Interictal pattern on scalp electroencephalogram predicts excellent surgical outcome of epilepsy caused by focal cortical dysplasia
Abstract
Objective
Focal cortical dysplasia (FCD) represents an essential cause of drug-resistant epilepsy with surgery as an effective treatment option. This study aimed to identify the important predictors of favorable surgical outcomes and the impact of the interictal scalp electroencephalogram (EEG) patterns in predicting postsurgical seizure outcomes.
Methods
We retrospectively evaluated 210 consecutive patients between 2015 and 2019. They were diagnosed with FCD by pathology, underwent resection, and had at least one year of postsurgical follow-up. Predictors of seizure freedom were analyzed.
Results
Based on the information at the latest follow-up, seizure outcome was classified as Engel Class I (seizure-free) in 81.4% and Engel Class II-IV (non-seizure-free) in 18.6% of patients. There were 43, 105, and 62 cases of FCD type I, type II, and type III, respectively. The interictal EEG showed a repetitive discharge pattern (REDP) in 87 (41.4%) patients, polyspike discharge pattern (PDP) in 41 (19.5%), and the coexistence of REDP and PDP in the same location in 32 (15.2%) patients. The analyzed patterns in order of frequency were repetitive discharges lasting 5 seconds or more (32.4%); polyspikes (16.7%); RED type 1 (11.4%); continuous epileptiform discharges occupying >80% of the recording (11.4%); RED type 2 (6.2%); brushes (3.3%); focal, fast, continuous spikes (2.4%); focal fast rhythmic epileptiform discharges (1.43%); and frequent rhythmic bursting epileptiform activity (1.4%). The coexistence of REDP and PDP in the same location on scalp EEG and complete resection of the assumed epileptogenic zone (EZ) was independently associated with favorable postsurgical prognosis.
Significance
Resective epilepsy surgery for intractable epilepsy caused by FCD has favorable outcomes. Interictal scalp EEG patterns were revealed to be predictive of excellent surgical outcomes and may help clinical decision-making and enable better presurgical evaluation.
Key Points
- Resective epilepsy surgery can be an effective treatment option for drug-resistant epilepsy caused by FCD.
- The coexistence of REDP and PDP in the same location on scalp EEG is highly predictive of excellent surgical outcome.
- Careful analysis of interictal scalp EEG can assist clinical decision-making.
- Complete resection is a positive prognostic indicator of seizure freedom.
1 INTRODUCTION
Focal cortical dysplasia (FCD) is the most common type of malformations of cortical development (MCD).1 It represents an important cause of drug-resistant epilepsy in adulthood, especially in children.2 In a study in Germany, researchers found that approximately 17% of patients have transient responsiveness to pharmacotherapy; however, secondary resistance commonly develops after a certain period.3 Despite the availability of new antiepileptic drugs, most patients remain unresponsive to medical treatment.2 Resection can be an effective treatment option, leading to seizure freedom in 55–64% of drug-resistant epilepsy caused by FCD.4-7 The goal of surgery is seizure control by resectioning the epileptogenic tissue while avoiding neurological deficits.8 This emphasizes the importance of seizure outcome prediction and careful selection of surgical candidates in presurgical decision-making.
Studies have been conducted to determine the predictors of postoperative outcomes. Several studies have shown that FCD type II, shorter duration of epilepsy, younger age at surgery, complete resection of the assumed epileptogenic zone (EZ), and unilobar localization were good prognostic factors for surgical outcome.5, 9-12 Moreover, it has been suggested that the presence of focal to bilateral tonic-clonic seizures (FBTCS) and FCD type I were predictors of poor outcome.13
Scalp electroencephalography (EEG) is an indispensable part of the noninvasive presurgical evaluation. The majority of previous studies primarily focused on the correlation between ictal patterns of scalp EEG and the prognosis of surgery.14, 15 It has been previously shown that focal low-voltage fast activity (LVFA) on scalp ictal EEG was positively associated with good surgical outcomes in frontal lobe epilepsy patients.15 Further, data are scarce on interictal scalp EEG patterns with prognostic factors in epilepsy patients with FCD. Some characteristic interictal EEG patterns have been regarded as biomarkers of focal cortical dysplasia.16 Nevertheless, the association between scalp EEG biomarkers and surgical prognosis remains unknown in FCD.
