and a real-time epileptic seizure detection algorithm to detect seizures. The device was validated in three patients with epilepsy who had HR changes. Tonic-clonic, generalized tonic, and hypermotor seizures were detected with a mean sensitivity of 75% and PPV of 70%.41 The remaining three studies with adults used a multimodal algorithm that was validated either prospectively28 or retrospectively.27, 31 In the prospective study, the multimodal sensor detected a median of 14 seizures, with a median sensitivity of 86%, median positive predictive value (PPV) of 49%, and a false positive (FP) rate of 0.25 per night.28 The authors also demonstrated that HR is a critical modality, as it accounted for 92% of the detection of true positives, whereas the ACC modality accounted for only 8% of detections. One retrospective study developed a seizure detection algorithm by analyzing HR, blood oxygen saturation (SpO2), and EDA biosignals acquired with a wrist-worn device. The personalized algorithm was able to detect seizures with 100% sensitivity and a FAR of 0.00/h in six out of 10 patients.31
In the study focused on HR-based seizure detection among children, an adapted algorithm was developed to reduce false alarms, where the alarm was only triggered when the participant was lying in a horizontal position. This algorithm detected 305 out of 384 seizures (median sensitivity: 93%), with a median PPV of 58% and a false negative alarm rate of 0.02/h.40 Studies that included both adults and children did not provide a clear distinction in algorithm performance between the two age groups, although one retrospective study used a leave-one-seizure-out method for evaluation across three patients and reported lower sensitivity and higher FAR in one pediatric patient compared to the other two adult patients (sensitivity: 67% vs 100%, FAR24: 41.52 vs 0.85-17.69).29 In this study, HR features were extracted from the BVP signals and combined with ACC and EDA features. An optimized model was also developed, which could detect focal motor seizures with a mean sensitivity of 75%, a mean FAR of 13.4/24 h, and a PPV of 2.1%.29
Two studies have used HRV as a parameter for seizure detection.33, 42 One retrospective validation study collected ECG, ACC, and behind-the-ear EEG signals from both adults and children using separate devices and extracted HRV measures such as Modified Cardiac Sympathetic Index (ModCSI) and Modified Cardiac Sympathetic Index with Slope (ModCSISlope) from the R peaks of the ECG signal.42 A support vector machine (SVM) algorithm, another machine learning tool, was used to classify seizure or non-seizure events based on the multimodal signal features extracted. This study reported the detection of focal tonic (sensitivity: 84%, FAR: 8 per 24 h) and focal non-motor seizures (sensitivity: 100%, FAR: 13 per 24 h) in three patients. In another retrospective study, the ECG signal collected using the chest-worn SmartCardia INYU wearable sensor was used to extract the R-R interval (RRI) and ECG-Derived Respiration (EDR) time series. For HRV analysis, time domain, frequency domain, Lorenz plot, and multifractality features were extracted from the RRI to assess changes in cardiac function. The random forest classifier, a machine learning tool, was applied to classify seizure and non-seizure segments. The algorithm was able to detect focal seizures with a sensitivity of 88.7% and a specificity of 85.7%.33
Six studies evaluated seizure detection based on both HR and HRV parameters.34, 36-39, 45 Three of these studies included both adult and child participants, two involved adults only, while the remaining study included children only. Among the studies with adult participants, one unimodal algorithm study analyzed and compared ECG and PPG wearable devices for seizure detection in patients with temporal lobe epilepsy (TLE).45 The seizure detection algorithm in this study utilized HRV and pulse rate variability extracted from ECG and PPG signals, respectively, and identified an HR increase before performing classification using a SVM classifier. The wearable ECG achieved the highest sensitivity (70%) compared to the hospital ECG and wearable PPG, with a comparable FAR of 2.11/h. The other study with adult participants retrospectively validated a multimodal algorithm combining HRV and HR with ACC and EDA, achieving a sensitivity of 67% and an FP rate of 0.03/h.36
