Risk factors that predict delayed seizure detection on continuous electroencephalogram (cEEG) in a large sample size of critically ill patients
Summary
Objective
Majority of seizures are detected within 24 hours on continuous EEG (cEEG). Some patients have delayed seizure detection after 24 hours. The purpose of this research was to identify risk factors that predict delayed seizure detection and to determine optimal cEEG duration for various patient subpopulations.
Methods
We retrospectively identified all patients ≥18 years of age who underwent cEEG at Cleveland clinic during calendar year 2016. Clinical and EEG data for all patients and time to seizure detection for seizure patients were collected.
Results
Twenty-four hundred and two patients met inclusion criteria. Of these, 316 (13.2%) had subclinical seizures. Sixty-five (20.6%) patients had delayed seizures detection after 24 hours. Seizure detection increased linearly till 36 hours of monitoring, and odds of seizure detection increased by 46% for every additional day of monitoring. Delayed seizure risk factors included stupor (13.2% after 48 hours, P = .031), lethargy (25.9%, P = .013), lateralized (LPDs) (27.7%, P = .029) or generalized periodic discharges (GPDs) (33.3%, P = .022), acute brain insults (25.5%, P = .036), brain bleeds (32.8%, P = .014), especially multiple concomitant bleeds (61.1%, P < .001), altered mental status (34.7%, P = .001) as primary cEEG indication, and use of antiseizure medications (27.8%, P < .001) at cEEG initiation.
Significance
Given the linear seizure detection trend, 36 hours of standard monitoring appears more optimal than 24 hours especially for high-risk patients. For awake patients without epileptiform discharges, <24 hours of monitoring appears sufficient. Previous studies have shown that coma and LPDs predict delayed seizure detection. We found that stupor and lethargy were also associated with delayed seizure detection. LPDs and GPDs were associated with delayed seizures. Other delayed seizure risk factors included acute brain insults, brain bleeds especially multiple concomitant bleeds, altered mental status as primary cEEG indication, and use of ASMs at cEEG initiation. Longer cEEG (≥48 hours) is suggested for these high-risk patients.
Key points
- We report 2402 consecutive adult patients who underwent cEEG during calendar year 2016, of whom 316 had subclinical seizures.
- Sixty-five (20.6%) patients had delayed seizure detection after 24 hours.
- Seizure detection increased linearly till 36 hours of monitoring, and odds of seizure detection increased by 46% for every additional day of monitoring.
- Delayed seizure risk factors included stupor, lethargy, LPDs, GPDs, acute brain insults, brain bleeds, especially multiple concomitant bleeds, altered mental status as primary cEEG indication, and use of ASMs at cEEG initiation.
- The aforementioned patient subpopulations are at risk of delayed seizure detection. Longer cEEG (≥48 hours) is suggested for these high-risk patients.
1 INTRODUCTION
Approximately 13%–20% of critically ill patients undergoing continuous electroencephalogram (cEEG) have electrographic seizures most of which are nonconvulsive (NCS) or subclinical.1 Timely seizure detection with cEEG might reduce medical and neurological complications.2-4 In addition, nonconvulsive status epilepticus increases mortality. While a recent trial showed no difference in outcomes at 6 months of follow-up between patients undergoing cEEG or routine EEG, other studies have reported cEEG monitoring is associated with reduced in-hospital mortality.5, 6. Hence, cEEG is recommended in critically ill with altered mental status (AMS) or unexplained encephalopathy.7-11
Majority of seizures are detected within 24 hours on continuous EEG (cEEG). Some patients have delayed seizures after 24 hours, which may be missed. Previous studies have shown that risk factors that predict delayed seizure detection include coma, lateralized periodic discharges (LPDs), and seizure history.1, 12 Therefore, current recommendation is to monitor for at least 48 hours in comatose patients and those with seizure history. For others, ≤24 hour of cEEG is recommended.1, 12
However, previous studies that have investigated the optimal time of cEEG have either looked at highly selected population,1, 13, 14 not considered all known EEG risk factors or studied their temporal relationship with respect to seizure occurrence,1, 14-16 or studied a limited indications for cEEG such as only those patients with AMS. Therefore, risk factors for delayed seizure detection remain unclear in different patient subpopulations.
The purpose of our research was to identify additional risk factors for delayed seizure detection with respect to mental status, electrographic features, etiology of presentation, and other clinical characteristics. We aimed to look at a diverse adult hospitalized population with a large sample size to identify subpopulations at risk of delayed seizures on cEEG who will require from longer cEEG monitoring to detect subclinical seizures.
2 METHODS
2.1 Study design and population
The current study is a retrospective study. After institutional review board approval, we used our prospectively maintained cEEG database to identify all adults (≥18 years of age) who underwent cEEG monitoring at Cleveland Clinic during the 2016 calendar year.