In this study, we reviewed clinical data and scalp EEG data in a cohort of 210 consecutive patients with pathologically confirmed FCD. The study aimed to investigate the postsurgical seizure outcomes in patients with FCD and evaluate the prognostic implications of clinical factors and interictal scalp EEG patterns.
2 METHODS
2.1 Patient selection
We retrospectively analyzed postoperative outcomes for all consecutive patients with a postoperative histological diagnosis of FCD who had undergone resective epilepsy operation at Beijing Tiantan-Fengtai Epilepsy Center between January 2015 and December 2019. We collected demographic, clinical, imaging, EEG, histopathological types of FCD, and follow-up data. For patients who underwent two resections, outcomes after the first surgery were employed to analyze the completeness of resection. We excluded patients with suboptimal EEG recording (e.g., electrodes with poor contact, absence of sufficiently long interictal EEG recordings including at least nonrapid eye movement [NREM] I and NREM II sleep recordings prior to epilepsy surgery). The study was approved by the ethics committee of the Beijing Tiantan Hospital, and informed consent was obtained from all the patients or their families.
2.2 Presurgical evaluation
All patients had a complete presurgical evaluation, including history, neurological examination, brain magnetic resonance imaging (MRI) with 3-T epilepsy protocols, and scalp EEG monitoring. Fluorodeoxyglucose positron emission tomography (FDG-PET) scanning and image postprocessing were performed if necessary. If these noninvasive methods did not yield consistent findings with a single resectable epileptogenic lesion or if the potential epileptogenic zone (EZ) overlapped with the eloquent cortex, then stereo-EEG (SEEG) was performed.
2.3 Interictal and ictal EEG pattern analysis
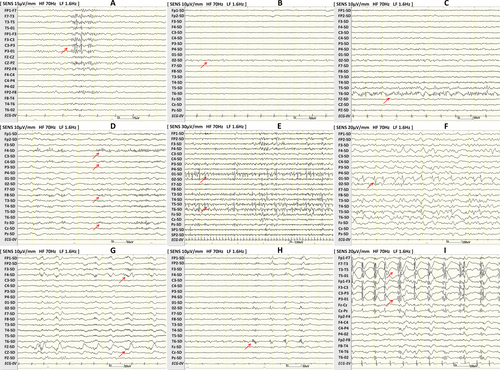
All patients underwent preoperative scalp EEG (EEG-1200; Nihon Kohden Corporation, Tokyo, Japan) according to the International 10–20 system of electrodes. The filters used were 120 Hz and 0.3 Hz. The EEG recordings were continually reviewed in referential and bipolar montages. To avoid the preictal change effects on interictal EEG patterns, the 30-minute segments before the seizure onset were excluded from the analysis. All EEG data of patients were reanalyzed by two trained epileptologists (H.J. Wan and W.H. Hu) who were blinded to the clinical data, and discrepancies were resolved by a senior epileptologist (X.Q. Shao). Interictal and ictal EEG patterns were only considered present when consensus was reached between the two independent reviewers. Scalp EEG data analysis was performed to detect interictal EEG patterns previously reported to be associated with FCD and the ictal EEG onset patterns.16, 17 Epitashvili et al. classified the characteristic interictal scalp EEG patterns of FCD into two broad categories as follows: repetitive discharges and polyspike discharges.16 Six interictal scalp EEG patterns were classified as repetitive discharge pattern (REDP) as follows: trains of repetitive, rhythmic 4- to 10-Hz sharp waves or spikes lasting 1–4 seconds (RED type 1);18 quasicontinuous, slower, rhythmic 2- to 7-Hz sharp wave activity (RED type 2);18, 19 focal, fast, continuous spikes;20 focal fast rhythmic epileptiform discharges (FREDs);21 continuous epileptiform discharges occupying >80% of the recording;22 and repetitive discharges lasting 5 seconds or more.22 Meanwhile, three interictal scalp EEG patterns were classified as polyspike discharge pattern (PDP) as follows: brushes,23 polyspikes of at least three consecutive spikes with a frequency of at least 10 Hz lasting at least 300 ms,22, 24 and frequent rhythmic bursting epileptiform activity (FRBEA)25 (Figure 1).