In the study with children, a multimodal seizure detection algorithm based on ECG and ACC signals was evaluated.34 The authors also developed a custom prototype unit to employ the algorithm and allow for real-time seizure detection. The cardiac algorithm, where both HR and HRV were analyzed, was able to detect tonic-clonic and focal seizures without focal to bilateral tonic-clonic features with an overall sensitivity of 72% and FAR or 0.04/h. Interestingly, when ECG and ACC parameters were combined, four seizures were detected faster, but the overall sensitivity did not improve.34 Studies that evaluated HRV and HR-based algorithms in both adults and children (n = 3) were all retrospective studies evaluating unimodal algorithms that achieved a combined sensitivity range of 87%-100%. However, differences in performance between adults and children were not reported.37-39
3.2 Studies on seizure prediction
Characteristics and wearable devices of studies on seizure prediction are listed in Table 3, and information on their algorithms is summarized in Table 4. Three studies employed multimodal seizure prediction algorithms and conducted retrospective validation.24, 47, 48 The first retrospective study evaluated a multimodal algorithm combining EDA, HR, and skin temperature collected with the wrist-worn Empatica E4 in three patients with refractory epilepsy. A naïve Bayes classifier was trained on a set of sample data and then evaluated using five-fold cross-validation for preictal and interictal classification during wakefulness, achieving a sensitivity of 78% and a specificity of 80%.47 The second retrospective study also used Empatica E4 to acquire ACC, EDA, PPG, and temperature data in adults and children with epilepsy. Out of 69 patients included in the analysis, seizure forecasting was significantly better than chance for 43.5%, of which achieved a mean improvement of chance (IoC) of 28.5 ± 2.6%, a mean sensitivity of 75.6 ± 3.8%, and a mean percentage of time spent in warning (TiW) of 47.2 ± 3.4% (mean ± SEM). The same study also assessed the effect of reducing the training dataset on seizure forecasting algorithm performance and reported improvements in IoC with larger datasets.24 Another retrospective study utilized a smartwatch to acquire PPG, sleep, and step count data, and a smartphone seizure diary app to monitor seizures in adults with refractory epilepsy. HR, HR cycles, and HRV features were extracted from the PPG signal for the algorithm to forecast periods of high and low risk of seizures. The hourly forecasts achieved a median accuracy of 86%, and the average time spent in high-risk (prediction time) prior to a seizure onset was 37 minutes. Meanwhile, the daily forecast achieved 83% median accuracy, and the average prediction time before a seizure was 3 days.48
| Studies included (Author, year) | Study characteristics | Wearable devices | |||||
|---|---|---|---|---|---|---|---|
| Study population | Adults/children | Study setting | Reference standard | Wearable device to collect cardiac data | Location of device | Physiological signal(s) collected | |
| Stirling et al 202148 | Adults (18 y and over) with a confirmed epilepsy diagnosis | Adults | Outpatient | Seizure events manually reported in a Smartphone diary app | FitBit | Wrist | PPG, sleep, step counts |
| Meisel et al 202024 | Patients (2-22 years old) with epilepsy admitted to the long-term video-EEG monitoring | Adults and children | Inpatient | Video-EEG | Empatica E4 | Wrist | ACC, EDA, PPG, TEMP |
| Yamakawa et al 202049 | Patients with refractory epilepsy admitted and underwent clinical video-EEG monitoring for presurgical evaluation or seizure assessment | Adults and children | Inpatient | Video-EEG | Custom wearable ECG device | Chest | ECG |
| Al-Bakri et al 201847 | Patients admitted for invasive presurgical evaluation at the University of Kentucky Medical Center | N/A | Inpatient | Video-EEG and iEEG | Empatica E4 | Wrist | EDA, PPG, TEMP |
- Abbreviations: ACC, accelerometry, ECG, electrocardiogram, EDA, electrodermal activity, EEG, electroencephalogram, iEEG, intracranial electroencephalogram, N/A, information not available, PPG, photoplethysmography, TEMP, temperature.