2.2 Clinical variables
Clinical data were gathered from review of electronic health records. Baseline demographic data (age, gender) and patient's mental status (wakefulness, lethargy, stupor, coma) were recorded at the time of cEEG initiation. Wakefulness was described as fully alert and responsive state. Lethargy was described as hypersomnolent state with reduced alertness but arousable to minimal stimulus. Stupor was described as unresponsiveness where patients could only be aroused to vigorous, repeated stimuli. Patient lapsed back into unresponsiveness when stimulus ceased. Coma was described as unarousable unresponsiveness with no understandable response to stimuli. Additional variables included primary etiology of presentation, history of epilepsy, whether a patient was on antiseizure medications (ASMs) at cEEG initiation, monotherapy or polytherapy, presence of acute brain insult {within preceding 7 days of cEEG initiation}, type of acute brain insults (ischemic stroke, brain bleed, types of brain bleed, autoimmune brain disease, postneurosurgery, central nervous system (CNS) infection, CNS tumor (new or recurrent tumors or tumor-related complications), venous sinus thrombosis (VST), posterior reversible encephalopathy syndrome (PRES), demyelination, vasculopathy, and Creutzfeldt-Jakob disease [CJD]), presence and type of remote brain insult, and duration of cEEG monitoring. The primary etiology of presentation was categorized into epilepsy-related breakthrough seizures, ischemic stroke, brain bleed, CNS tumor, CNS infection, autoimmune brain disease, hypoxic ischemic encephalopathy (HIE), toxic/metabolic/infectious (TMI) encephalopathy, postneurosurgery, VST, PRES, demyelination, vasculopathy, CJD, decreased level of consciousness (LOC), or witnessed event of unclear etiology and psychogenic nonepileptic seizures (PNES). Brain bleeds were further subcategorized into subarachnoid hemorrhage (SAH), intracranial hemorrhage (ICH), subdural hematoma (SDH), or mixed bleeds (more than one type of concomitant bleeds). Indications for performing cEEG were classified as altered mental status, witnessed seizure or seizure-like event (paroxysmal, mostly motor, events such as myoclonic jerks or transient unilateral posturing in comatose patients), or hypothermia protocol among cardiac arrest patients.
For analysis, some variables had category levels combined to account for low frequency. In the variable “Primary Etiology of Presentation,” “PNES” and “Decreased LOC or Witnessed event of other or unclear etiology” were combined into “Dec LOC/Event/Unclear or PNES.” In both “primary etiology of presentation” and in “acute brain insults,” the category of “other causes of acute brain insult” consists of "autoimmune brain disease," "CNS infection," "postneurosurgery," "PRES," “VST,” “demyelination,” “vasculopathy,” and “CJD.” The number of patients in types of “acute brain insults” varies within these two categories because some patients could have a different etiology of presentation even in the presence of an acute brain insult.
2.3 EEG variables
CEEGs were recorded according to the international 10-20 system. CEEG database was used to identify patients with NCS EEG seizures or status epilepticus (Salzburg criteria17). Other interictal epileptiform discharges (IEDs) included isolated interictal EDs (sharp waves (SW) or spikes),18 lateralized periodic discharges (LPDs, formerly PLEDs)/lateralized rhythmic delta activity,16 and generalized periodic discharges (GPDs).19 EDs preceding seizures were recorded. For seizure patients, time of cEEG initiation and time of first electrographic seizure were recorded to calculate time to detect first seizure.
2.4 Statistical analysis
Continuous variables are summarized with mean and standard deviation, and categorical variables with frequencies and percentages. Mann-Whitney U tests are used for continuous variables, and Pearson chi-square tests (or Fisher's exact test) are used for categorical variables. Logistic regression is used to identify risk factors associated with seizure occurrence. Variables with low frequency (EEG status epilepticus), or with high variance inflation factors (cEEG indication and acute brain insults) are excluded from regression model. A logistic regression model predicting delayed seizures (after 24 hours) is presented. A cumulative incidence graph is used to depict seizure risk by time. A Cox proportional hazards model is built; however, the proportional hazards assumptions are violated. Analysis is done in R (v4); p-values<0.05 are considered significant (bolded p-values).
3 RESULTS
3.1 Study cohort
Among 2425 patients who underwent cEEG during 2016, 339 (14.0%) experienced seizures. Twenty-three patients with exclusively clinical seizure or with exclusive postanoxic myoclonia were excluded from analysis. Twenty-four hundred and two patients were included, of whom 316 (13.2%) had at least one NCS or subclinical seizure. The mean age of 2402 patients was 59.44 ± 17.4 years, and 1191 (49.6%) of them were female. Most common primary etiologies of presentation were TMI encephalopathy, brain bleeds, and ischemic strokes. Most common indication for monitoring was witnessed seizure-like event.
3.2 Seizure on cEEG
Of 316 patients with NCS, 38(12.0%) had EEG status epilepticus. The median age of seizure patients was 60 (IQR): 45-72) years with 147 (46.5%) females (Table 1). Nonseizure patients were more likely to have awake mentation (p-0.011). Seizure patients had a higher frequency of IEDs (sharp waves/spike [P < .001], LPDs [P < .001], and GPDs [P < .001],) epilepsy-related breakthrough seizures (P < .001), any type of acute brain insults(P < .001), any brain bleeds (P = .001), SDH (P = .003), mixed bleeds (P = .002), other causes of acute brain insults (P < .001), remote brain insults (P < .001), and remote history of autoimmune brain disease (P = .047).
