Leukotriene antagonists reduce epileptic seizures-related hospitalization in older adult populations with allergic rhinitis or asthma: A population-based cohort study using the Shizuoka Kokuho database: The Shizuoka study
Abstract
Objective
Managing the risk of epileptic seizures in older adults is increasingly important as the population ages. Leukotriene receptor antagonists (LTRAs) are commonly used to treat asthma or allergic rhinitis. Preclinical studies suggest that LTRAs have antiepileptic effects; however, few population-based etiological studies on this topic have been available. Our study explored whether LTRAs reduce hospitalization risk associated with epileptic seizures in older individuals with asthma or allergic rhinitis.
Methods
We conducted a new-user design analysis using the Shizuoka Kokuho database. We included all individuals aged 60-89 years who had at least one episode of allergic rhinitis or asthma during the study period. We compared individuals who newly started LTRAs with those who did not take LTRAs. Propensity score matching was used to balance the baseline characteristics of the participants. We compared the hazard ratios for seizure-related hospitalization between new LTRA users and non-users and performed subgroup analyses.
Results
Our matched cohorts consisted of 64 724 new users and non-users of LTRAs who were aged 60-89 years and had asthma or allergic rhinitis. During the observation period, 377 (0.58%) and 595 (0.92%) incidents were observed in the LTRA new-user and non-user groups, respectively. The hazard ratio for seizure-related hospitalization was 0.75 (95% confidence interval [CI]: 0.62-0.92) in the LTRA new-user group compared with the non-user group. Subgroup analysis revealed that the hazard ratio was weak in diabetic patients (1.31; 95% CI: 0.72-2.38).
Significance
This study indicated that LTRAs reduced seizure-related hospitalization in older adult patients with allergic rhinitis or asthma. We could not evaluate the severity and related diseases of epileptic seizures during LTRAs. Further studies, including observational studies, detailed multicenter prospective studies, and clinical trials, are needed to validate these findings.
Plain Language Summary
This study examined if leukotriene receptor antagonists (LTRAs), commonly used for asthma or allergies, could lower seizure risk in older adults. Analyzing health records of 60-89 year-olds with asthma or allergies, we found a reduced rate of seizure-related hospitalizations in those starting LTRAs, though this was not as evident in diabetic patients. Our results suggest potential benefits of LTRAs in preventing seizures in older adults with respiratory issues, but further research is needed to confirm these findings.
Key points
- The study targets older patients with allergic rhinitis or asthma to assess the potential effects of leukotriene receptor antagonists (LTRAs).
- The aim is to evaluate the impact of LTRAs on epileptic seizure-related hospitalization in older adult populations with allergic rhinitis or asthma.
- LTRAs are associated with a decreased risk of seizure-related hospitalization, especially in patients without diabetes.
- Detailed studies and trials are necessary to fully assess the effects of LTRAs on epileptic seizures.
1 INTRODUCTION
Epileptic seizures are paroxysmal episodes characterized by transient motor, sensory, mental, autonomic, and visual signs and symptoms resulting from abnormal synchronous neuronal electrical activity propagating through one or more brain circuits, including cortical and subcortical structures.1
Older adults are at a higher risk of developing epileptic seizures than individuals in other age groups.2 According to United States Medicare administrative claims data, the incidence of epileptic seizures in older adults is 2.4 per 1000 person-years.3, 4 As the population continues to age in many countries including Japan, the number of seizures and associated hospitalizations among older adults is expected to increase.2 Therefore, research aimed at reducing the risk of epileptic seizures that are associated with hospitalization in older adults is essential.
Most epileptic seizures are currently treated with pharmacotherapy. Other known treatments include surgery, neuromodulation therapy, and the ketogenic diet.5 Leukotriene receptor antagonists (LTRAs) have not been considered antiseizure medications (ASMs).