| Studies included (Author, year) | Type of validation | Total participants recruited | No. of patients analyzed | Adults/children | Modality | Cardiac parameter used for algorithm | Seizure type | Results of algorithm performance |
|---|---|---|---|---|---|---|---|---|
| Stirling et al 202148 | Retrospective | 39 | 11 | Adults | Multimodal | HR cycles, HRV, HR | N/A |
Median accuracy (hourly forecast): 86% Median accuracy (daily forecast): 83% |
| Meisel et al 202024 | Retrospective | 317 | 69 | Adults and children | Multimodal | BVP | Primary and secondary generalized, and focal seizures |
Mean IoC = 28.5 ± 2.6% Mean sensitivity = 75.6 ± 3.8% Mean TiW (ie, the percentage of time spent in warning) = 47.2 ± 3.4% (mean ± SEM) |
| Yamakawa et al 202049 | Prospective | 14 | 7 | Adults and children | Unimodal | HRV | FIAS, FBTCS, FAS |
Sensitivity: 85.7% FP rate: 0.62/h |
| Al-Bakri et al 201847 | Retrospective | 3 | 3 | N/A | Multimodal | HR | N/A |
Sensitivity = 78% Specificity = 80% Cohen's kappa = 55% |
- Abbreviations: BVP, blood volume pulse, FAS, focal aware seizures, FBTCS, focal to bilateral tonic–clonic seizures, FIAS, focal impaired awareness seizures, FP, false positive, FPAR, false positive alarm rate, HR, heart rate, HRV, heart rate variability, IoC, improvement over chance, N/A, information not available, SEM, standard error of mean, TiW, time in warning.
Only one study prospectively validated their seizure prediction algorithm and extracted both time-domain and frequency-domain HRV features.49 A custom wearable ECG device for seizure prediction was developed in this study, which consists of an RR interval telemeter connected to a custom smartphone app via Bluetooth connection. The smartphone app is able to receive and analyze RRI, which is used to extract HRV features. A machine learning tool known as multivariate statistical process control for seizure prediction was employed, where a successful prediction was defined as a seizure identified between 15 minutes and immediately before seizure onset. The custom seizure prediction system demonstrated the ability to predict focal impaired awareness seizures, focal to bilateral tonic-clonic seizures, and focal aware seizures in both adults and children (sensitivity: 85.75%, FAR: 0.62/h).49
3.3 Quality assessment
The studies included in this review are of mixed quality (Table 5). Some studies did not provide a clear description of the recruitment process or reference standard; therefore, it was difficult to assess the limitations and quality of these studies. Although a majority of the studies used EEG or video-EEG as the reference standard (n = 19), only five studies28, 37, 39, 44, 45 explicitly reported blinded annotation of seizures. It was unclear in the remaining studies whether the reference standard was reviewed without knowledge of the cardiac data. In the flow and timing domain, studies mostly had a low risk of bias as the data from the reference standard was collected concurrently with the cardiac data, and all patients received the same reference standard. Due to the heterogeneity in study design and algorithm performance indicators reported in the studies, we could not conduct a meta-analysis in this present study.
| Studies included (Author, year) | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Seizure detection | |||||||
| Ali and Alam 202027 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| Arends et al 201828 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| Bottcher et al 202229 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| Bruno et al 202130 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| Cogan et al 201532 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| Cogan et al 201731 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| Forooghifar et al 201933 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| Hegarty-Craver et al 202134 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| Henze et al 202135 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| Jahanbekam et al 202136 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| Jeppesen et al 201738 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| Jeppesen et al 201939 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| Jeppesen et al 202037 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| Lazeron et al 202240 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| Masse et al 201341 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| Munch Nielsen et al 202242 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| Tang et al 202143 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| van Andel et al 201744 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| Vandecasteele et al 201745 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| Zsom et al 201946 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| Seizure prediction | |||||||
| Al-Bakri et al 201847 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| Meisel et al 202024 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| Stirling et al 202148 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
| Yamakawa et al 202049 | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ | ♦ |
- ♦ = High risk of bias; ♦ = Unclear risk of bias; ♦ = Low risk of bias.
