Add-on cannabidiol significantly decreases seizures in 3 patients with SYNGAP1 developmental and epileptic encephalopathy
Abstract
Mutations in SYNGAP1 are associated with developmental delay, epilepsy, and autism spectrum disorder (ASD). Epilepsy is often drug-resistant in this syndrome with frequent drop attacks. In a prospective study of add-on cannabidiol (CBD), we identified three patients with SYNGAP1 mutations: two boys and one girl. Seizure onset was at 3.5, 8, and 18 months (M), respectively, with numerous atypical absences per day associated with eyelid myoclonia (2/3 patients), upper limb myoclonic jerks (2/3 patients), and drop attacks (all patients). Seizures were resistant to at least 5 antiepileptic drugs (AEDs). After CBD introduction, two patients were responders since M2 and achieve a seizure reduction of 90% and 80%, respectively, at M9 with disappearance of drop attacks. EEGs showed an improvement regarding background activity and interictal anomalies. The last patient showed a late response at M7 of treatment with an 80% decrease in seizure frequency. Caregiver in all three evaluated as much improved the status of their children. Treatment was well-tolerated in all, and no major adverse events (AEs) were reported. CBD showed efficacy in patients with drug-resistant epilepsy due to SYNGAP1 mutations. Other patients with rare genetic developmental and epileptic encephalopathies with drug-resistant epilepsies might benefit from CBD.
1 INTRODUCTION
SYNGAP1 encephalopathy is a rare developmental and epileptic encephalopathy characterized by developmental delay, intellectual disability, epilepsy, and autism spectrum disorder.1, 2 Epilepsy is reported in 84%–98% of patients.2, 3 In most of the cases, seizures are numerous daily and include absences and myoclonic-atonic seizures with drop attacks.3 Reflex seizures are also frequent, sometimes self-induced.3-5 The main antiepileptic drugs (AEDs) used in this syndrome are valproate (VPA), lamotrigine (LTG), and levetiracetam (LVT).2, 3 However, at least 50% of patients present a drug-resistant epilepsy.1-3, 5 Since September 2019, European Medicines Agency authorized cannabidiol (CBD, Epidyolex®) as clobazam adjunctive therapy for patients older than 2 years in Dravet or Lennox-Gastaut syndromes. In these indications, CBD allowed a decrease in more than 50% of the seizures in almost 40% of patients.6-8 However, some open-label studies highlighted the possible efficacy of CBD in other drug-resistant epilepsies with a similar efficacy rate.9
We aimed to study the efficacy and safety of CBD in patients presenting SYNGAP1 developmental and epileptic encephalopathy with drug-resistant epilepsy followed at our center.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| SYNGAP1 mutation |
c.1966G > T p.(Glu656*) |
c.992T > C (p.Trp308Arg) |
c.2059C > T p.(Arg687*) |
| Sex | M | F | M |
| Onset of epilepsy (years) | 3.5 | 1.5 | 0.66 |
| Age at CBD introduction (years) | 6 | 7.5 | 3.5 |
| Maximum CBD dose | 10 mg/kg/d | 17 mg/kg/d | 23 mg/kg/d |
| Concomitant AEDs | VPA, LTG | LTG, ZNS, CLB | CLB |
| Seizure type | AA, M, DA | AA, DA | MA, DA |
| Seizure frequency (per day) | 20-30 | 20 | 40-100 |
| Follow-up duration (months) | 11 | 14 | 13 |
| Seizure frequency reduction | |||
| At M2 | 85% | 50% | 0% |
| At M9 | 95% | 80% | 80% |
- Abbreviations: AA, atypical absence; AEDs, antiepileptic drugs; CBD, cannabidiol; CLB, clobazam; DA, drop Attack; F, female; LTG, lamotrigine; M, male; M, myoclonia; MA, myoclonic absence; VPA, valproate; ZNS, zonisamide.
2 METHODS
This study was a prospective study on safety and efficacy of CBD for patients aged from 2 to 18 years with pharmacoresistant epilepsies starting CBD therapy as add-on, between March and September 2019. We included in this study 3 patients with SYNGAP1 mutations presenting drug-resistant epilepsy and followed in our institution. CBD was obtained through a nominative temporary authorization for use (nATU), a special regimen for medicine administration before its availability on the market requiring the French Medicine Agency approval for each patient.