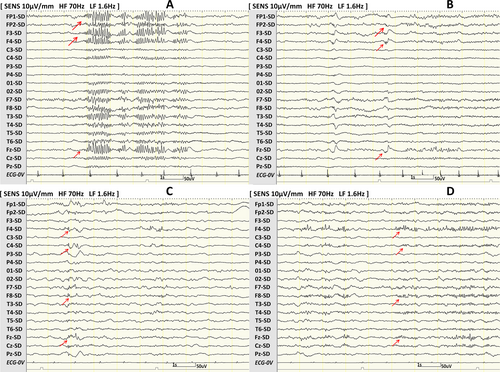
Furthermore, we particularly focused on patients with REDP and PDP in the same location (Figure 2). We counted the number of characteristic interictal scalp EEG patterns simultaneously present in one patient. Due to low numbers, the simultaneous presence of three to five characteristic interictal scalp EEG patterns was merged. Ictal EEG onset patterns were classified into the following three categories: low-voltage fast activity, spikes or sharp waves, and slow waves.17
2.4 Surgery and pathology
The surgical plan was decided based on the available data collected during the presurgical assessment. Complete resection of the assumed EZ was defined by resection of the visible lesion on MRI and the ictal onset zone that was confirmed during presurgical evaluation.7 Pathological cortical dysplasia was diagnosed based on the International League Against Epilepsy classification by an expert neuropathologist.26
2.5 Postsurgical evaluation and outcome definitions
All patients underwent a postoperative 3.0T MRI or 1-mm CT scan. The completeness of resection was defined by the resection of all the planned EZ on presurgical evaluation. Given the impact of the distance between the epileptic generator and recording electrodes on EEG patterns, patients were classified as having a lesion mainly involving the lateral (areas of the lateral frontal, temporal, parietal, or occipital lobes, excluding the basal or mesial areas) or medial cortex (areas of the mesial frontal, temporal, parietal, occipital lobes, or insula) according to the surgical resection sites. All patients were followed up until June 30, 2021, with a minimum follow-up of one year. Postsurgical seizure outcomes were determined at the latest available follow-up in the outpatient department or by telephone interview, according to Engel’s classification scheme.27 We defined a favorable outcome as class I (seizure-free) and an unfavorable outcome as class II or higher (not seizure-free) at the latest available follow-up.
2.6 Statistical analysis
Descriptive statistics were used for each variable. Continuous variables that did not follow a normal distribution were expressed as the median and quartile. Categorical variables were presented as the number of cases and percentages. The data were analyzed using the t-test, Wilcoxon rank-sum, and chi-square tests to compare patients who were seizure-free with those with postoperative recurrence. Two multivariate logistic regression models were conducted.
In multivariable model 1, variables with P < 0.05 in univariable analysis and relevant factors based on previous literature were included. In multivariable model 2, variables in model 1 plus possible confounders (cortex and FCD subtypes) were included. The odds ratios (OR) and their 95% confidence intervals were calculated. Data analysis was performed using SPSS 22.0 (IBM, Armonk, New York), and a P-value <0.05 was considered statistically significant.
