The AMPA receptor antagonist perampanel suppresses epileptic activity in human focal cortical dysplasia
Gavin L. Woodhall and Mark O. Cunningham contributed equally.
Abstract
Focal cortical dysplasia (FCD) is one of the most common malformations causing refractory epilepsy. Dysregulation of glutamatergic systems plays a critical role in the hyperexcitability of dysplastic neurons in FCD lesions. The pharmacoresistant nature of epilepsy associated with FCD may be due to a lack of well-tolerated and precise antiepileptic drugs that can target glutamate receptors. Here, for the first time in human FCD brain slices, we show that the established, noncompetitive α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist, perampanel has potent antiepileptic action. Moreover, we demonstrate that this effect is due to a reduction in burst firing behavior in human FCD microcircuits. These data support a potential role for the treatment of refractory epilepsy associated with FCD in human patients.
KEY POINTS
- FCD IIb lesions are frequently pharmacoresistant and require alternative therapeutic options
- Ex vivo electrophysiological studies in human FCD IIb brain slices obtained from neurosurgery reveal that a clinically approved AMPA receptor antagonist can inhibit seizure activity at therapeutically relevant concentrations
- These findings suggest that pharmacological approaches that antagonise AMPA receptors may be worth exploring in the treatment of seizures associated with FCD IIb
1 INTRODUCTION
Focal cortical dysplasia type IIb (FCD IIb) lesions are notoriously epileptogenic and associated with drug-refractory epilepsy. From a histological point of view, FCD IIb presents with cytoarchitectural abnormalities of the neocortex, often with the presence of balloon cells, dysmorphic neurons, and hypomyelinated white matter. Due to the propensity for pharmacoresistance in FCD IIb, a significant proportion of patients will undergo surgical resection of the lesion in order to control seizures. The ability to conduct a “complete” resection can be complicated by a number of factors. Crucially, due to the fact that FCD IIb lesions frequently occur close to eloquent cortex and important fiber tracts, it is not always possible to conduct a full resection as this may lead to postsurgical neurological deficits. Therefore, alternative therapeutic approaches for epilepsy arising from this particular type of lesion are required.
There is evidence to suggest a role for glutamate in the refractory nature of FCD IIb. Increased glutamatergic input and altered expression/function of N-methyl-D-aspartate receptors has been demonstrated in surgical samples from patients with FCD IIb. An abundance of vesicular glutamate transporter 1 (VGLUT1)–positive synapses on dysmorphic neurons in the epileptogenic focus of a FCD IIb1 is likely to lead to increased cortico-cortical excitatory input to dysplastic regions. Moreover, there have also been reports of increased glutamate signal in some patients with cortical developmental malformations as detected by magnetic resonance imaging.2 Indeed, seizure generation in human microcircuits is dependent upon the emergence of population glutamatergic activity.3
Given the refractory nature of epilepsy caused by FCD IIb lesions and the hypothesis that glutamate may be elevated in and around FCD IIb lesions, we have aimed to examine the impact of an antiepileptic drug that acts to noncompetitively antagonize α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. A number of compounds have been demonstrated to antagonize the AMPA receptor. These include experimental compounds such as 6-nitro-2,3-dioxo-1,4-dihydrobenzo[f]quinoxaline-7-sulfonamide (NBQX) and LY293558. A number of AMPA receptor antagonists have been employed in clinical trials (eg, talampanel), but only one has been approved for clinical use, This drug, perampanel (Fycompa), is capable of maintaining the closure of AMPA receptors even in the presence of elevated glutamate levels. Perampanel is currently licensed for the adjunctive treatment of partial-onset seizures with or without generalized seizures in children (≥12 years old) and adults and has also been approved for use in genetic generalized epilepsy.4 Previous ex vivo studies have examined the impact of perampanel on epileptiform activity induced by the addition of picrotoxin5 and altered extracellular potassium (K+) and magnesium (Mg2+) levels in the artificial cerebrospinal fluid (ACSF) in resected hippocampal slices from a single juvenile patient with refractory temporal lobe epilepsy.6 A recent paper has demonstrated the ability of perampanel to suppress spontaneous epileptiform activity via a selective inhibitory effect on excitatory posy-synaptic currents.7 Here, for the first time, we demonstrate the efficacy of perampanel on ex vivo human ictal activity arising from a neocortical developmental abnormality associated with intractable epilepsy.