Preclinical studies using animal models have previously suggested that LTRAs may exert an antiepileptic effect by suppressing inflammation in many diseases, including those of the central nervous system.6 However, few cohort studies have examined the causal relationship between LTRAs and the development of epileptic seizures. In a single-arm, open-label study of patients, the LTRA pranlukast suppressed seizures in two-thirds of patients and increased attacks in one-third of patients.7 A disruption of the immune system is reportedly involved in the development of epilepsy. LTRAs may act on the immune system to potentiate the effects of ASMs.8 Furthermore, except for neuropsychiatric side effects, the long-term use of LTRAs has a well-established safety profile.9 Given the confirmed antiepileptic effect of LTRAs, these drugs may be essential in controlling epileptic seizures and suppressing seizure severity.
This background led to the hypothesis that taking LTRAs may reduce the risk of epileptic seizure-related hospitalization. Our study aimed to test this hypothesis in a large population-based cohort study using the Japanese Shizuoka Kokuho database (SKDB).
2 METHODS
2.1 Setting and data source
Located in the country's center, Shizuoka Prefecture is representative of Japan because it includes characteristics of nature, climate, industry, economy, and culture that are typical to the country. The SKDB includes information on a regional population-based longitudinal cohort of 2 398 393 Japanese individuals (women, n = 1 303 667, 54.4%) living in Shizuoka Prefecture (population: approximately 3.6 million)10 and has been used as a data source for several studies.11-13 Comprehensive personally linked data were collected and all individuals were uniquely identified. The dataset includes basic information from the subscriber list (sex, age, zip code, observation period, and reason for disenrollment, including death). The database includes claims data from public health insurance organizations (National Health Insurance [NHI] for individuals <75 years of age and the late-stage elderly medical care system [LSEMCS] for those ≥75 years of age). The SKDB is a suitable database for studies because it contains accurate information on deaths and loss to follow-up from Japan's Basic Resident Registration Network System.
2.2 Study population and new-user design
The study used the SKDB. The study period was from 1 April 2012 to 30 September 2019 (Figure 1). All participants were subscribers aged ≥60 years enrolled in the NHI scheme or the LSEMCS during the study period. The cohort entry date was defined as the registration date with one of these health insurance agencies or 1 April 2012—whichever occurred later. The study population was patients aged 60-89 years with asthma or allergic rhinitis. The exclusion assessment window was 1 year before the index date. Patients already prescribed LTRAs or ASMs or those diagnosed with brain injury, brain tumors, or epileptic or febrile seizures were excluded (Tables S1 and S2).
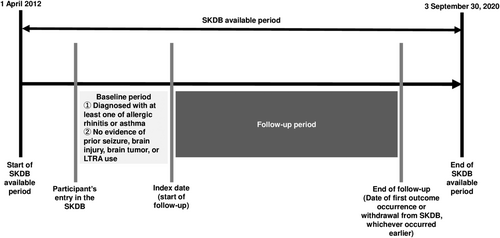
This study used a new-user design to estimate with limited bias the causal effect of LTRA exposure on the incidence of hospital admissions associated with epileptic seizures. For the LTRA-exposed group, the index date was defined as the day LTRAs were first prescribed; for the LTRA-unexposed group, the index date was the cohort entry date. The end date of the follow-up period was defined as the first date of epileptic seizure-related hospitalization, the study end date (30 September 2020), or the withdrawal date from the NHI scheme or the LSEMCS—whichever occurred first.
2.3 Incidence of epileptic seizure-related hospital admissions
The primary outcome was time to hospital admission associated with an epileptic seizure, which was identified using codes G40 and G41 of the 10th revision of the International Statistical Classification of Diseases and Related Health Problems.14 The secondary outcome was the time to occurrence of an epileptic seizure, regardless of hospitalization, using codes G40 and G41 of the classification. To accurately assess these outcomes, patients diagnosed with a brain tumor, brain injuries, or febrile seizures (Table S1) or those who redeemed a prescription for ASM (Table S2) after study entry were censored on the date of the first occurrence to exclude provoked or chronic seizures—as in a previous study.15
2.4 Potential confounders
The covariate assessment window was 1 year before the index date. We considered the following candidate confounders: Charlson and Elixhauzer comorbidities,16 attention-deficit hyperactivity disorder, allergic conjunctivitis, allergic rhinitis, Alzheimer's disease, anaphylaxis, angioneurotic edema, anxiety, asthma, atopic dermatitis, autism, bipolar disorder, cerebral palsy, encephalopathy, head injury, infection, intellectual illness, meningitis, hypoxemia, sleep disorder, substance use disorder, and urticaria. Related confounders were age, sex, season, comorbidities, and use of drugs, including H1 antihistamines, inhaled corticosteroids, topical tacrolimus, xanthine for asthma, systemic steroids, and topical steroids (Tables S1 and S2).