3 RESULTS
3.1 Clinical features
Among the 210 patients with FCD, 115 were male and 95 were female. The median age at surgery was 21 years (range: 13-27); the median age at seizure onset was eight years (range: 4-14); and the median duration of epilepsy was ten years (range: 4-16). Histopathology of brain tissue was confirmed to be FCD type I in 43 cases (20.5%), FCD type II in 105 cases (50%), and FCD type III in 62 cases (29.5%). SEEG recording was performed in 121 (57.6%) patients. A family history of epilepsy was present in 10 patients (4.8%). Complete MRI data were available from 209 patients. Of these, 110 (52.6%) patients had a visible lesion on MRI, and 99 patients (47.4%) were considered MRI-negative. The surgical resection site mainly involved the lateral cortex and medial cortex in 181 (86.2%) and 29 (13.8%) patients, respectively. Six patients underwent incomplete resection of the assumed EZ because of overlap with the eloquent cortex. Time to follow-up ranged from 17 to 76 months (median: 49 months). Overall, at the latest available follow-up visit, 171 patients (81.4%) remained seizure-free (Engel Class I), and 39 (18.6%) failed to achieve seizure-free results (Engel Class II-IV) (Table 1).
| Characteristics | N = 210 | Seizure-free (N = 171) | Non-seizure-free (N = 39) | P value |
|---|---|---|---|---|
| Sex (male), N (%) | 115 (54.8 %) | 94 (55%) | 21 (53.9%) | 0.899 |
| Age at surgery, year | 21 (13,27) | 21(12,27) | 24(17,29) | 0.074 |
| Age at surgery, N (%) | ||||
| <18 | 79 (37.6 %) | 68 (39.8 %) | 11 (28.2 %) | 0.179 |
| ≥18 | 131 (62.4 %) | 103 (60.2 %) | 28 (71.8 %) | |
| Age at onset, year | 8 (4,14) | 8 (4,14) | 9 (4,14) | 0.803 |
| Preoperative duration of epilepsy, year | 10(4,16) | 9(4,16) | 12(7,19) | 0.062 |
| Preoperative duration of epilepsy, N (%) | ||||
| <5 | 55 (26.2 %) | 48 (28.1%) | 7 (18 %) | 0.195 |
| ≥5 | 155 (73.8 %) | 123 (71.9 %) | 32 (82.1%) | |
| Side of surgery, left, N (%) | 92(43.8 %) | 78 (45.6 %) | 14 (35.9 %) | 0.270 |
| Presence of aura, N (%) | 105 (50%) | 85 (49.7 %) | 20 (51.3 %) | 0.859 |
| Presence of FBTCS, N (%) | 110 (52.4 %) | 85 (49.7 %) | 20 (64.1 %) | 0.104 |
| Medical history, N (%) | ||||
| Encephalitis | 11 (5.3 %) | 9 (5.33%) | 2 (5.13%) | 0.960 |
| Febrile seizures | 25 (11.9 %) | 20 (11.7 %) | 5 (12.8 %) | 0.846 |
| Cranial trauma | 21 (10 %) | 16 (9.36 %) | 5 (12.8 %) | 0.527 |
| Perinatal injury | 14 (6.7 %) | 13 (7.6 %) | 1 (2.56 %) | 0.208 |
| Family history of epilepsy | 10 (4.8 %) | 8 (4.68 %) | 2 (5.13 %) | 0.906 |
| MRI findings, N (%) | ||||
| Positive | 110 (52.6 %) | 93 (54.7 %) | 17 (43.6 %) | 0.210 |
| Negative | 99 (47.4 %) | 77 (45.3 %) | 22 (56.4%) | |
| FCD subtypes, N (%) | ||||
| I | 43 (20.5 %) | 30 (17.5%) | 13 (33.3%) | 0.075 |
| II | 105(50 %) | 90 (52.6%) | 15 (38.5 %) | |
| III | 62 (29.5 %) | 51 (29.8 %) | 11 (28.2 %) | |
| Cortex location, N (%) | ||||
| Lateral cortex | 181 (86.2 %) | 149 (87.1 %) | 32 (82.1%) | 0.406 |
| Medial cortex | 29 (13.8 %) | 22 (12.9 %) | 7 (18 %) | |
| SEEG, N (%) | 121 (57.6 %) | 98 (57.3 %) | 23 (59 %) | 0.850 |
| Completeness of surgery, N (%) | ||||
| Complete resection | 204 (97.1 %) | 168 (98.3 %) | 36 (92.3 %) | 0.077 |
| Incomplete resection | 6 ( 2.9 %) | 3 (1.7 %) | 3 (7.7 %) | |
- Abbreviations: FBTCS, focal to bilateral tonic-clonic seizures; FCD, focal cortical dysplasia; SEEG, stereoelectroencephalography.