| Variable | Level | Seizure not detected | Seizure detected | P.overall |
|---|---|---|---|---|
| Age, median [25th; 75th] | 62.0 [49.0;72.0] | 60.0 [45.0;72.0] | .062 | |
| Gender, N (%) | Female | 1045 (50.1%) | 147 (46.5%) | .261 |
| Male | 1041 (49.9%) | 169 (53.5%) | ||
| Awake at EEG monitoring start, N (%) | 1036 (49.7%) | 132 (41.8%) | .011 | |
| Coma at EEG monitoring start, N (%) | 183 (8.77%) | 31 (9.81%) | .619 | |
| Lethargy at EEG monitoring start, N (%) | 460 (22.1%) | 85 (26.9%) | .065 | |
| Stupor EEG monitoring start, N (%) | 407 (19.5%) | 68 (21.5%) | .448 | |
| Monitoring duration (days), median [25th; 75th] | 1.50 [1.00;2.50] | 4.50 [3.00;8.50] | <.001 | |
| Lateralized periodic discharges, N (%) | 51 (2.44%) | 112 (35.4%) | <.001 | |
| Sharp waves or spikes, N (%) | 259 (12.4%) | 179 (56.6%) | <.001 | |
| Generalized periodic discharges, N (%) | 138 (6.62%) | 51 (16.1%) | <.001 | |
| Hours to 1st seizure, median [25th; 75th] | - | 3.42 [0.21;18.8] | ||
| Primary etiology of presentation | ||||
| Brain bleed, N (%) | 253 (12.1%) | 61 (19.3%) | .001 | |
| CNS tumor, N (%) | 140 (6.71%) | 29 (9.18%) | .139 | |
| Ischemic stroke, N (%) | 238 (11.4%) | 27 (8.54%) | .156 | |
| Other causes of acute brain insult, N (%) | 135 (6.47%) | 44 (13.9%) | <.001 | |
| Epilepsy-related breakthrough seizures, N (%) | 143 (6.86%) | 99 (31.3%) | <.001 | |
| Hypoxic ischemic encephalopathy, N (%) | 159 (7.62%) | 16 (5.06%) | .130 | |
| TMI encephalopathy, N (%) | 822 (39.4%) | 37 (11.7%) | <.001 | |
| Dec LOC/event/unclear or PNES, N (%) | 196 (9.40%) | 3 (0.95%) | <.001 | |
| Brain bleed type | ||||
| Brain bleed-intracranial hemorrhage, N (%) | 95 (4.55%) | 21 (6.65%) | .140 | |
| Brain bleed-subarachnoid hemorrhage, N (%) | 63 (3.02%) | 6 (1.90%) | .352 | |
| Brain bleed-subdural hematoma, N (%) | 44 (2.11%) | 16 (5.06%) | .003 | |
| Brain bleed-mixed, N (%) | 51 (2.44%) | 18 (5.70%) | .002 | |
| Indication for cEEG | ||||
| Indication for cEEG-witnessed seizure-like event, N (%) | 1136 (54.5%) | 229 (72.5%) | <.001 | |
| Indication for cEEG-altered mental status, N (%) | 841 (40.3%) | 75 (23.7%) | <.001 | |
| Indication for cEEG-cardiac arrest, N (%) | 109 (5.23%) | 12 (3.80%) | .345 | |
| Epilepsy history, N (%) | 379 (18.2%) | 133 (42.1%) | <.001 | |
| ASMs, N (%) | 955 (45.8%) | 198 (62.7%) | <.001 | |
| Monotherapy or polytherapy, N (%) | Monotherapy | 691 (72.5%) | 106 (53.5%) | <.001 |
| Polytherapy | 262 (27.5%) | 92 (46.5%) | ||
| Acute brain insults, N (%) | 778 (37.3%) | 161 (50.9%) | <.001 | |
| Type | ||||
| Acute brain insult type—brain bleed, N (%) | 261 (12.5%) | 63 (19.9%) | <.001 | |
| Acute brain insult type—ischemic stroke, N (%) | 246 (11.8%) | 27 (8.54%) | .109 | |
| Acute brain insult type—CNS tumor-related, N (%) | 141 (6.76%) | 30 (9.49%) | .100 | |
| Acute brain insult type—other causes, N (%) | No | 125 (5.99%) | 42 (13.3%) | <.001 |
| Remote brain insult, N (%) | 661 (31.7%) | 163 (51.6%) | <.001 | |
| Remote tumor, N (%) | 181 (27.4%) | 37 (22.7%) | .265 | |
| Remote stroke, N (%) | 288 (43.6%) | 63 (38.7%) | .294 | |
| Remote neurosurgery, N (%) | 131 (19.8%) | 41 (25.2%) | .163 | |
| Remote brain bleed, N (%) | 107 (16.2%) | 32 (19.6%) | .350 | |
| Remote CNS infection, N (%) | 21 (3.18%) | 8 (4.91%) | .403 | |
| Remote TBI, N (%) | 28 (4.24%) | 7 (4.29%) | 1.000 | |
| Remote autoimmune brain disease, N (%) | 6 (0.91%) | 5 (3.07%) | .047 | |
| Remote PRES, N (%) | 1 (0.15%) | 1 (0.61%) | .357 | |
- Abbreviations: ASM, antiseizure medications; CNS, central nervous system; EEG, electroencephalogram; LOC, level of consciousness; PNES, psychogenic nonepileptic seizures; PRES, posterior reversible encephalopathy syndrome; TBI, traumatic brain injury; TMI, toxic/metabolic/infectious encephalopathy.
- Bold and italics indicate significant p values.
Seizures were more frequent in patients with cEEG indication of witnessed seizure-like events (P < .001). More than twice as many patients in the seizure compared with nonseizure group had a history of epilepsy (P < .001), and patients with seizures on cEEG were more likely to be on antiseizure medications (ASMs) (P < .001), especially on polytherapy (P < .001) at the time of cEEG initiation. Monitoring duration was significantly longer in patients with seizures than those without seizures (4.5 vs 1.5 days, P < .001).
