Modeling PCDH19 clustering epilepsy by Neurogenin 2 induction of patient-derived induced pluripotent stem cells
Abstract
Background
Loss of function mutations in PCDH19 gene causes an X-linked, infant-onset clustering epilepsy, associated with intellectual disability and autistic features. The unique pattern of inheritance includes random X-chromosome inactivation, which leads to pathological tissue mosaicism. Females carrying PCDH19 mutations are affected, while males have a normal phenotype. No cure is presently available for this disease.
Methods
Fibroblasts from a female patient carrying frameshift mutation were reprogrammed into human induced pluripotent stem cells (hiPSCs). To create a cell model of PCDH19-clustering epilepsy (PCDH19-CE) where both cell populations co-exist, we created mosaic neurons by mixing wild-type (WT) and mutated (mut) hiPSC clones, and differentiated them into mature neurons with overexpression of the transcriptional factor Neurogenin 2.
Results
We generated functional neurons from patient-derived iPSC using a rapid and efficient method of differentiation through overexpression of Neurogenin 2. Was revealed an accelerated maturation and higher arborisation in the mutated neurons, while the mosaic neurons showed the highest frequency of action potential firing and hyperexcitability features, compared to mutated and WT neurons.
Conclusions
Our findings provide evidence that PCDH19 c.2133delG mutation affects proper metaphases with increased numbers of centrosomes in stem cells and accelerates neuronal maturation in premature cells. PCDH19 mosaic neurons showed elevated excitability, representing the situation in PCDH19-CE brain. We suggest Ngn2 hiPSC-derived PCDH19 neurons as an informative experimental tool for understanding the pathogenesis of PCDH19-CE and a suitable approach for use in targeted drug screening strategies.
Key points
- PCDH19 c.2133delG mutation increases the number of centrosomes in stem cells.
- Ngn-2 hiPSC-derived PCDH19-mutated neurons maturate earlier than wild type.
- Ngn-2 hiPSC-derived PCDH19 mosaic neurons show an elevated excitability.
1 INTRODUCTION
Protocadherin 19 (PCDH19) is one of the genes in which pathogenic variant mosaicism causes epilepsy-related neurodevelopmental disorder1 and is the second most relevant gene in epilepsy after SCN1A.2 Heterozygous loss-of-function (LOF) mutations in PCDH19 located on the X-chromosome cause an epileptic encephalopathy characterized by seizure onset in infancy, mild to severe intellectual disability (ID) and often accompanied by autistic spectrum disorder (ASD) and psychosis.3
PCDH19 encodes delta2-protocadherin protein, which is a cell adhesion molecule. PCDH19 is highly expressed during development and it is supposed to mediate cell–cell interaction within neuronal circuits and to be involved in neuronal migration or establishment of synaptic connections.1, 2 PCDH19 gene is located on the short arm of the X-chromosome (Xq22.1). Different from the most X-linked diseases, the PCDH19 epilepsy demonstrates a unique pattern of inheritance. The disease is caused by a random X-chromosome inactivation, which leads to tissue mosaicism where the part of the cells expresses wild-type PCDH19 and part—mutant PCDH19. Males carrying PCDH19 mutations have normal phenotype without seizures and ID while females are affected. This mechanism is so-called “cellular interference” implying that the disease mechanism is based on co-existence of two populations of cells, one expressing the wild-type and the other the mutant allele.4
Rare affected males are somatic mosaics carrying postzygotic somatic mutations occurring in some cells of an embryo5 and show a similar neuropsychiatric profile to females.6, 7 Transmitting males who can be null for PCDH198 do not show clinical signs of the disease, which suggests that the function of the protein might be dispensable in the developing brain. With an increasing number of studies on affected males, a new term was proposed for the disease – PCDH19 Clustering Epilepsy (PCDH19-CE)7 instead of female-restricted epilepsy with ID, which we are using in this manuscript.
Studies of neuronal functions in human disease mechanisms are limited by the difficulties to obtain patients' cells from the CNS. Human induced pluripotent stem cell (hiPSC) technologies have made it possible to study human neurons and explore physiopathology in vitro.9-11 In this study we used direct differentiation of hiPSC into mature neurons with overexpression of the transcription factor Neurogenin 2, which provides electrophysiologically active neurons in 3 weeks.12-14
Here, we recapitulated PCDH19-CE in vitro model through the generation of patient's hiPSC-derived neurons to investigate the pathogenetic mechanisms underlying the disease and assess the functional and electrical activity properties.