3.2 Interictal and ictal EEG pattern
The interictal EEG showed REDP in 87 (41.4%) patients, PDP in 41 (19.5%), and coexistence of REDP and PDP in the same location in 32 (15.2%) patients. The analyzed separate patterns in order of frequency were repetitive discharges lasting 5 seconds or more (32.4%), polyspikes (16.7%), RED type 1 (11.4%); continuous epileptiform discharges occupying >80% of the recording (11.4%); RED type 2 (6.2%); brushes (3.3%); focal, fast, continuous spikes (2.4%); FREDs (1.43%); and FRBEA (1.4%) (Table 2).
| Characteristics | N = 210 | Seizure-free (N = 171) | Non-seizure-free (N = 39) | P value |
|---|---|---|---|---|
| Interictal EEG pattern, N (%) | ||||
| Repetitive discharges pattern | 87 (41.4 %) | 75 (43.9 %) | 12 (30.8 %) | 0.134 |
| RED type 1 | 24 (11.4 %) | 21 (12.3 %) | 3 (7.7 %) | 0.396 |
| RED type 2 | 13 (6.2 %) | 12 (7 %) | 1 (2.6 %) | 0.252 |
| Focal, fast, continuous spikes | 5 (2.4 %) | 4 (2.3 %) | 1 (2.6 %) | 1.000 |
| FREDs | 3 (1.43 %) | 3 (1.8 %) | 0 (0 %) | 1.000 |
| Continuous epileptiform discharges occupying >80% of the recording | 24 (11.4 %) | 19 (11.1 %) | 5 (12.8 %) | 0.765 |
| Repetitive discharges lasting 5 seconds or more | 68 (32.4 %) | 57 (33.3 %) | 11 (28.2 %) | 0.537 |
| Polyspike discharges pattern | 41 (19.5 %) | 36 (21 %) | 5 (12.8 %) | 0.242 |
| Brushes | 7 (3.3 %) | 6 (3.5 %) | 1 (2.6 %) | 0.760 |
| Polyspikes | 35 (16.7 %) | 30 (17.5 %) | 5 (12.8 %) | 0.475 |
| FRBEA | 3 (1.4 %) | 3 (1.8 %) | 0 (0 %) | 1.000 |
| Number of interictal EEG pattern, N (%) | ||||
| 0 | 110 ( 52.4%) | 86 ( 50.3%) | 24 ( 61.5%) | 0.556 |
| 1 | 45 (21.4 %) | 38 (22.2%) | 7 ( 18.0%) | |
| 2 | 34 ( 16.2%) | 30 (17.5%) | 4 ( 10.3%) | |
| 3-5 | 21 ( 10%) | 17 (9.9%) | 4 ( 10.3%) | |
| Coexistence of REDP and PDP in the same location, N (%) | 32 (15.2 %) | 31 (18.13 %) | 1 (2.56 %) | 0.015 |
| Regional or focal ictal EEG pattern, N (%) | ||||
| LVFA | 33 (15.8 %) | 29 (17 %) | 4 (10.3 %) | 0.325 |
| Spikes or sharp wave | 72 (34.3 %) | 59 (34.5 %) | 13 (33.3 %) | 0.973 |
| Slow waves | 25(12 %) | 21 (12.3 %) | 4(10.3 %) | 0.76 |
- Abbreviations: EEG, electroencephalography; FRBEA, frequent rhythmic bursting epileptiform activity; FREDs, focal fast rhythmic epileptiform discharges; LVFA, focal low-voltage fast activity; PDP, polyspike discharge pattern; RED, repetitive discharge; REDP, repetitive discharge pattern. Differences with P ≤ 0.05 were considered significant. Significant p values are bolded.