2.5 Statistical analysis
We summarized data as means (interquartile range) for continuous variables and frequencies (percentage) for categorical variables for the groups. We used a multivariable logistic regression model to estimate individual propensity scores (PSs) for LTRA exposure as the outcome variable based on the above-selected potential confounders as covariates. Patients were paired based on their PSs using a one-to-one nearest-neighbor matching method without replacement. A caliper width of 0.2 times the standard deviation of the logit PS was used for this matching process. Standardized mean differences with a cutoff value of 0.10 were then adopted to assess the balance of covariates between the two groups in the overall and the PS-matched populations. Hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) for the risk of epileptic seizure-related hospital admission were estimated using the Cox proportional hazards regression model.
To investigate the dose-response differences in the antiepileptic effect of LTRAs, we conducted a sensitivity analysis in patients with asthma or allergic rhinitis. The analysis was designed to consider the fact that allergic rhinitis patients only take LTRAs during specific seasons, whereas asthma patients regularly take LTRAs regardless of the season. We then performed an analysis similar to the main analysis. To account for bias due to the choice of starting point and the resulting asynchrony, we added the calendar year as a covariate for PS estimation and performed an analysis similar to the main evaluation as a further sensitivity analysis. We also truncated the observation period to 1 year to reduce differences in follow-up periods between LTRA-exposed and unexposed groups and performed an analysis similar to the main analysis.
Supplemental subgroup analyses were performed by age, sex, and the presence and absence of comorbidities, hypertension, diabetes, and renal and liver disease.
We did not impute missing values. All statistical tests were two-sided and we considered CIs not overlapping 1.0 to indicate statistical significance. Because of the exploratory nature of the study, multiplicity adjustment was not made in this study. All statistical analyses were conducted using SAS, version 9.4 (SAS Institute Inc.).
2.6 Ethics
We anonymized data from the SKDB.10 Japanese medical ethics guidelines do not require that informed consent be obtained for this research. The ethics committee of the Shizuoka Graduate University of Public Health approved the study protocol (#SGUPH_2021_001_039).
3 RESULTS
3.1 Study population
Figure 2 shows a flowchart of cohort selection and the construction of the cohorts. The source cohort consisted of 2 398 393 participants. Table S3 reports the unmatched baseline characteristics after we applied the exclusion criteria and exposure definitions. Of the 297 141 asthma or allergic rhinitis patients between 60 and 90 years of age, 99 103 (33.4%) took LTRAs and 198 038 (66.6%) did not.
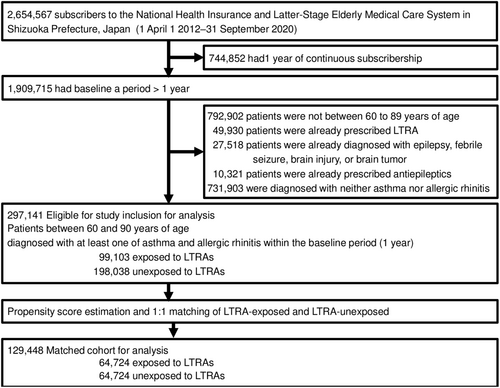
3.2 Patient characteristics in the matched cohort
Following PS estimation and matching, the cohorts in the analyses included 64 724 patients each to allow comparison of the LTRA-exposed vs unexposed groups (matched in a 1:1 ratio). Table 1 includes the baseline characteristics of the matched cohorts for the primary outcome. The baseline characteristics were well balanced between the groups (ie, with less than a 10% standardized mean difference).