3.3 Drivers of seizure detection
Patients presenting with epilepsy-related breakthrough seizures had over 3.6 times odds of having a seizure detected than whose with CNS tumor (OR=3.65 (CI: 1.66-8.05), P = .001). Patients presenting with TMI encephalopathy had about one-third the odds of having seizures (OR = 0.33 (0.16, 0.67), P = .002), and those with decreased LOC /seizure-like event of unclear etiology or PNES had about 1/9th the odds of having seizures (OR = 0.11 (0.03, 0.45), P = .002) compared with CNS tumor patients (Table 2). Patients not on ASMs had 0.38 times the odds of having a seizure than patients on ASMs (OR = 0.38 (0.25, 0.59), P < .001). For every additional day of monitoring, odds of seizure detected increased by 46% (OR = 1.46 (1.37, 1.56), P < .001).
| Variable | Level | Odds ratio (95% CI) | P-value |
|---|---|---|---|
| Intercept | 0.06 (0.02,0.14) | <.001 | |
| Age | 0.99 (0.98,1) | .09 | |
| Gender | Male (vs Female) | 1.21 (0.86,1.69) | .277 |
| cEEG monitoring duration | 1.46 (1.37,1.56) | <.001 | |
| Etiology of presentation | Other causes of acute brain insults (versus CNS tumor) | 1.21 (0.57,2.59) | .617 |
| Brain Bleed (vs CNS tumor) | 1.04 (0.52,2.11) | .904 | |
| Dec LOC/Event/Unclear or PNES (vs CNS tumor) | 0.11 (0.03,0.45) | .002 | |
| Epilepsy-related breakthrough seizures (vs CNS tumor) | 3.65 (1.66,8.05) | .001 | |
| Hypoxic ischemic encephalopathy (vs CNS tumor) | 0.5 (0.18,1.43) | .194 | |
| Ischemic stroke (vs CNS tumor) | 0.6 (0.27,1.35) | .218 | |
| TMI encephalopathy (vs CNS tumor) | 0.33 (0.16,0.67) | .002 | |
| Epilepsy history | 1.18 (0.65,2.13) | .591 | |
| Not on antiseizure medications | 0.38 (0.25,0.59) | <.001 | |
| Remote brain insult | 1.27 (0.88,1.83) | .199 | |
| Mental status | Coma (vs awake) | 0.95 (0.37,2.44) | .908 |
| Lethargy (vs awake) | 1.43 (0.82,2.51) | .209 | |
| Stupor (vs awake) | 0.56 (0.28,1.1) | .09 | |
| Sharp waves (for awake patients) | 5.05 (3.01,8.46) | <.001 | |
| Period pattern (for awake patients) | 8.39 (2.25,31.21) | .002 | |
| Periodic lateralized epileptiform discharges (for awake patients) | 12.88 (5.31,31.24) | <.001 | |
| Mental status: SW interaction | Coma | 0.46 (0.1,2.04) | .307 |
| Lethargy | 0.27 (0.12,0.64) | .003 | |
| Stupor | 0.67 (0.27,1.67) | .388 | |
| Mental status: GPD interaction | Coma | 0.09 (0.01,0.64) | .016 |
| Lethargy | 0.1 (0.02,0.5) | .005 | |
| Stupor | 0.16 (0.03,0.77) | .022 | |
| Mental status: LPD interaction | Coma | 4.72 (0.58,38.36) | .147 |
| Lethargy | 0.67 (0.2,2.18) | .504 | |
| Stupor | 1.08 (0.32,3.68) | .9 |
Note
- Logistic regression results are shown with a very high c-index of 0.91, indicating model reliability with regard to discrimination (ability to correctly rank patients by risk); the model has an index of prediction accuracy of 0.43, indicating a well calibrated model (reliable/accurate in prediction). The odds ratios presented in the table are exponentiated coefficient estimates, and for the variables related to the interaction terms, do not represent the actual relationships of the variables. The interaction terms are multipliers for the abnormality variables.
- Abbreviations: ASM, antiseizure medications; cEEG, continuous electroencephalogram; CNS, central nervous system; GPD, generalized periodic discharges; LOC, Level of consciousness; LPD, lateralized periodic discharges; PNES, psychogenic nonepileptic seizures; PRES, posterior reversible encephalopathy syndrome; SW, sharp wave; TBI, traumatic brain injury; TMI, toxic/metabolic/infectious encephalopathy.
- Bold and italics indicate significant p values
3.4 EEG findings, mental status, and seizure activity
Depending on patients’ mental status, there were different effects of IEDs on seizure detection (Table 2). The interaction terms in Table 2 are multipliers for the abnormality variables. For all of IEDs that are statistically significant in predicting seizure detection, these abnormalities have the largest effect for patients who are awake.