We evaluated the efficacy and safety at 1 month (M1), 2 months (M2), 6 months (M6), and 9 months (M9) after the introduction of CBD. We followed prospectively the number of seizures compared with a baseline period of one month prior to CBD introduction, adverse events (AE) as noted by the families with biological follow-up for liver function at M1, M2, and M6, and global evaluation of the parents and the physician by the clinical global impression-improvement scale at each visit.
This study was approved by the ethics committee of our institution Necker Hospital, APHP. All participants or their legal guardians signed an informed consent to be included in the data analysis.
3 RESULTS
Three patients with SYNGAP1 encephalopathy with drug-resistant epilepsy were included: 2 boys and 1 girl. They were 4.5, 7, and 8 years old at inclusion. All presented with cognitive impairment, ASD, and drug-resistant epilepsy (Table 1).
3.1 Patient 1
He was a boy born at term to healthy, nonconsanguineous parents after an uneventful pregnancy. He had early delayed psychomotor milestones with walking at 19 months and single-word sentences around 18 months. Behavior was marked by motor stereotypies and heteroaggressivity, and he had a diagnosis of ASD at 2 years.
He had two simple febrile seizures around 2.5 years. At the age of 3.5 years, he developed photosensitivity with episodes of eyelid myoclonia and absences with drop attacks in addition to upper limb myoclonic jerks from the age of 4 years. Episodes were short (5-7 seconds) and occurred several times a day (20-40/day). Atypical absences and myoclonia were activated by sleep and intermittent light stimulation (IPS).
Magnetic resonance imaging (MRI) showed nonspecific delayed myelination of both temporal lobes. A de novo heterozygous mutation c.1966G>T p.(Glu656*) was identified in SYNGAP1. Seizures persisted despite the administration of valproate (VPA) in combination, respectively, with levetiracetam (LEV), ethosuximide (ESM), lamotrigine (LTG), and ketogenic diet (KD).
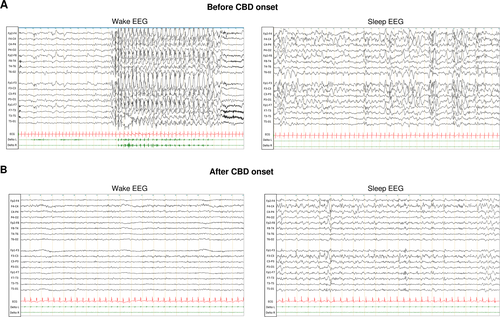
Add-on CBD to VPA and LTG was started at the age of 5.6 years and titrated to reach 10 mg/kg/d in 1 month. Parents reported disappearance of drop attacks after 2 weeks and a significant reduction in frequency and duration of absences with eyelid myoclonia. He showed at M2 a decrease of 85% in seizure frequency. After 9 months of treatment, he had a reduction of 95% in seizure frequency with few seizure-free days. EEG at M6 was markedly improved in terms of background activity and interictal anomalies compared with the recording before CBD (Figure 1). CBD treatment was well-tolerated without AEs reported by the family and no change in the liver enzymes at M9. Efficacy was maintained at the last visit at M11.
3.2 Patient 2
A girl was born at term to nonconsanguineous parents after an uneventful pregnancy without any family history of neurological disorders. She had psychomotor delay and achieved head control at 9 months and walking at 3.5 years. She presented speech delay, motor stereotypies, and ASD.
Onset of epilepsy was at 18 months with several atypical absence episodes per day and since the age of 3 additional atonic seizures with drop attacks. EEG showed slow background activity with no physiological features of sleep. Spikes, spike waves, and, more rarely, polyspikes in occipital regions with tendency to diffusion and numerous atypical absences with atonic component activated by sleep were reported.
Genetic analyses have revealed a de novo heterozygous SYNGAP1 mutation c.992T>C (p.Trp308Arg).
Treatment with VPA, ESM, zonisamide (ZNS), clobazam (CLB), LTG, and KD, in monotherapy or in combination with vagus nerve stimulation (VNS), did not show any efficacy.
CBD therapy was started, as add-on to LTG and ZNS, at age of 7.5 years. A dose of 10 mg/kg/d was reached in one month and gradually increased to 17 mg/kg/d. At M2, family reported a 50% reduction in seizure duration and frequency. She had persistent isolated myoclonic seizures but without drop attacks.
At M6, we added CLB after secondary increase seizure frequency. This association has led to a rapid decrease of 80% in seizure frequency. LTG was stopped progressively from M9. Her last EEG was improved with less epileptic anomalies. She is at the last follow-up at M14 on CBD, CLB, and ZNS with a maintained efficacy. Parents and caregivers did not report any AE. A slight increase (x1.5) in liver enzymes was reported and was stable during follow-up.