| Variable | Category or unit | LTRA user (n = 64 724) | LTRA non-user (n = 64 724) | SMD |
|---|---|---|---|---|
| Demographic statistics | ||||
| Age | 1 year | 73.6 [67.7, 79.0] | 73.2 [66.6, 78.4] | 0.08 |
| 60 to 69 | 23 834.0 (36.8) | 25 121.0 (38.8) | 0.00 | |
| 70 to 79 | 28 564.0 (44.1) | 29 252.0 (45.2) | 0.00 | |
| 80 to 89 | 12 326.0 (19.0) | 10 351.0 (16.0) | 0.00 | |
| Sex | Women | 37 413 (57.8) | 35 913 (55.5) | 0.05 |
| Season | Spring | 29 488 (45.6) | 31 743 (49.0) | |
| Summer | 10 662 (16.5) | 10 327 (16.0) | ||
| Autumn | 11 409 (17.6) | 11 030 (17.0) | ||
| Winter | 13 165 (20.3) | 11 624 (18.0) | ||
| Comorbidities in baseline period | ||||
| ADHD | Presence | 4 (0.0) | 3 (0.0) | 0.00 |
| Allergic conjunctivitis | Presence | 17 480 (27.0) | 18 295 (28.3) | −0.03 |
| Allergic rhinitis | Presence | 50 041 (77.3) | 50 876 (78.6) | −0.03 |
| Alzheimer's disease | Presence | 1801 (2.8) | 1540 (2.4) | 0.03 |
| Anaphylaxis | Presence | 127 (0.2) | 110 (0.2) | 0.01 |
| Angioneurotic edema | Presence | 13 (0.0) | 16 (0.0) | 0.00 |
| Anxiety | Presence | 4542 (7.0) | 4217 (6.5) | 0.02 |
| Asthma | Presence | 29 145 (45.0) | 27 751 (42.9) | 0.04 |
| Atopic dermatitis | Presence | 1649 (2.5) | 1525 (2.4) | 0.01 |
| Autism | Presence | 1 (0.0) | 1 (0.0) | 0.00 |
| Bipolar disorder | Presence | 127 (0.2) | 117 (0.2) | 0.00 |
| Birth-related brain injury or cerebral palsy | Presence | 10 (0.0) | 10 (0.0) | 0.00 |
| Encephalopathy | Presence | 30 (0.0) | 25 (0.0) | 0.00 |
| Head injury | Presence | 3385 (5.2) | 2982 (4.6) | 0.03 |
| Infection | Presence | 25 152 (38.9) | 24 229 (37.4) | 0.03 |
| Intellectual disabilities | Presence | 13 (0.0) | 12 (0.0) | 0.00 |
| Meningitis | Presence | 31 (0.0) | 27 (0.0) | 0.00 |
| Hypoxemia | Presence | 932 (1.4) | 813 (1.3) | 0.02 |
| Sleep disorder | Presence | 18 486 (28.6) | 17 367 (26.8) | 0.04 |
| Substance use disorders | Presence | 13 (0.0) | 10 (0.0) | 0.00 |
| Urticaria | Presence | 3600 (5.6) | 3332 (5.1) | 0.02 |
| Cerebrovascular disease | Presence | 14 303 (22.1) | 13 363 (20.6) | 0.04 |
| Any malignancy | Presence | 7577 (11.7) | 7384 (11.4) | 0.01 |
| Dementia | Presence | 2208 (3.4) | 1901 (2.9) | 0.03 |
| Myocardial infarction | Presence | 1624 (2.5) | 1588 (2.5) | 0.00 |
| Renal disease | Presence | 2769 (4.3) | 2685 (4.1) | 0.01 |
| Rheumatic disease | Presence | 2591 (4.0) | 2419 (3.7) | 0.01 |
| Peptic ulcer disease | Presence | 13 633 (21.1) | 12 833 (19.8) | 0.03 |
| Mild liver disease | Presence | 12 134 (18.7) | 11 585 (17.9) | 0.02 |
| Diabetes without chronic complication | Presence | 1653 (2.6) | 1609 (2.5) | 0.00 |
| Diabetes with chronic complication | Presence | 4418 (6.8) | 4256 (6.6) | 0.01 |
| Moderate or severe liver disease | Presence | 176 (0.3) | 156 (0.2) | 0.01 |
| AIDS/HIV | Presence | 9 (0.0) | 14 (0.0) | −0.01 |
| Congestive heart failure | Presence | 9731 (15.0) | 9077 (14.0) | 0.03 |