2 MATERIALS AND METHODS
2.1 Study subject and sample collection
A 19-year-old female patient was diagnosed with PCDH19-CE at the Medical Genetics Unit of the Azienda Ospedaliero-Universitaria Senese (Siena, Italy) after Sanger sequencing of PCDH19 gene. Previously, she was tested for mutations in the SCN1A gene—the most common gene causing epilepsy2 and resulted negative. Genomic DNA of the patient was isolated from EDTA peripheral blood samples using a QIAamp DNA Blood Kit according to the manufacturer's protocol (Qiagen, Hilden, Germany). The frameshift mutation c.2133delG (p.Thr712Pro) was revealed in the blood cells' DNA. The mutation is not yet reported in the literature. The patient has focal epilepsy, mild ID, mental retardation, and motor deficits.
A skin specimen containing epidermis, dermis, and superficial fat was obtained from a punch biopsy of the forearm under local anesthesia. The sample was stored in phosphate buffered saline at room temperature and processed shortly after collection. The study protocol was approved by the Institutional Ethics Committee of the University of Siena/Azienda Ospedaliero-Universitaria Senese, Siena, Italy. The patient (and/or her parents) provided and signed a written informed consent at the Medical Genetics Unit for clinical data usage, and the use of DNA samples for both research and diagnosis purposes.
2.2 Cell cultures
All types of cells were cultured in the CO2 incubator (Heracell, 37°C, 95% humidity, and 5% CO2). Skin biopsy material from the patient was cut with a sterile blade into pieces of 1–2 mm and put into complete CHANG Medium C (Irvine Scientific, FUJIFILM) for the expansion of fibroblasts. The medium was changed every day until cells started to proliferate and reached 80% confluence. Primary dermal fibroblasts were cultured in the same complete medium. hiPSCs were grown in complete StemFlex medium (Thermo Fisher Scientific, USA) on Geltrex (Thermo Fisher Scientific, USA) coated plates. Cells were routinely passed with appropriate enzymes as soon as they reached 80%–90% confluence. All primary cells and cell cultures were grown in a medium with 1% penicillin–streptomycin (10 000 U/mL, Thermo Fisher Scientific, USA).
2.3 Generation of human-induced pluripotent stem cell and human-induced pluripotent stem cell–derived neurons
PCDH19-mutated hiPSC clones used in the study were generated and characterized by Marina Cardano from the Cell Technology Facility (CIBIO—University of Trento). HiPSC clones were obtained from patient's skin fibroblasts using Sendai virus particles delivering Yamanaka factors (CytoTune-iPS 2.0 Sendai Reprogramming Kit, Thermo Fisher Scientific, USA). Mature neurons were obtained from hiPSC through the overexpression of transcription factor Neurogenin-2 (Ngn2) by infection with lentiviral vectors to stably integrate transgenes into the genome of hiPSCs (Figure 1A). The protocol of generation of induced neurons from hiPSCs was modified from.12-14 Briefly, we produced ultra-concentrated Lentivirus encoding Ngn2 and rtTA plasmids (a present from Prof. Anna Margherita Corradi, Department of Experimental Medicine, University of Genoa) using calcium phosphate method of transfection with third generation lentiviral packaging vectors in HEK293T cells.15 RtTA virus encodes a Tet-On Advanced transactivator and the second virus encodes Ngn2 under the control of Tet-controlled promoter, eGFP cassette and blasticidin resistance gene as a fusion protein linked by P2A and T2A sequences (Figure 1B). Doxycycline-induced lentiviral Ngn2 gene expression based on the Tet-On system allowed us to generate electrophysiologically active, mature neurons in 3 weeks.
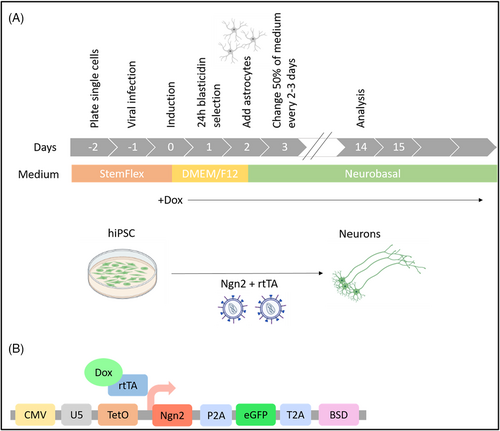
2.4 Molecular biology methods
Genomic DNA and total RNA were isolated from pelleted cells. DNA from cells was extracted using QIAmp DNA Mini Kit (Qiagen, Germany). Total cellular RNA was extracted using TRIzol Reagent and RNeasy mini Kit (Life Technologies, USA). RNA concentration was quantified using Nanodrop-1000 spectrophotometer (Thermo Fisher Scientific, USA). In analyses, only samples of DNA with a 260/280 ratio of 1.8–2.0 and RNA with 260/230 ratio of 2.0–2.2 were used. cDNA was synthesized starting from 500 ng of total RNA with QuantiTect Reverse Transcription Kit (Qiagen, Germany) according to manufacturer's protocol.