Fifty-five patients (26.2%) had several characteristic interictal scalp EEG patterns simultaneously. Among them, 34 (16.2%) had two, 17 (8.1%) had two, 34 (16.2%) had three, three (1.4%) had four, and one (0.5%) had five characteristic interictal scalp EEG patterns. There were 110 (52.4%) patients without characteristic interictal scalp EEG patterns significantly associated with FCD (Table 2). The ictal EEG showed LVFA in 33 (15.8%) patients, spikes or sharp waves in 72 (34.3%), and slow waves in 25 (12%) patients (Table 2).
3.3 Predictors of outcome
On univariate analysis, the incidence of REDP and PDP in the same location showed significant differences between the seizure-free and non-seizure-free groups (P = 0.015), while differences in the other clinical variables were nonsignificant (Table 1 and Table 2). All patients but one with this interictal pattern had a seizure-free outcome.
On multivariate logistic regression models analysis (models 1 and 2), coexisting REDP and PDP in the same location on scalp EEG and the complete resection of the assumed EZ were independently associated with being seizure-free postoperatively (Engel Class I). Other clinical variables of FBTCS, age at surgery, cortex location, and FCD subtypes had no significant association with the postsurgical outcome (Table 3).
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Model 1 | |||
| Coexistence of REDP and PDP in the same location | 0.09 | 0.01-0.787 | 0.03 |
| Presence of FBTCS | 1.749 | 0.808-3.788 | 0.156 |
| Age at surgery | 1.157 | 0.508-2.637 | 0.728 |
| Complete resection | 0.126 | 0.019-0.853 | 0.034 |
| Model 2 | |||
| Coexistence of REDP and PDP in the same location | 0.084 | 0.009-0.752 | 0.027 |
| Presence of FBTCS | 1.804 | 0.819-3.972 | 0.143 |
| Age at surgery | 1.209 | 0.52-2.815 | 0.66 |
| Complete resection | 0.12 | 0.017-0.825 | 0.031 |
| Cortex location | 0.771 | 0.343-1.736 | 0.53 |
| FCD subtypes | 0.646 | 0.388-1.077 | 0.094 |
- Abbreviations: CI, confidence interval; EEG, electroencephalogram; FBTCS, focal to bilateral tonic-clonic seizures; FCD, focal cortical dysplasia; OR, odd ratio; PDP, polyspike discharge patternREDP, repetitive discharge pattern. Differences with P ≤ 0.05 were considered significant. Significant p values are bolded.
3.4 Interictal EEG pattern in either FCD subtype
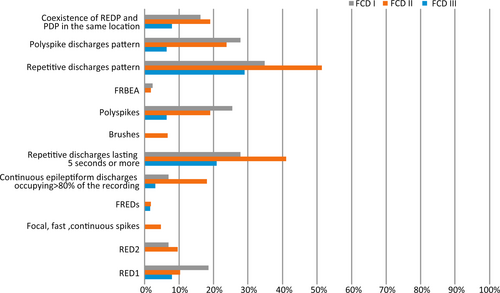
Patients with histologically verified FCD type II were found to have all nine patterns, two broad category patterns (REDP and PDP), and the coexistence of REDP and PDP in the same location. However, focal, fast, continuous spikes, FREDs, and brushes were not observed in patients with histologically verified FCD type I. Furthermore, focal, fast, continuous spikes, FREDs, brushes, and RED type 2 were not observed in patients with histologically verified FCD type III. The incidence of all the characteristic interictal scalp EEG patterns in patients with FCD type III was lower than those with FCD types I and II (Figure 3).
4 DISCUSSION
Past studies on the relationship between noninvasive EEG and postoperative prognosis mostly focused on the ictal onset patterns. To the best of our knowledge, this is the first study regarding the predictive role of interictal scalp EEG patterns in the postsurgical prognosis of epilepsy caused by FCD in a large cohort of patients (n = 210). The main results revealed that resective epilepsy surgery led to seizure freedom in 81.4% of patients with FCD. The strongest predictors of seizure freedom were the coexistence of REDP and PDP in the same location on interictal scalp EEG and the complete resection of the assumed EZ. The results remained statistically significant even after adjustment for confounding factors (i.e., cortex location and FCD subtypes).