| Peripheral vascular disease | Presence | 8494 (13.1) | 8067 (12.5) | 0.02 |
| Chronic pulmonary disease | Presence | 35 919 (55.5) | 34 104 (52.7) | 0.06 |
| Hemiplegia or paraplegia | Presence | 446 (0.7) | 421 (0.7) | 0.00 |
| Metastatic solid tumor | Presence | 824 (1.3) | 785 (1.2) | 0.01 |
| Weight loss | Presence | 295 (0.5) | 272 (0.4) | 0.01 |
| Fluid and electrolyte disorders | Presence | 7749 (12.0) | 7106 (11.0) | 0.03 |
| Deficiency anemia | Presence | 5119 (7.9) | 4883 (7.5) | 0.01 |
| Alcohol abuse | Presence | 361 (0.6) | 347 (0.5) | 0.00 |
| Drug abuse | Presence | 17 (0.0) | 13 (0.0) | 0.00 |
| Depression | Presence | 4205 (6.5) | 3908 (6.0) | 0.02 |
| Psychoses | Presence | 866 (1.3) | 788 (1.2) | 0.01 |
| Cardiac arrhythmias | Presence | 10 442 (16.1) | 9772 (15.1) | 0.03 |
| Valvular disease | Presence | 3735 (5.8) | 3511 (5.4) | 0.02 |
| Pulmonary circulation disorders | Presence | 250 (0.4) | 220 (0.3) | 0.01 |
| Hypertension, uncomplicated | Presence | 38 696 (59.8) | 37 885 (58.5) | 0.03 |
| Hypertension, complicated | Presence | 622 (1.0) | 610 (0.9) | 0.00 |
| Other neurological disorders | Presence | 916 (1.4) | 839 (1.3) | 0.01 |
| Diabetes, uncomplicated | Presence | 1513 (2.3) | 1472 (2.3) | 0.00 |
| Diabetes, complicated | Presence | 4476 (6.9) | 4313 (6.7) | 0.01 |
| Hypothyroidism | Presence | 1890 (2.9) | 1744 (2.7) | 0.01 |
| Renal failure | Presence | 2765 (4.3) | 2679 (4.1) | 0.01 |
| Liver disease | Presence | 12 168 (18.8) | 11 614 (17.9) | 0.02 |
| Peptic ulcer disease excluding bleeding | Presence | 13 358 (20.6) | 12 573 (19.4) | 0.03 |
| Lymphoma | Presence | 400 (0.6) | 369 (0.6) | 0.01 |
| Solid tumor without metastasis | Presence | 7203 (11.1) | 7037 (10.9) | 0.01 |
| Rheumatoid arthritis/collagen vascular diseases | Presence | 3258 (5.0) | 3029 (4.7) | 0.02 |
| Coagulopathy | Presence | 835 (1.3) | 804 (1.2) | 0.00 |
| Obesity | Presence | 308 (0.5) | 283 (0.4) | 0.01 |
| Blood loss anemia | Presence | 120 (0.2) | 117 (0.2) | 0.00 |
| Medications in baseline period | ||||
| Non-sedating antihistamines | Presence | 35 531 (54.9) | 35 978 (55.6) | −0.01 |
| Sedating antihistamines | Presence | 10 653 (16.5) | 10 179 (15.7) | 0.02 |
| Inhaled steroids without beta-stimulant (asthma) | Presence | 2336 (3.6) | 2032 (3.1) | 0.03 |
| Inhaled steroids with beta-stimulant (asthma) | Presence | 9378 (14.5) | 9036 (14.0) | 0.02 |
| Nasal steroids | Presence | 12 966 (20.0) | 12 919 (20.0) | 0.00 |
| Topical steroid skin drugs | Presence | 17 857 (27.6) | 17 108 (26.4) | 0.03 |
| Systemic steroids | Presence | 23 601 (36.5) | 22 471 (34.7) | 0.04 |
| Topical tacrolimus | Presence | 577 (0.9) | 523 (0.8) | 0.01 |
| Xanthine for asthma (theophylline) | Presence | 5200 (8.0) | 4621 (7.1) | 0.03 |
- Abbreviations: ADHD, attention deficit hyperactivity disorder; LTRA, leukotriene receptor antagonist; SMD, standardized mean difference.