2 METHODS
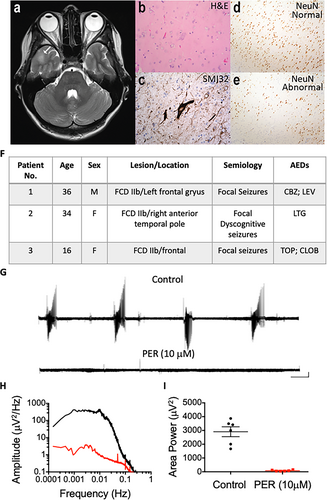
The electrophysiological data obtained from slice studies were derived from three patients with medically intractable epilepsy who were undergoing elective neurosurgical tissue resection for the removal of a suspected FCD. Before surgery, all patients gave their informed consent for the use of the resected brain tissue for scientific studies. This study was approved by the County Durham & Tees Valley 1 Local Research Ethics Committee (06/Q1003/51) (date of review 03/07/06) and had clinical governance approved by the Newcastle upon Tyne Hospitals NHS Trust (CM/PB/3707). Studies at Aston were approved by the Black Country Local Ethics Committee (10/H1202/23; 04/30/10), the Aston University ethics committee (Project 308 cellular studies in epilepsy), and through the Research and Development Department at Birmingham Children's Hospital (IRAS ID12287).
2.1 In vitro human neocortex recordings
Briefly, human cortical samples were derived from material removed as part of surgical treatment of medically intractable cortical epilepsy from the left parietal lobe, left frontal lobe, and right temporal lobe regions with written informed consent of the patients (N = 3). Slice preparation and extracellular recording were conducted using methods as previously described.8 The time between resection and slice preparation was <5 minutes. Multielectrode array (MEA) recordings were conducted using Buzsaki-style probes (64-electrode; NeuroNexus Technologies) connected to an Intan RHD2000-series amplifier system (Intan Technologies). Signals were amplified and digitized (20 kHz) using the Intan system and downsampled to 2 kHz for offline analysis of the extracellular local field potential (LFP) using MATLAB (MathWorks).
2.2 Extracellular data and statistical analysis
All statistical calculations were performed with GraphPad Prism (La Jolla, California).
3 RESULTS
Similar to previous studies, spontaneous epileptic activity was not observed in slices prepared from resected tissue from patients with FCD.10, 11 In order to elicit epileptiform activity, slices were perfused with modified ACSF containing reduced Mg2+ (0.25 mM) and elevated K+ (8 mM). Previous studies have demonstrated that the use of this modified ACSF (mACSF) is known to induce ictal events in brain slices obtained from patients with a history of epilepsy.3 Preliminary data from our group have shown that application of this mACSF to nonepileptic comparison tissue does not elicit epileptic activity (Cunningham et al, unpublished data). These findings suggest that this particular type of human epileptic tissue demonstrates a reduced threshold for ictal behavior due to alterations in cellular and network excitability.10 Following application of the mACSF, recurring ictal events emerged (Figure 1G), and once established, a mean (± standard error of the mean) area power value of 2807 ± 418.1 µV2 was observed across all samples examined (N = 6). Upon application of perampanel (10 µM), ictal events were abolished and power spectral analysis revealed that the area power was significantly reduced to 114.6 ± 30.1 µV2 (paired student t test, P < .05; Figure 1H,I).
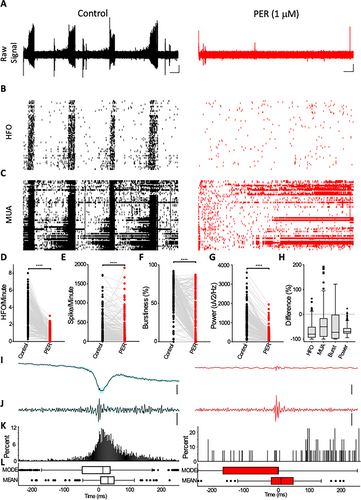
In patients with epilepsy, plasma concentrations of perampanel have been reported to vary between 1.06 and 3.26 µM.12 In order to examine the effect of a therapeutic concentration of the drug on FCD epileptic activity, we next tested the drug at a lower concentration (1 µM). In these studies, we used MEA technology in an attempt to capture the activity of single neurons in tandem with LFP epileptic activity and, therefore, understand the impact of perampanel on collective neuronal firing behavior. As was observed in the glass microelectrode studies (Figure 1G-I), the addition of perampanel (1 µM) significantly reduced the power of ictal LFP activity (Figure 2A) (control – median: 872.6 μV2/Hz, interquartile range [IQR]: 384.5-1434 vs perampanel – median: 214.3 μV2/Hz, IQR: 110-352.8; reduction of 69.1%, IQR: −76.96 to −55.59) recorded using MEAs (Figure 2D). Recordings with MEAs revealed bursts of single-unit action potentials occurring concurrently with the ictal LFP event (Figure 2C). The addition of perampanel significantly reduced the spike count (control – median: 201.3 spikes/minute, IQR: 72.7-439.7 vs perampanel – median: 65.9 spikes/minute, IQR: 4.28-274.6; reduction of 49.96%, IQR: −89.97 to −15.95), and bursting behavior (control – median: 61.9%, IQR: 46.3-77.5 vs perampanel – median: 11.9%, IQR: 0.02-51.3%; reduction of 72.3%, IQR −97.6 to −1.5 (Figure 2E-G)). High-frequency oscillations (HFOs), a hallmark of epileptogenic networks, were also significantly reduced by the application of the drug (control – median: 3.06 HFO/minute, IQR: 2.02-4.38 vs perampanel – median: 0.53 HFO/minute, IQR: 0.27-1.05; reduction of 79.51%, IQR: −93.48 to −50.19 (Figure 2D)). Perampanel was also observed to significantly reduce the degree of cross correlation between multiunit activity and HFOs (Figure 2I-L).