Most of the studies are categorized as phase 1 (n = 19) according to the proposed standards by Beniczky and Ryvlin (Table 6). Three studies are categorized as phase 2,28, 37, 39 while the remaining two studies are phase 0.30, 48 Although all studies used a dedicated device and a majority used video recording or video-EEG (n = 22) as the reference standard, some studies could not be classified as phase 2 due to an inadequate number of patients or because the safety of the device was not addressed. Thirteen studies trained and tested their algorithms on the dataset, and 10 studies used predefined algorithms and cutoff values. Only four studies evaluated their algorithms in real time.28, 40, 41, 49
| Studies included (Author, year) | Study phasea | Subjects | Recordings | Analysis and alarms | Reference standard | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Simulation/healthy subjects | No. of pts with seizures | No. of seizures | Conventional methods | Dedicated device | Continuous | MultiCentre | Offline/Retrospective | Training & testing using the dataset | Predefined algorithm and cutoff values | Real time | Blinded | Video or video-EEG recordings | Information from pt and care-givers | ||
| Seizure detection | |||||||||||||||
| Ali and Alam 202027 | 1 | + | 1-10 | 15-30 | − | + | − | − | + | − | + | − | − | − | − |
| Arends et al 201828 | 2 | − | 20-50 | ≥75 | − | + | − | + | − | − | + | + | + | + | − |
| Bottcher et al 202229 | 1 | − | 1-10 | 15-30 | − | + | + | + | + | + | − | − | − | + | − |
| Bruno et al 202130 | 0 | − | 10-20 | 30-75 | + | + | + | + | − | − | − | − | − | + | − |
| Cogan et al 201532 | 1 | − | 1-10 | 1-15 | − | + | + | − | + | + | − | − | − | + | − |
| Cogan et al 201731 | 1 | − | 1-10 | 1-15 | − | + | + | − | + | + | − | − | − | + | − |
| Forooghifar et al 201933 | 1 | − | 10-20 | ≥75 | + | + | + | − | + | + | − | − | − | + | − |
| Hegarty-Craver et al 202134 | 1 | − | 10-20 | 15-30 | − | + | + | − | + | − | + | − | − | + | − |
| Henze et al 202135 | 1 | − | 10-20 | 15-30 | − | + | + | − | + | + | − | − | − | + | − |
| Jahanbekam et al 202136 | 1 | − | 20-50 | 30-75 | − | + | + | − | + | + | − | − | − | + | − |
| Jeppesen et al 201738 | 1 | − | 10-20 | − | + | + | − | + | + | − | − | − | + | − | |
| Jeppesen et al 201939 | 2 | − | 20-50 | ≥75 | − | + | + | + | + | − | + | − | + | + | − |
| Jeppesen et al 202037 | 2 | − | 10-20 | 30-75 | − | + | + | + | + | − | + | − | + | + | − |
| Lazeron et al 202240 | 1 | − | 20-50 | ≥75 | − | + | − | + | − | − | + | + | − | + | + |
| Masse et al 201341 | 1 | + | 1-10 | 15-30 | − | + | − | − | + | − | + | + | − | + | − |
| Munch Nielsen et al 202242 | 1 | − | 1-10 | 30-75 | − | + | + | − | + | + | − | − | − | + | − |
| Tang et al 202143 | 1 | − | ≥50 | ≥75 | − | + | + | − | + | + | − | − | − | + | − |
| van Andel et al 201744 | 1 | − | 20-50 | ≥75 | − | + | + | + | + | − | + | − | + | + | − |
| Vandecasteele et al 201745 | 1 | − | 10-20 | 30-75 | + | + | + | − | + | − | + | − | + | + | − |
| Zsom et al 201946 | 1 | − | 10-20 | 30-75 | − | + | + | − | + | + | − | − | − | + | − |
| Seizure prediction | |||||||||||||||
| Al-Bakri et al 201847 | 1 | − | 1-10 | N/A | + | + | + | − | + | + | − | − | − | + | − |
| Meisel et al 202024 | 1 | − | ≥50 | ≥75 | − | + | + | − | + | + | − | − | − | + | − |
| Stirling et al 202148 | 0 | − | 10-20 | ≥75 | − | + | + | − | + | + | − | − | − | − | + |
| Yamakawa et al 202049 | 1 | + | 1-10 | 1-15 | + | + | + | + | − | − | + | + | − | + | − |
- Abbreviations: N/A, information not available; No., number; pt, patient.