3.3 Hospital admission associated with epileptic seizures
Before matching, we compared asthma or allergic rhinitis patients who were exposed to LTRAs with those who were unexposed. Hospital admissions with epileptic seizures occurred in 264 patients who were exposed to LTRAs (0.75 cases per 1000 person-years) compared with 959 unexposed patients (0.90 cases per 1000 person-years); the corresponding HR was 0.86 (95% CI: 0.75-0.99). After matching, hospital admissions for epileptic seizures occurred in 182 patients exposed to LTRAs (0.78/1000 person-years) compared with 308 patients unexposed to LTRAs (1.04/1000 person-years); the HR was 0.75 (95% CI: 0.62-0.92). In the sensitivity analysis, the HR was 0.81 (95% CI: 0.66-1.01) when the calendar year was added as a confounding factor; the HR was 0.65 (95% CI: 0.43-0.96) when the observation period was truncated by 1 year.
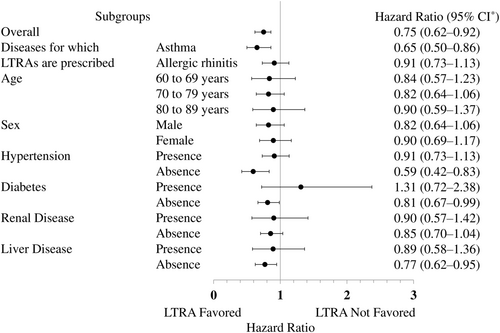
After matching, the HRs in the subgroup analysis were 0.65 (95% CI: 0.50-0.86) in asthma patients with the possibility of relatively high LTRA doses, and 0.91 (95% CI: 0.73-1.13) in allergic rhinitis patients (Figure 3). In addition, HR pointed not to favor LTRAs in patients with diabetes (1.31; 95% CI: 0.72-2.38).
3.4 Epileptic seizures without relationship with hospitalization
Before matching, we compared asthma or allergic rhinitis patients who were exposed to LTRAs vs those who were unexposed. All of the new-onset epileptic seizures occurred in the 1377 patients who were exposed to LTRAs (3.9/1000 person-year) compared with 4467 unexposed patients (4.2/1000 person-years); the HR was 0.95 (95% CI: 0.89-1.01). After matching, all of the new-onset of epileptic seizures occurred in 909 patients exposed to LTRAs (3.9/1000 person-year) compared with 1233 unexposed patients (1233/294 891: 4.2/1000 person-year); the HR was 0.96 (95% CI: 0.88-1.05).