4.1 Association between characteristic interictal EEG pattern and favorable postsurgical prognosis
Several scholars have reported the characteristic interictal EEG patterns of FCD.16, 18, 20-22, 24, 25, 28-31 However, the relationship between interictal EEG patterns and the surgical outcome remains unclear. A study revealed no relationship between the presence of brushes and Engel class I surgical outcome,23 consistent with this study’s conclusion. Based on our findings, brushes, other separate interictal EEG patterns, or the two major categories of REDP and PDP could not predict good postoperative outcomes in patients with FCD. Interestingly, the coexistence of REDP and PDP in the same location was observed in only approximately 15.2% of patients with drug-resistant epilepsy caused by FCD in our cohort. Despite the relatively low rate of visual detection of this interictal scalp EEG pattern, the chance of favorable postsurgical seizure control is high (31/32) when the pattern is present. Consequently, in presurgical evaluation, this interictal scalp EEG pattern may identify a subgroup of patients with drug-resistant epilepsy caused by FCD who respond favorably to resective surgery. Additionally, several characteristic interictal scalp EEG patterns were simultaneously present in one patient. However, the number of interictal EEG patterns was not associated with the prognosis of surgery. The incidence of characteristic interictal scalp EEG patterns in our study was lower than that previously reported.16 We speculated that the low incidence was partly due to the heterogeneous in-patient population. The recently published study by Epitashvili et al.16 did not include patients with FCD type III who had a lower incidence of characteristic interictal scalp EEG patterns.
Intrinsic hyperexcitability of the dysplastic cortex has been confirmed repeatedly.32-34 Complicated interactions between neurons and the upregulation of excitatory amino acid receptors and the downregulation of inhibitory receptors are the known mechanisms of epileptogenesis in FCD.35, 36 The mechanisms underlying these interictal electrophysiological patterns are not fully known. We found some patterns with core graph elements and variable duration, repetitions, or frequency, similar to the findings of Epitashvili et al.16 For example, repetitive, rhythmic, and continuous, or quasicontinuous are the core repetitive discharge patterns. The presence of REDs on scalp EEG of patients with FCD is strongly associated with the occurrence of continuous epileptiform discharges (CEDs) on ECoG or SEEG recordings.18, 37 The spontaneous pacemaker GABA receptor-mediated synaptic activity,38 which is characterized by rhythmic synaptic events (usually 5-10 Hz), could represent the cellular substrate of REDs and CEDs on the EEG of FCD cases38 and may contribute to epileptogenesis by facilitating neuron synchrony.39 High frequency and bursting discharges are core features of the polyspike discharge pattern. In addition, the presence of brushes is associated with balloon cells.23 Generally, balloon cells do not contribute to the epileptogenic network.34 However, the high expression of gap junction-forming connexin 43 on astrocytes and balloon cells from FCD IIb may be implicated in the development of the hypersynchronous discharge of neuronal networks, thus enabling rapid propagation of electrical activity.22, 39, 40
Although our results confirmed that the coexistence of REDP and PDP in the same location was independently associated with good surgical prognosis, the mechanism underlying this relationship remains unknown. It can be assumed that the dysplastic areas of patients with REDP and PDP in the same location on scalp EEG may have a more complex epileptogenic network. The dysplastic areas may generate special interictal patterns that may spatially correlate with the anatomic extent of the lesion, hence leading to better surgical results. However, this study did not provide mechanistic data to support these speculations. Nevertheless, these findings would help guide future basic research.