For awake patients, the presence of sharp waves (SWs) increased the odds of seizure detection by 5 times (OR = 5.05 (3.01, 8.46), P < .001), GPDs increased the odds by a factor of 8.39 (OR = 8.39 (2.25, 31.21), P =.002), and LPDs/PLEDs increased the odds of seizure detection over 12.5 times higher (OR = 12.88 (5.31, 31.24), P < .001). For lethargic patients, the effect of SWs was significantly reduced compared with awake patients, with the odds of having a seizure in the presence of SWs being 1.36 (=5.05*0.27), that is, a 36% higher odds of seizure detection for lethargic patients with SWs. The effect of GPDs was reduced 10-fold, with the odds of seizures in the presence of GPDs being 0.839 (=8.39*0.1); that is, GPDs in lethargic patients were associated with ~16% lower odds of seizure detection compared to lethargic patients without GPDs. For stuporous patients, the effect of GPDs was reduced to 1.34 (=8.39*0.16), that is, a 34% higher odds of seizure for stuporous patients with GPDs compared to stuporous patients without GPDs. For patients in coma, the effect of GPDs was decreased to one-eleventh (OR = 0.76 (= 8.39*0.09)), that is, 24% lower odds of seizure detection for comatose patients with GPDs compared to coma patients without GPDs. The effect of LPDs showed no evidence of change depending on mental status.
3.5 Time to record first seizure on cEEG
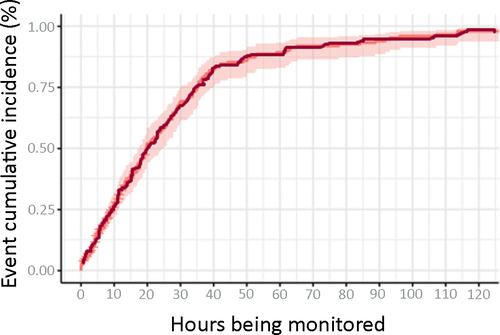
Of the 316 seizure patients, 251 (79.4%) had their first seizure detected during 24 hours of cEEG monitoring (Table 3). Sixty-five (20.6%) had seizures detected after 24 hours. Forty-three (13.6%) patients had seizures detected between 24 and 48 hours, and 22 (7.0%) had seizures detected after 48 hours. Figure 1 is the cumulative incidence curve depicting first seizure detected on cEEG over time. Probability of seizure detection on cEEG increases steadily and linearly from 1 to 36 hours.
| Variable | Level | First seizure within 24 h (n = 251) | First seizure after 24 h (n = 65) | P-value | First seizure within 48 h (n = 294) | First seizure after 48 h (n = 22) | P-value |
|---|---|---|---|---|---|---|---|
| Age, median [25th; 75th] | 59.0 [45.0;72.5] | 65.0 [52.0;72.0] | .142 | 60.0 [45.0;72.8] | 61.5 [49.5;68.8] | .728 | |
| Gender, N (%) | Female | 116 (46.2%) | 31 (47.7%) | .942 | 135 (45.9%) | 12 (54.5%) | .575 |
| Male | 135 (53.8%) | 34 (52.3%) | 159 (54.1%) | 10 (45.5%) | |||
| Awake at EEG monitoring start, N (%) | 112 (44.6%) | 20 (30.8%) | .061 | 130 (44.2%) | 2 (9.09%) | .003 | |
| Lethargy at EEG monitoring start, N (%) | 63 (25.1%) | 22 (33.8%) | .208 | 78 (26.5%) | 7 (31.8%) | .772 | |
| Stupor EEG monitoring start, N (%) | 52 (20.7%) | 16 (24.6%) | .608 | 59 (20.1%) | 9 (40.9%) | .031 | |
| Coma at EEG monitoring start, N (%) | 24 (9.56%) | 7 (10.8%) | .954 | 27 (9.18%) | 4 (18.2%) | .252 | |
| Monitoring duration (days), median [25th; 75th] | 4.50 [3.00;7.00] | 7.00 [4.50;11.5] | <.001 | 4.50 [3.00;8.38] | 7.50 [5.50;17.4] | .001 | |
| EEG status epilepticus, N (%) | 33 (13.1%) | 5 (7.69%) | .322 | 36 (12.2%) | 2 (9.09%) | 1.000 | |
| Lateralized periodic discharges, N (%) | 81 (32.3%) | 31 (47.7%) | .029 | 105 (35.7%) | 7 (31.8%) | .891 | |
| Sharp waves or spikes, N (%) | 140 (55.8%) | 39 (60.0%) | .637 | 163 (55.4%) | 16 (72.7%) | .175 | |
| Generalized period discharges, N (%) | 34 (13.5%) | 17 (26.2%) | .022 | 44 (15.0%) | 7 (31.8%) | .064 | |
| Hours to 1st seizure, median [25th; 75th] | 1.45 [0.12;6.40] | 36.3 [29.6;59.0] | <.001 | 2.81 [0.15;13.5] | 80.9 [59.7;104] | <.001 | |
| Primary etiology of presentation | |||||||