4 DISCUSSION
To the best of our knowledge, this is the first population-based cohort study using a database to report the negative relationship between the exposure of LTRAs and epileptic seizure-related hospitalization. Preclinical studies using animal models have suggested that LTRAs exert an antiepileptic effect by suppressing inflammation in many diseases, including those of the central nervous system.6 Currently, a definitive cure for epilepsy is not available, and safety and affordability are the main challenges of current ASMs. LTRAs which bind to the leukotriene receptor and are used to treat asthma and seasonal allergies, have been identified as a potential alternative treatment for epilepsy. The anti-inflammatory effect of LTRAs helps maintain the integrity of the blood-brain barrier (BBB), and their neuroprotective and antioxidant properties reduce the incidence of epileptic seizures.6
Given the characteristics captured in the SKDB and the fact that epileptic seizures in the elderly are often due to age-related changes, we focused on older adults in this study. Some of the previous studies that investigated the association between allergies or asthma and epilepsy was cross-sectional study in children.17 Furthermore, the only evidence of an association between LTRA prescription and the onset of epileptic seizure-related hospitalization in older adults is from the results of this study. In addition, the mechanism of LTRA's preventive effect on epileptic seizures is still unknown, and age-specific effects cannot be ignored.
In this study, LTRAs significantly reduced epileptic seizure-related hospitalization, but all forms of epileptic seizures were not suppressed by LTRAs. This may be caused by the presence of all form patients including medication for other diseases with names similar to epilepsy; however, this could not be confirmed. Furthermore, we could not completely rule out that hospitalization might be mainly caused by epileptic seizures-unrelated disorders. In a previous single-arm, open-label study in patients with severe epileptic seizures, the LTRA pranlukast suppressed seizures in two-thirds of patients.7
In our study, PS analysis was used to balance confounding factors between the two groups, suggesting that LTRA administration influences hospital admissions associated to epileptic seizures, as evidenced by an HR of 0.75 (95% CI: 0.62-0.92). In a sensitivity analysis in which we considered the year as a confounder, the HR was 0.81 (95% CI: 0.66-1.01), confirming the minimal changes in medical practices, exposures, and population characteristics over time and the consistent directionality of the primary outcome. Moreover, a truncated sensitivity analysis at 1 year yielded an HR of 0.65 (95% CI: 0.43-0.96). This analysis aimed to mitigate the study's potential temporal variations—especially changes in medical practices—and suggests a more pronounced impact of the intervention in the short term. Collectively, these results provide significant insights into how the timing and duration of interventions influence patient outcomes, showcasing the study's high consistency while highlighting potential variations under different conditions and assumptions.
The unique anatomical and physiological interface between the BBB, the central nervous system, and the peripheral circulation is essential to the function of neural circuits. The BBB, cerebral vessels, neurons, and pericytes form a dynamic functional unit known as the neurovascular unit.18 Crosstalk between the neurovascular unit and the BBB is essential in regulating neuronal firing and synaptic plasticity. Age-related blood-brain barrier dysfunction (BBBD) is necessary for status epilepticus superimposition. Typically, BBBD is observed within the first hour of status epilepticus superimposition and is known to persist for months in epileptogenic brain regions. Critical signaling pathways associated with BBBD-induced neurovascular dysfunction include transforming growth factor-beta cytokine-inflammatory paths. Furthermore, we have confirmed that transforming growth factor beta is elevated in the acute phase of cerebral infarction.19 As a blocker of transforming growth factor-beta, the angiotensin antagonist losartan was shown to block epileptogenesis by lowering BBBD. Leukotriene antagonists may similarly contribute to epilepsy suppression related to BBBD.
Studies suggest that seizures are more frequent among people with diabetes. Baviera et al20 observed that diabetes mellitus is an independent risk factor for attacks in older adults and that the risk of seizures increases with comorbidities. Huang et al21 found that diabetes may aggravate epileptic seizures and that severe episodes may be associated with recurrent seizures in patients with diabetes. Sander et al22 found that the prevalence of type 1 diabetes was roughly two times greater in people with epilepsy than in the general population. These findings suggest that seizures are more common and severe among people with diabetes. However, the precise pathogenesis of epileptic attacks in patients with diabetes remains undetermined, according to Yun and Xuefeng23 In this study, LTRAs were found to have no inhibitory effect on epileptic seizures in a subpopulation of patients with diabetes (HR: 1.31; 95% CI: 0.72-2.38). Thus, epileptic seizures and diabetes appear to be deeply linked but the cause of the association is unknown. This study also suggests that diabetic epileptic seizures cannot be prevented by taking LTRAs.