- a Studies are categorized into the following phases: Phase 0: initial studies for starting or developing a novel method. Phase 1: proof-of-principle studies. Phase 2: studies using dedicated seizure detection device with safety of the device addressed. Phase 3: studies on final confirmation of safety and accuracy. Phase 4: in-field studies of seizure detection devices patients home environments, addressing usability aspects.
4 DISCUSSION
The findings from this systematic review highlighted both the promise and challenges of the feasibility of cardiac-based seizure detection and prediction using non-invasive wearable devices. There is a clear feasibility with utilizing cardiac-based algorithms in non-invasive wearable devices for seizure detection, especially in adult populations of epilepsy with generally good cardiovascular health; however, the feasibility of them for seizure prediction, whether in adults or children, may be too soon to conclude given the lack of data/clinical studies on their real-world prospective usage. Moreover, the reliability, validity, and sensitivity of these devices when taking into account the effect of cardiovascular abnormalities, such as a history of bradycardia or tachycardia, among people with epilepsy have not been investigated. The findings were based on articles that were either in phase 1 (proof-of-principle) or phase 2 (safety of device addressed) of their study, with none being in phase 3 (confirmation of safety and accuracy) or phase 4 (in-field, usability aspects), suggesting that the feasibility of these cardiac-based seizure detection and prediction devices is still in the early stages of development and validation, mainly in a controlled environment. Real-world effectiveness accounting for patient clinical heterogeneity in not only seizure development but also general health, as well as patient usability in their daily lives, still requires further research. Since the risk of bias was found to be mostly unclear for a number of the studies due to a lack of information in the reference standards used, the conclusions from these studies could be potentially biased. Although a dedicated device was used in all studies, most of them lack an assessment of device safety and even have low sample sizes, contributing to a lack of feasibility information and data interpretation biasness. Nevertheless, findings from these studies may still contribute as an important stepping stone toward the epilepsy diagnostic and possibly therapeutic avenue upon further validation.
Some of the studies have trained and validated their own machine learning algorithms to detect changes in physiological signals that indicate seizure events,43 while others have also used pre-trained algorithms. It is unclear at this point in time whether there are any confounding variables to be considered with either one style of algorithm but a recent paper stated that while pre-trained models may speed up optimization of the algorithm, they may have biased notions from previous machine learning training that will lead to inaccuracies in current data interpretation.50 Moreover, multiple different machine learning techniques have been employed for seizure detection and prediction across the studies, resulting in diverse performance results, thereby creating a more convoluted conclusion. However, the diversity in machine learning techniques used may bring to light the limitations and advantages of each technique, thereby providing future studies with a better idea of which technique would be best suited for further testing.