| Brain bleed, N (%) | 41 (16.3%) | 20 (30.8%) | .014 | 56 (19.0%) | 5 (22.7%) | .779 | |
| CNS tumor-related, N (%) | 23 (9.16%) | 6 (9.23%) | 1.000 | 28 (9.52%) | 1 (4.55%) | .706 | |
| Ischemic stroke, N (%) | 24 (9.56%) | 3 (4.62%) | .307 | 24 (8.16%) | 3 (13.6%) | .417 | |
| Other causes, N (%) | 32 (12.7%) | 12 (18.5%) | .325 | 38 (12.9%) | 6 (27.3%) | .100 | |
| Epilepsy-related breakthrough seizures, N (%) | 83 (33.1%) | 16 (24.6%) | .246 | 95 (32.3%) | 4 (18.2%) | .254 | |
| Hypoxic ischemic encephalopathy, N (%) | 13 (5.18%) | 3 (4.62%) | 1.000 | 15 (5.10%) | 1 (4.55%) | 1.000 | |
| TMI encephalopathy, N (%) | 33 (13.1%) | 4 (6.15%) | .178 | 35 (11.9%) | 2 (9.09%) | 1.000 | |
| Decreased LOC/Event/Unclear or PNES, N (%) | 2 (0.80%) | 1 (1.54%) | .500 | 3 (1.02%) | 0 (0.00%) | 1.000 | |
| Brain bleed type | |||||||
| Brain bleed-intracranial hemorrhage, N (%) | 18 (7.17%) | 3 (4.62%) | .585 | 20 (6.80%) | 1 (4.55%) | 1.000 | |
| Brain bleed-subarachnoid hemorrhage, N (%) | 5 (1.99%) | 1 (1.54%) | 1.000 | 6 (2.04%) | 0 (0.00%) | 1.000 | |
| Brain bleed-subdural hematoma, N (%) | 11 (4.38%) | 5 (7.69%) | .337 | 15 (5.10%) | 1 (4.55%) | 1.000 | |
| Brain bleed-mixed, N (%) | 7 (2.79%) | 11 (16.9%) | <.001 | 15 (5.10%) | 3 (13.6%) | .120 | |
| Indication for cEEG-Witnessed seizure-like event, N (%) | 193 (76.9%) | 36 (55.4%) | .001 | 217 (73.8%) | 12 (54.5%) | .088 | |
| Indication for cEEG-cardiac arrest, N (%) | 9 (3.59%) | 3 (4.62%) | .717 | 11 (3.74%) | 1 (4.55%) | .586 | |
| Indication for cEEG-altered mental status, N (%) | 49 (19.5%) | 26 (40.0%) | .001 | 66 (22.4%) | 9 (40.9%) | .089 | |
| Epilepsy history, N (%) | 109 (43.4%) | 24 (36.9%) | .421 | 126 (42.9%) | 7 (31.8%) | .431 | |
| ASMs, N (%) | 143 (57.0%) | 55 (84.6%) | <.001 | 180 (61.2%) | 18 (81.8%) | .090 | |
| Monotherapy or polytherapy, N (%) | Monotherapy | 74 (51.7%) | 32 (58.2%) | .513 | 94 (52.2%) | 12 (66.7%) | .356 |
| Polytherapy | 69 (48.3%) | 23 (41.8%) | 86 (47.8%) | 6 (33.3%) | |||
| Acute brain insult, N (%) | 120 (47.8%) | 41 (63.0%) | .036 | 146 (49.7%) | 15 (68.2%) | .146 | |
| Acute brain insult type | |||||||
| Brain bleed, N (%) | 43 (17.1%) | 20 (30.8%) | .023 | 58 (19.7%) | 5 (22.7%) | .782 | |
| Ischemic stroke, N (%) | 24 (9.56%) | 3 (4.62%) | .307 | 24 (8.16%) | 3 (13.6%) | .417 | |
| CNS tumor-related, N (%) | 22 (8.76%) | 7 (10.8%) | .631 | 29 (9.86%) | 1 (4.55%) | .707 | |
| Other causes of acute brain insults, N (%) | 31 (12.4%) | 11 (16.9%) | .446 | 36 (12.2%) | 6 (27.3%) | .094 | |
| Remote brain insult, N (%) | 130 (51.8%) | 33 (50.8%) | .994 | 152 (51.7%) | 11 (50.0%) | 1.000 | |
| Remote tumor, N (%) | 27 (20.8%) | 10 (30.3%) | .350 | 34 (22.4%) | 3 (27.3%) | .714 | |
| Remote stroke, N (%) | 52 (40.0%) | 11 (33.3%) | .616 | 58 (38.2%) | 5 (45.5%) | .751 | |
| Remote neurosurgery, N (%) | 32 (24.6%) | 9 (27.3%) | .929 | 38 (25.0%) | 3 (27.3%) | 1.000 | |
| Remote brain bleed, N (%) | 25 (19.2%) | 7 (21.2%) | .992 | 30 (19.7%) | 2 (18.2%) | 1.000 | |
| Remote CNS infection, N (%) | 6 (4.62%) | 2 (6.06%) | .664 | 8 (5.26%) | 0 (0.00%) | 1.000 | |
| Remote TBI, N (%) | 5 (3.85%) | 2 (6.06%) | .630 | 6 (3.95%) | 1 (9.09%) | .393 | |
| Remote autoimmune brain disease, N (%) | 5 (3.85%) | 0 (0.00%) | .584 | 5 (3.29%) | 0 (0.00%) | 1.000 | |
| Remote PRES, N (%) | 1 (0.77%) | 0 (0.00%) | 1.000 | 1 (0.66%) | 0 (0.00%) | 1.000 | |
- Abbreviations: ASM, antiseizure medications; CNS, central nervous system; EEG, electroencephalogram; LOC, level of consciousness; PNES, psychogenic nonepileptic seizures; PRES, posterior reversible encephalopathy syndrome; TBI, traumatic brain injury; TMI, toxic/metabolic/infectious encephalopathy.