3.3 Patient 3
He was a boy born at term from nonconsanguineous parents with his paternal grandmother presenting an unspecified epilepsy.
Seizures initiated at 8 months with upward rolling of the eyes (up to 180 episodes/d), eyelid myoclonia, and several drop attacks per day occasionally associated with upper limb myoclonic jerks.
He had normal psychomotor milestones but showed language delay and developmental slowing from the age of 2.5 years. Revised Brunet-Lezine score showed a global developmental quotient of 53 at age of 32 months.
Reflex seizures self-induced by eye closure started at 3 years, and EEG recorded myoclonic absences with 2-Hz generalized polyspike wave discharges. Genetic testing identified a heterozygous de novo mutation c.2059C>T p.(Arg687*) in SYNGAP1. MRI was normal.
He did not respond to ESM, LEV, LTG, VPA, KD, CLB, ZNS, and perampanel (PER). Add-on CBD to CLB was introduced at the age of 3.5 years and increased to reach a 23 mg/kg/d dose at M6. A sleep disorder was noted during CBD treatment, improved with melatonin prescription. No other AEs were reported.
Treatment with CBD did not decrease seizures but was maintained after M6 because of parents' perception and rehabilitation teams’ perception of improvement with an improved alertness and interaction. At M7, without any other drugs or nonpharmacological approaches added, parents reported a seizure decrease of 80%, especially for drop attacks. The response was maintained at M13 at the last visit.
4 DISCUSSION
Recently described as causative of developmental and epileptic encephalopathy, reports of patients with SYNGAP1 mutations are rapidly increasing.10-12 More than 120 patients are reported, 98% had intellectual disability (122/124), 91% seizures (113/124), and 55% ASD (68/124). Seizures are often frequent reaching more than 100 seizures per day, with significant drug resistance, as in our 3 patients.2 In a large cohort, six patients were reported to have CBD and remained on this treatment without further details.3
All 3 patients in our report had a decrease in seizure frequency ranging from 80% to 95%. This decrease in drop attacks and myoclonic seizures had a positive impact on patients' general condition especially that these two types of seizures are significantly associated with a high risk of seizure-related injuries.13
The efficacy of CBD in patients with SYNGAP1 mutations may be supported by the functional impact of SYNGAP1 mutations and the mechanisms of action of CBD on the transient receptor potential cation channel subfamily V member 1 (TRPV1). Syngap1 heterozygous mutations generate an increase in different proteins of Ras-dependent pathways in particular TRPV1.14-16 In fact, the increase in TRPV1 might be one of the mechanisms promoting excitatory/inhibitory imbalance in epilepsy17, 18 and specifically in drug-resistant epilepsy related to SYNGAP1 mutations. CBD is known to generate a rapid activation and desensitization of TRPV1 in a dose-dependent manner19-22 and might target in this way the TRPV1 increase induced by SYNGAP1 mutations resulting in seizures’ reduction.
The response to CBD add-on was obtained within M1-M2 in 2 patients, and surprisingly, the third had a late response at M7. Although we cannot confirm that the response is exclusively due to CBD, this could argue for a long trial duration before asserting that CBD is not effective on seizures, especially when it shows benefits on alertness and behavior and a good tolerance.
Response to CBD was maintained after 11 to 14 months of therapy. Patient 1 had CLB in association as recommended by the EMA.23 Patient 2 had CLB adjunction after partial losings of efficacy on CBD, and efficacy was secondarily restored. CBD-CLB concomitant use might increase the efficacy and should be tried in patients presenting a partial response without CLB comedication.
Studies to evaluate the efficacy of CBD in patients with SYNGAP1 over a larger population and on a longer follow-up period would be of interest to confirm these preliminary results.
5 CONCLUSION
CBD add-on therapy in patients with SYNGAP1 encephalopathy showed a good response in three patients with a good safety profile and a late response in one patient. This therapy should be included in the treatment algorithm of patients with SYNGAP1 mutations presenting drug resistance epilepsy and might be expanded to other rare genetic epilepsies that might not be included in formal trials.
ACKNOWLEDGMENTS
We would like to thank the families of these three patients for their active participation in this study. This work was supported (RN) by state funding from the Agence Nationale de la Recherche under “Investissements d'avenir” Program (ANR-10-IAHU-01) and the “Fondation Bettencourt Schueller”.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.