In addition, diverse algorithmic performance was also reported when comparing seizure detection between adults and children. Among studies that use HR as an input for the seizure detection algorithm, a generally higher FAR was observed in children. However, one prospective study involving children only achieved an improvement in FAR by using an adapted algorithm.40 In studies that used HRV and HR, higher detection sensitivity was reported among children compared to the studies involving adults, possibly due to the utilization of a patient-dependent algorithm in the study with children. It is difficult to compare the performance between adults and children among the studies that reported on both, as they did not provide a clear difference between the two age groups. Resting HR was found to increase with age51 and a pre-ictal decrease in HR has been reported exclusively in studies on pediatric population,18 therefore, the large variation in HR among different age groups may contribute to diverse performance results. While training and testing algorithms on one specific age group at a time could potentially improve the detection or prediction performance of the cardiac-based devices, it should also be noted that adults and children have distinct seizure profiles and requirements and therefore should always be treated as separate subject groups in future studies, unlike some studies reviewed in this manuscript.24, 29, 30, 43, 44, 49
In regards to the cardiac parameters utilized, it was found that HR was most commonly extracted and analyzed for seizure detection, with a few studies reporting the use of HRV or the combination of HRV and HR. There is a high incidence of pre-ictal HR increase more specifically in studies involving TLE patients, adults, or patients receiving antiseizure medications (ASM).18 However, there may be limitations, as not all seizures have changes in HR52 and may be prone to fluctuations contributed by medication, stress, age, sleep quality, and exercise.53 Some studies have provided a possible solution to this by using a multimodal algorithm and comparing it with a unimodal algorithm,24, 30, 44 and others have also asked patients to perform an exercise or stress test to sample real-life situations.37, 39 Another meaningful cardiac measurement is the HRV, and studies that used this parameter as a basis for seizure detection achieved a slightly higher sensitivity compared to those using HR only. HRV is regulated by the balance between the sympathetic and parasympathetic nervous systems; therefore, changes in HRV serve as an indicator of the ANS function. Studies investigating the correlation between interictal HRV and epileptic seizures reported a lower HRV, suggesting an imbalance that shifts more toward sympathetic activity.54 This is in line with another study, which also reported an increase in peri-ictal (pre-ictal and post-ictal) sympathetic activity in generalized tonic-clonic seizures.55 Taken together, it is clear that both HR and HRV cardiac parameters should be included in the device algorithm to improve the sensitivity and specificity of seizure detection and prediction, which could also greatly improve with the addition of other physiological parameters such as skin temperature and electrodermal activity. The combination of cardiac and other physiological parameters may also reduce the chances of false alarms, which will encourage greater patient compliance and usability.
Unfortunately, detection of seizures was more frequently assessed and reported among the studies compared to seizure prediction. Since the latter is more aimed at notifying patients and caregivers of an imminent seizure,56 it is imperative to accurately determine pre-ictal periods when training the algorithm.24 Accurate prediction of seizures will allow patients and caregivers to take the necessary precautions (medications or safe space) prior to their seizures, thus eliminating possible scenarios that may reduce their quality of life. Among the seizure prediction studies, there were variations in the performance metrics reported, maybe due to the different forms of prediction evaluated. Some studies provided a binary prediction (yes or no), while others forecast periods of high or low seizure likelihood, which could present more benefits as it allows users to plan activities and manage treatment according to the different periods of seizure likelihood.11, 12 At present, it is difficult to compare prediction performance between age groups as we did not find any studies that evaluated seizure prediction exclusively in pediatric patients. The studies included either adults only or both adults and children. In the studies that included both adults and children, they did not provide separate performance metrics for either population. Similar to seizure detection studies, multimodal algorithms or patient-specific algorithms may help to improve prediction rates, especially since there is no one-size-fits-all approach to developing a seizure prediction algorithm.57 Billeci et al58 developed a patient-specific algorithm for seizure prediction and found that optimal performance was achieved in patients with more conventional seizures. A considerable number of studies have also used multiple modalities, combining cardiac parameters with other physiological data in an effort to improve algorithm performance. Nevertheless, one or two modalities could be sufficient, depending on the type of seizures or the presence of ictal tachychardia.42
Wearable technology has made a remarkable impact in healthcare by allowing non-invasive monitoring of patients' health status and providing easier access to information for physicians. The studies included in the analysis have used a wide range of wearable devices, collecting multiple physiological signals that are utilized for seizure detection or prediction algorithms. Most studies used devices that are currently available in the market, and studies that have developed custom wearable devices are currently at the prototype stage and have reported preliminary data. Further clinical testing, particularly on validity and reliability, as well as an evaluation of user acceptance, may still be needed. For instance, a study investigating signal quality in wearable devices used for epilepsy management and monitoring has evaluated the patient experience and revealed their significant preference for using wrist-worn devices.59 Despite the ease and convenience associated with wearable devices, motion artifacts caused by normal daily activities should also be taken into consideration. Signal quality may also differ between individuals due to device or battery failures, consequently resulting in a lack of usable data for analysis. Yamakawa and colleagues have suggested that an ECG that can be worn like clothing may be an option to improve signal and reduce motion artifacts.49 Nevertheless, most of the currently available studies were conducted in an inpatient setting, where data from wearable sensors was collected either prospectively or from an existing dataset, and algorithms were validated retrospectively. Hence, the real-world factors such as motion artifacts and battery failures (loss of signal) that could influence the sensitivity of the seizure prediction still lack clarity.