- Bold and italics indicate significant p values
3.6 Delayed seizure detection (first seizure after 24 or 48 hours)
Table 3 shows subcohorts of patients with delayed seizure detection on cEEG, after 24 and 48 hours. Awake patients were more likely to have their first seizures detected in <48 hours (P = .003). Stuporous patients were more likely to require >48 hours to detect seizures (P = .031). Patients with LPDs (P = .029), GPDs (P = .022), any type of acute brain insults (P = .036), primary etiology of brain bleed (P = .014), mixed type of brain bleeds (P < .001), and primary cEEG indication of altered mental status (P = .001) and those on ASMs at the time of cEEG initiation (P < .001) were more likely to require monitoring for >24 hours to detect seizures. Patients whose indication for cEEG was witnessed seizure-like event(s) were more likely to have their seizures detected in <24 hours (P = .001).
Patients with early seizure detection had shorter median monitoring duration (4.5 days) compared to those with delayed seizure detection after 24 hours (median: 7.25 days, IQR: 4.5-11.9, P < .001) and those with seizure detection after 48 hours (median: 7.5 days, IQR: 5.5-17.4, P = .001).
3.7 Drivers of delayed seizure detection
Table 4 shows the results for the logistic regression model identifying drivers of delayed seizure detection after 24 hours. Patients on ASMs had over 5 times the odds of delayed seizure detection (OR = 5.15 [2.57, 10.33], P < .001). Lethargic patients had a 2.24 times the odds of a delayed seizure detection compared with awake patients (OR = 2.24 [1.18, 4.25], P = .013).
| Variable | Level | Odds ratio (95% CI) | P-value |
|---|---|---|---|
| Intercept | 0 (0,0.01) | <.001 | |
| cEEG monitoring duration | 1.1 (1.07,1.15) | <.001 | |
| Antiseizure medications | 5.15 (2.57,10.33) | <.001 | |
| Mental status | Coma (vs awake) | 1.8 (0.66,4.97) | .254 |
| Lethargy (vs awake) | 2.24 (1.18,4.25) | .013 | |
| Stupor (vs awake) | 1.69 (0.83,3.45) | .149 |
- Bold and italics indicate significant p values.
4 DISCUSSION
In this retrospective study of 2402 adult hospitalized patients, we investigated risk factors for delayed seizure detection on cEEG. NCS were recorded in ~13.2% of patients, which is comparable with prior studies (8 to 34%).1, 2, 20-23
Our study showed that the NCS detection on cEEG increased linearly till 36 hours of monitoring and the odds of seizure detection increased by 46% for every additional day of cEEG monitoring. This highlights the need for longer cEEG monitoring especially in high-risk patients. Given the linear seizure detection trend, monitoring of 36 hours appears more optimal than 24 hours.
Previous studies have shown increased seizure risk and delayed seizure detection in comatose patients, especially those with prior history of seizures/epilepsy and those with LPDs.1, 12, 14 Current recommendation is to monitor for 24 hours in noncomatose patients and 48 hours if they are comatose, especially in those with co-existent history of seizures.1, 12 However, these studies have only considered comatose and noncomatose patients. Noncomatose ICU patients may still have some degree of altered mentation. Accordingly, our patients were divided into awake, lethargic, stuporous, and comatose.
Our findings show that 84.8% of awake patients had seizures detected within 24 hours. Therefore, in the absence of IEDs, for awake patients less than 24 hours of monitoring is sufficient. Stuporous patients were more likely to have delayed seizure detection after 48 hours, and lethargic patients had a 2.24 odds of delayed seizure detection after 24 hours in comparison with awake patients. In addition to comatose patients, stuporous and lethargic patients likely require at least 48 hours of monitoring to detect subclinical seizures.
However, it must be noted that in our study the percentage of comatose patients who had seizures detected on their cEEG was 9.81%, which is lower compared to previous reports of ~20%, which is probably because of our more detailed classification of mental status. In previous studies, stuporous patients were likely also included in the comatose patients group. Therefore, these differences must be kept in mind when comparing the results of our study to the previously published data.
LPDs are associated with delayed seizure detection, and one previous study showed that 21% of LPD patients have their first seizure detected after 24 hours of cEEG.1 However, the temporal relationship between the appearances of individual IEDs and seizures has not been assessed. We found that preceding LPDs (27.7%) and GPDs (33.3%) are risk factors for delayed seizure detection and should warrant 48 hours of monitoring.
Etiology of presentation is a key factor driving clinical management. We studied individual reasons for presentations and their association with delayed seizure detection. A high proportion of acute brain insult patients (25.5%) and brain bleed patients (32.8%) especially those with mixed bleeds (61.1%) had seizures detected after 24 hours. Therefore, acute brain insult patients should undergo 48 hours of monitoring. Patients with brain bleeds especially those with multiple concomitant bleeds have the highest risk of delayed seizure detection. These findings are especially interesting and useful since a previous study found that their proposed algorithm failed to predict optimal recording duration for acute brain insult patients.24
We studied common indications for cEEG during hospitalization including altered mental status, witnessed seizure-like events, and hypothermia protocol. Even though witnessed seizure-like event patients had a high seizure occurrence risk, only 15.9% of them had seizures after 24 hours. Therefore, for this patient population 24 hour of monitoring is sufficient. Among altered mental status patients, 34.7% patients had delayed seizure detection. Therefore, for patients undergoing cEEG for the primary indication of altered mental status, 48 hours of monitoring should be considered. The reason why 24 hours of monitoring was found sufficient for witnessed seizure-like event patients, despite their high seizure risk, is likely because at baseline given their high risk of seizure, their first seizure is likely to be occur on EEG be detected earlier compared to those with altered mental status patients.