5 LIMITATIONS
This study had several limitations. First, we acknowledge that the study is significantly limited by the lack of validation of epileptic seizure-related hospitalization. Although the claims-based SKDB can have lower specificity for disease diagnosis, the potential misclassification, which could have introduced bias into our findings, must be considered. The database does not include detailed clinical characteristics such as the type, frequency, and duration of seizures, thus raising the potential for both false-positive and false-negative diagnoses and potentially leading to the over- or under-estimation of the reported associations. Furthermore, the lack of validation studies for investigated diseases other than allergic rhinitis or asthma should be considered when interpreting the results of this study. Second, in keeping with the high coverage of older adults in the databases used, the study population in this study was limited to older adults; future studies of age groups other than older adults are desirable. Third, the study did not provide detailed clinical information about the severity of allergic diseases, seizure type, and the frequency and duration of an epileptic seizure; additional studies using data sources that include detailed clinical information are needed to provide details on epileptic seizure-related hospitalization. Fourth, this study was conducted in the general Japanese population; its applicability to other Asian populations or across racial groups is unknown. Fifth, examining the exact dose-response relationship of LTRAs was difficult because this receipt study determined the actual amount of drugs billed but did not specify whether drugs were taken. However, compared with patients with allergic rhinitis, epileptic seizure suppression was higher in patients with asthma who took LTRAs continuously. Therefore, long-term use and higher doses of LTRAs can enhance epileptic seizure suppression.
Sixth, in the Japanese healthcare system, it is common for ICD-10 codes G40-41 to be assigned to cases diagnosed with epileptic seizures. Although febrile convulsions (R56.0) may also be selected as a diagnostic code, this code is typically used in pediatric cases; thus, excluding this code for the older adult population targeted in this study was appropriate. Additionally, valproic acid and lamotrigine are prescribed for epileptic seizures. However, because these drugs are also covered by insurance for bipolar disorder, we did not use prescriptions for these drugs in the outcome definition. Electroencephalogram (EEG) testing is used to differentially diagnose psychogenic non-epileptic seizures and exclude other conditions; however, the mere presence of an EEG test does not necessarily confirm a diagnosis of epileptic seizures. Therefore, we did not use EEG testing for the outcome definition. Incorporating ASMs and EEG testing is expected to increase the positive predictive value but at the cost of reduced sensitivity. Despite these limitations, this study provides new evidence of the association between LTRAs and epileptic seizure-related hospitalization.
The study results suggest that LTRAs augment the effects of ASMs when used in combination with these drugs. However, it remains a matter of future research to evaluate the impact of LTRAs on epileptic seizure suppression in seizure-prone populations other than patients with diabetes. Further similar studies in a wider age range, more detailed multicenter prospective studies, and clinical trials to determine efficacy are required.
6 CONCLUSIONS
This study suggests that LTRAs may reduce the risk of epileptic seizure-related hospitalization in older adult patients with allergic rhinitis or asthma other than those with diabetes. Further studies, including repetitive observations, detailed multicenter prospective studies, and clinical trials, are needed to validate these findings.
AUTHOR CONTRIBUTIONS
YI and EN contributed equally to this work. YI performed statistical analysis. YI and EN interpreted the data. YI wrote the draft of the manuscript. YI, EN, NK, YF, KM and AS contributed to the critical revision of the manuscript. All authors have read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
The drug code search was conducted in a database of the Japan Pharmaceutical Information Center. The Shizuoka Graduate University of Public Health conducts contract research projects for public health in Shizuoka Prefecture and receives funding from Shizuoka Prefecture, including for the current study. We thank Anahid Pinchis from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflict of interest to disclose.
ETHICS STATEMENT
We confirm that we have read the journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
According to Shizuoka Prefecture's data use agreement with local insurers, readers cannot access the analyzed data. Researchers interested in accessing this dataset may apply to Shizuoka Prefecture to request access. Please contact the staff of the Shizuoka Graduate University of Public Health (Email: [email protected]).