This systematic review is limited by the lack of statistical analysis or meta-analysis to objectively compare the different cardiac parameters used in the seizure detection and prediction algorithms. This is due to the large heterogeneity in study design, setting, and population among the included studies, representing a challenge that may be overcome in the future by following guidelines for conducting and reporting seizure detection or prediction studies.26, 60, 61 Developing studies using these guidelines will ensure that studies are comparable and data can be shared across different seizure detection or prediction research groups, subsequently improving the quality of evidence.
Based on current advancements in technology and digital health, there is a possibility that patient-specific algorithms with an integration of multiple physiological parameters that enhance the accuracy and reliability of cardiac-based seizure detection and prediction devices may be available in the near future. Indeed, real-time validation is first required, especially for the seizure prediction device, to ensure patient compliance and acceptance do not confound the validity and reliability of the seizure prediction. Moreover, with real-time clinical studies, preferably long-term studies, the safety, cost-effectiveness, logistics, and practical utility of the devices can be assessed as well. Large-scale and long-term patient data are required to develop and refine patient-specific algorithms. In addition, prospective validation of the algorithms in a real-world setting and assessment of signal quality would also be useful, taking into account any artifacts and noise that could be contributed by normal daily activities. A recent systematic review discovered that performance, design, comfort, and cost are crucial factors that determine the acceptance of wearable devices in real-world settings although this was not specific to seizure detection or prediction.62 Additionally, people with epilepsy highly prefer non-stigmatizing devices that can be seamlessly integrated into their daily lives thereby justifying the need for real-world usability studies for these cardiac-based seizure detection and prediction devices.61
Once validated, the cardiac-based seizure device, particularly the seizure prediction device, will be a game-changer in epilepsy management, as treatment against seizures can be utilized more efficiently in a proactive manner than the current reactive seizure management strategies, thereby ensuring timely prevention of seizures and reducing the occurrence of drug adverse effects and resistance caused by overloading of current ASMs. In fact, by utilizing the cardiac-based seizure detection and prediction device, treatment against seizures could also become more automated, leaving children with epilepsy to be more independent in managing their condition and adults to have better adherence to their treatment plan. Thus, successful implementation of these cardiac-based tools into clinical practice may improve current methods of epilepsy management, possibly preventing seizures before their manifestation, thereby ensuring the preservation of quality of life among people with epilepsy.
5 CONCLUSION
Altogether, the studies analyzed in this systematic review have collectively demonstrated the feasibility of utilizing cardiac parameters as a tool for seizure detection or prediction. The integration of machine learning tools and non-invasive wearable devices signifies a promising advancement in epilepsy care and management. However, future research should focus on refining the detection or prediction performance and providing stronger evidence with more large-scale, multicenter studies conducted in an outpatient, real-life setting. Evaluation of user experience and feedback would be equally important to provide more insight into the clinical value of seizure detection or prediction using non-invasive wearable devices.
AUTHOR CONTRIBUTIONS
All authors have contributed to the preparation of this manuscript. EAS performed the literature search, critical analysis of the articles, and drafted the manuscript; HHMY and MFS performed the literature screening and selection; IWN performed the quality analysis of the literature; JW, JX, AA, CSK, AK, and MFS conceptualized, reviewed, edited, and approved the final manuscript.
ACKNOWLEDGMENTS
Open access publishing facilitated by Monash University, as part of the Wiley - Monash University agreement via the Council of Australian University Librarians.
FUNDING INFORMATION
This project is funded by Monash University Malaysia NEED (NEtwork for Equity through Digital Health) Grant Scheme 2020 (MED/NEED/11-2020/002). EAS, IWN and HHMY are supported by Monash University Malaysia Graduate Research Excellence Scholarship.
CONFLICT OF INTEREST STATEMENT
None of the authors have any conflict of interest to disclose.
ETHICS STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.