Patients with epilepsy history and others with high index of suspicion for seizures are typically started on ASMs before cEEG initiation. Frequently, the question arises as to how long should we monitor these patients with cEEG who are already on ASMs? We found that 27.8% of patients on ASMs at the time of cEEG initiation had seizures after 24 hours and had over 5 times odds of delayed seizure detection. Therefore, patients on ASMs should undergo 48 hours of monitoring.
Compared with previous studies, we found similar frequencies of electrographic seizures in patients with ischemic strokes (10.2% vs 6%–26%),1, 2, 20, 25-27 ICH (18% vs 13%–28%),1, 2, 20, 25, 28-32 SAH (8.7% versus 3%–26%),33-42 SDH (26.7% vs 2.2%–43%),43, 44 HIE (10.1% vs 10%–30%),1, 45 and CNS infection (23.8% vs 10%–33%).1, 25, 46 We found lower seizure frequencies among brain tumor (17.2% vs 23%–54%)1, 25 and TMI encephalopathy (4.3% vs 18%–60%)1, 25 patients compared with previous reports. These differences could be secondary to variation in sample size, variability of population, definition of electrographic seizures, and subjective decision about when to order cEEG. Additionally, these seizure frequencies are likely an overestimation because cEEG was initiated based on clinical suspicion representing a selection bias.
Electrographic seizures were more frequent in patients with any IEDs1, 12, 14 including isolated IEDs (40.9%), LPDs (68.7%), and GPDs (27.3%), in the presence of brain bleeds,1, 2, 20, 25, 28-42 history of epilepsy1 (26.0%), and clinical seizure-like event1 (16.8%), similar to previous studies. Seizures were more common in patients on ASMs (17.2%), especially those on polytherapy (26.0%) at cEEG initiation, but this is likely because many of these patients had epilepsy history. Seizures were less frequent in awake patients (11.3%) and those with cEEG indication of altered mental status (8.2%).
Additionally, seizures were more frequent in any type of acute brain insults (17.2%), acute SDH (26.7%), mixed bleeds (26.1%), less common causes of acute brain insults (25.1%) including CNS infection, postneurosurgery, PRES, VST, demyelination, autoimmune brain disease, vasculopathy, and CJD, any remote brain insults (19.8%), and remote autoimmune disease (45.5%). When patients presented with epilepsy-related breakthrough seizures, the risk of seizures was significantly higher. The seizure risk was significantly lower in TMI encephalopathy patients and those without a clear etiology of presentation. These identify some additional subpopulations at higher or lower risk of NCS.
Despite low seizure frequency among awake patients, presence of IEDs (SW, GPDs, and/or LPDs) increased seizure detection by several folds. Irrespective of mental status, LPDs increased seizure risk by several folds. SWs increased seizure risk among lethargic patients, and GPDs increased seizure risk in stuporous and comatose patients as well but by much lower percentage in comparison with their effect on seizure risk among awake patients.
Our study has several limitation including retrospective nature, variety of neurological diagnosis, and nonuniform monitoring duration. Median monitoring duration was longer in seizure than in nonseizure patients. Suboptimal monitoring duration among nonseizure patients is a concern, especially for those with <24 hour of monitoring. The newly found effect of noncoma alterations of consciousness (stupor and lethargy) could be because of the difference in definition of altered mental status (more categories) and previous studies. However, the additional categories of altered mental status included in our study will likely be helpful in clinical practice. Another limitation of our study is that a large number of comparisons were made without adjusting the statistical threshold for multiple comparisons.
- Given the linear trend of seizure detection, standard monitoring duration of 36 hours appears more optimal than 24 hours. As this duration represents an average across subgroups of patients, it is most relevant when little information is available with regard to other clinical and EEG risk factors.
- For awake patients, seizure risk is low and detection is early. Hence, in the absence of IEDs, less than 24 hour of monitoring is likely sufficient.
- In addition to comatose patients as previously established, stuporous (>48 hours) and lethargic patients (~48 hours) should undergo at least 48 hour of monitoring.
- Presence of preceding LPD and GPDs increase risk of delayed seizure detection. Their presence on cEEG should warrant 48 hour of monitoring.
- Patients with any type of acute brain insults should undergo 48 hour of monitoring. Of these, brain bleed represents the highest risk group for delayed detection, especially those with multiple concomitant bleeds.
- Even though witnessed seizure-like event patients are at high seizure risk, most are detected early on, and hence, 24 hour of monitoring is sufficient for them. If the indication for cEEG is altered mental status, 48 hours of monitoring should be considered.
- Patients on ASMs at cEEG initiation have higher odds of delayed seizure detection and should undergo 48 hours of monitoring.
ACKNOWLEDGMENT
This research did not receive grant support from public, commercial, or not-for-profit sector funding agencies.
CONFLICT OF INTEREST
All other authors declare no conflicts of interest relevant to this study. We confirm that we have read the Journal's position on issues involved in ethical publications and affirm that this report is consistent with those guidelines.
AUTHORS CONTRIBUTIONS
IZ and SH conceived and designed the study. IZ, SH, and IB acquired and analyzed the data. IZ drafted a significant portion of the manuscript and figure. Statistical analysis was performed by Mr Isaac Briskin MS.