4 DISCUSSION
These electrophysiological and pharmacological data demonstrate that the use of perampanel is effective in reducing seizure-like activity in neocortical slices resected from human patients with FCD, suggesting potential benefit for the use of this drug in treating pharmacoresistant epilepsy in this patient group.
Mechanistically, perampanel has been demonstrated to be a potent noncompetitive inhibitor of AMPA receptors.13 Studies conducted in rodent hippocampal slices have demonstrated the direct inhibition of AMPA-receptor–mediated currents,14 and AMPA-mediated synaptic transmission.15 AMPA receptors are crucial for generation, synchronization, and spread of epileptic discharges.16 In cortical tissue removed from patients with partial-onset epilepsy, AMPA receptor density is increased,17 and the sensitivity of the receptor to glutamate enhanced by altered RNA editing.18
Previous work has suggested that AMPA receptors are a potential target for therapeutic intervention in patients with epilepsy associated with FCD. In one study examining mRNA expression, AMPA receptor subunit transcripts (GluR4) were increased in dysplastic neurons.19 The mammalian target of rapamycin (mTOR) pathway has emerged as a primary pathogenic mechanism underlying cortical lesions such as FCD IIb. Preclinical and clinical studies have demonstrated the effectiveness of mTOR inhibitors in treating FCD, although the mechanism remains unclear. Studies in neuronal cultures have demonstrated that mTOR inhibitors significantly reduced the surface expression of AMPA receptors on cortical neurons,20 thus supporting a potential role for AMPA receptors in epileptic FCD networks. Pharmacological blockade of AMPA receptors constitutes a more readily available therapeutic option. Extracellular recordings in human epileptic tissue have previously demonstrated that ictal events are sensitive to AMPA receptor antagonism using a competitive blocker (6-nitro-2,3-dioxo-1,4-dihydrobenzo[f]quinoxaline-7-sulfonamide; NBQX).3 Whole-cell patch clamp recordings in human FCD brain slices have shown that excitatory postsynaptic currents mediated via AMPA receptors where abolished by perampanel, whereas inhibitory events mediated via GABAA receptor were relatively unaltered.7 This differential effect of perampanel is likely to underlie the profound antiseizure effect we report in our current study. In the present study, concentrations similar to those used by Wright et al (2020)7 (10 μM) produced a complete suppression of ictal activity. In addition, we have showed that the suppression of ictal LFP activity and associated HFOs is maintained by concentrations of perampanel likely to be observed in plasma concentrations in human patients (c. 1 μmol/L).12 The MEA recordings in human tissue revealed that the pathological bursting activity of neurons (which is coincidental with ictal discharges) is strongly inhibited. Interestingly, in the presence of the lower concentration of perampanel, a large proportion of neurons are still active. This finding supports the notion that AMPA-mediated synaptic conductances are critical for bursting behavior that drives ictal activity16 and perampanel is capable of blocking these specific synaptic conductances and thus limiting associated epileptic activity (Figure 2H) in FCD neuronal microcircuits.
Additional work is required to understand the role of AMPA receptors in FCD and, in particular, the therapeutic potential of perampanel in this patient group. Our ex vivo human tissue findings are, to a degree, corroborated clinically by a retrospective analysis study21 showing seizure suppression with perampanel in adolescent patients with FCD. It remains to be seen if the results presented here are translationally robust in a clinical setting. A recent observational multicenter study has shown efficacy and safety for perampanel as an adjunctive therapy in a drug-resistant focal epilepsy patient cohort that had a significant number of participants with focal cortical dysplasias.22 In that respect, a randomized, controlled trial examining the clinical efficacy of perampanel in patients with FCD is warranted.
ACKNOWLEDGMENTS
This work has been supported by investigator-initiated grants from Eisai (MOC and GLW), Birmingham Children's Hospital Charities grant BCHRF349 (SS, GLW) and Aston Brain Centre (GLW), the Wellcome Trust/EPSRC (102037; RGW and MOC), and a CAPES-funded (Brazil) PhD studentship (BEX-0437-14-0; ABDS, RGW, and MOC). Editorial support, under the direction of the authors, was provided by Stephanie Agbu, PhD, of CMC AFFINITY, a division of Complete Medical Communications Inc, Hackensack, NJ, USA, funded by Eisai Inc, in accordance with Good Publication Practice (GPP3) guidelines.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose.
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
AUTHOR CONTRIBUTIONS
MRB, SS, RSGJ, GLW, and MOC contributed to the conception and design of the study; ABdS, BH, SDG, RW, JP, KC, DB, RWW, AJ, HK, CN, MRB, GLW, and MOC contributed to the acquisition and analysis of data; and GLW, RSGJ, MRB, and MOC provided a substantial contribution to drafting the paper.