4.2 Complete resection as a positive prognostic indicator of seizure freedom
In our study, the complete resection of the assumed EZ was also a predictive factor for seizure freedom, according to previous reports.5, 9, 11, 12, 41 Overlap of the assumed EZ and eloquent cortex was the leading cause of incomplete resection in our series. Theoretically, a subtotal resection to avoid the risk of postoperative neurological deficit can result in postsurgical seizures because of the intrinsically epileptogenic nature of FCD.32, 33 Three out of the six patients with incomplete resection exhibited early seizure recurrence. Nevertheless, from the first follow-up to their latest available follow-up, three patients remained seizure-free despite incomplete resection of the assumed EZ. Similar phenomena of “unexpectedly successful surgeries” were reported in other studies.9, 29, 42 Krsek et al. speculated that the EZ occupied only a portion of the dysplastic cortex in some FCD cases, which may account for this phenomenon.9 The balloon cells were not involved in the process of generation and propagation of epileptic discharges compared with the dysmorphic cytomegalic and immature neurons in FCD II, which may support the hypothesis.39 Thus, being seizure-free after incomplete resection of the assumed EZ of FCD should be the exception rather than the rule.9 However, our data were not sufficient to confirm these speculations; they only generated interesting hypotheses that can be studied further.
4.3 Relationship between postsurgical prognosis and other clinical variables
LVFA is the common intracranial and scalp EEG pattern on ictal onset among different pathologies, including FCD15, 43, 44. It is typically associated with a good prognosis after surgical resection.43, 44 However, our findings did not confirm the results of a previous study.15 Furthermore, we found that the incidence of LVFA (15.8%) in our study was lower than that in a previous study (25%).15 It is well known that the distance from the seizure onset zone to the surface and motion artifacts during the ictal phase could influence the surface seizure expression. The tissues overlying the brain may attenuate cortical potentials, particularly those occurring at faster frequencies (i.e., LVFA).45 The LVFA at the seizure onset reflects more superficial sources, which are generally neocortical.46 Therefore, the EZ location may impact the incidence of LVFA. Hence, the low incidence of LVFA in our patients may limit the predictive value of LVFA for surgical prognosis.
Furthermore, we found that clinical variables, such as the pathological type of FCD, age at surgery, and MRI-negative, did not affect the surgical outcome, contrary to previous findings.5, 11, 13, 47 Additionally, there was difference in seizure-free rate after surgery in this study (81.4%) compared with previous studies (55–64%). Generally, the prognosis of patients with epilepsy after surgical treatment mainly depends on the precise localization and complete resection of the EZ. All patients included in this study underwent surgery between 2015 and 2019. Therefore, these discrepancies may be attributed to the advancements in preoperative evaluation, the evolution of surgical techniques, and varied standards of patient selection. Regardless of the pathological types of FCD and differences in MRI findings, improving the accuracy of epileptogenic zone localization will result in better surgical outcomes. Patients with FCD type II constituted the majority of the study population (50%). A previous study by our research group found that the vast majority of FCD type II malformation is bottom-of-sulcus-rooted.48 Two image postprocessing techniques, including morphometric analysis program and normalized FLAIR signal intensity, with higher sensitivities for FCD detection than conventional visual inspection, were used during presurgical evaluation in our epilepsy center. The imaging postprocessing findings combine the directional clues of seizure semiology and scalp EEG and may facilitate a more precise prediction of the EZ in MRI-negative patients. We adopted a surgical strategy called “sulcus-centered resection” for patients with FCD type II and achieved a better surgical outcome (seizure-free rate after surgery was 90%).49
4.4 Limitations
Our study has some limitations, including the imbalance between the sample sizes of the three reported FCD subtypes and the low number of some interictal scalp EEG patterns. This limited the statistical analysis of the association between one specific pattern and postsurgical outcome. Therefore, future prospective research should expand the sample size to test these findings. Again, the detailed mechanisms underlying the specific interictal EEG pattern would need further exploring and investigation in future.
5 CONCLUSION
In conclusion, the surgical outcomes of patients with intractable epilepsy associated with FCD are often favorable. Patients with characteristic interictal scalp EEG patterns—the coexistence of REDP and PDP in the same location—were good candidates for surgery. Complete resection of the assumed EZ was a good prognostic factor. The FCD subtypes, cortex location, age at epilepsy onset, age at surgery, presence of LVFA, and presence of FBTCS did not have a significant impact on the long-term postsurgical outcome. Careful analysis of interictal EEG patterns on scalp EEG can assist clinical decision-making and enable better presurgical evaluation.
Acknowledgments
The authors declare that there are no other contributors or funders, and the work has not been presented previously.
DISCLOSURE
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.