The presence of PCDH19 mutation in the DNA of the patient's fibroblasts was confirmed by Sanger sequencing. Genomic DNA isolated from human primary dermal fibroblasts was amplified by PCR with primers flanking mutation sites (GGGCTCTGTGAACTTGTCCT; CAGAGGAGAAAGTGAGCCTA). Primers were designed using the online tool Primer3web version 4.1.0.16 PCR products were purified with Agencourt AMPure XP magnetic beads (Beckman Coulter, USA) and loaded for sequencing reaction with BigDye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific, USA) according to manufacturer's protocol. Samples were loaded on ABI PRISM 3130 Genetic Analyzer (Applied Biosystems, USA) and data were analyzed with Sequencher software V.4.9 (Gene Codes, USA). Sanger sequencing was also performed on coding DNA of hiPSC clones using the same protocol.
X-inactivation analysis was performed on genomic DNA template by standard human androgen receptor (HUMARA) X-chromosome inactivation assay as described in Ref. [17]. using a forward primer labeled with a fluorescent dye (5′-FAM) GCTGTGAAGGTTGCTGTTCCTCAT and reverse primer TCCAGAATCTGTTCCAGAGCGTGC. Samples were loaded with 0.25 μL of GeneScan 500 LIZ dye Size Standard (Thermo Fisher Scientific, USA) on ABI PRISM 3130 Genetic Analyzer (Applied Biosystems, Thermo Fisher Scientific; USA). To analyze PCDH19 expression in patients' cells, cDNA was amplified with the same primers used for genomic amplification as they were designed on two different exons and flanking mutation sites and sequenced on ABI PRISM 3130 Genetic Analyzer (Applied Biosystems, Thermo Fisher Scientific; USA) as described before.
2.5 Characterization of human induced pluripotent stem cells and neurons
Antibodies for immunostaining were used at the following dilutions: PCDH19 (1:300, HPA001461, Sigma, Germany), NeuN (1:300, ab177487, Abcam, UK), SOX2 (1:200, GTX101507, Genetex, USA), Nanog (1:200, sc-376915, Santa Cruz, USA), b3-Tubulin (1:300, sc-80005, Santa Cruz, USA), FOXA2 (1:100, sc-374376, Santa Cruz, USA), T-Brachyury (1:100, sc-166962, Santa Cruz, USA) goat anti-mouse or anti-rabbit IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 568 (1:2500, A-11004 and A-11011, Invitrogen, USA). Samples were analyzed by a confocal laser microscope Leica TCS SP8 (Leica, Germany) with 405 lex and 440–480 lem, 488 lex and 490–540 lem and 561 lex and 578–625 lem for DAPI, GFP and Alexa Fluor 568, respectively. All images were processed using ImageJ software (National Institutes of Health, USA).
2.6 Electrophysiology
Whole-cell patch-clamp electrophysiological recordings were performed by Prof. Maria Passafaro and Luca Murru in the NeuroMI Milan Center for Neuroscience, at the University of Milano-Bicocca with a Multiclamp 700B amplifier (Axon CNS Molecular Devices, USA) and using an infrared-differential interference contrast microscope Nikon Eclipse FN1 (Nikon, Japan). Patch electrodes (borosilicate capillaries with a filament and an outer diameter of 1.5 μm; Sutter Instruments, USA) were prepared with a four-step horizontal puller (Sutter Instruments, USA) and had a resistance of 3–5 MΩ. Voltage- and Current-clamp experiments were performed using an intracellular solution containing (in mM): 126 K-gluconate, 4 NaCl, 1 EGTA, 1 MgSO4, 0.5 CaCl2, 3 ATP (magnesium salt), 0.1 GTP (sodium salt), 10 glucose, and 10 HEPES–KOH (pH 7.28), and an external solution containing (in mM) 138 NaCl, 4 KCl, 2 CaCl2, 1.2 MgCl2, 10 HEPES, and 10 d-glucose, adjusted to physiological pH 7.4 with NaOH. To evaluate hiPSC-derived neurons active and passive membrane properties, a series of depolarizing current steps (0–230 pA) were injected (10 pA per step, 0.5 s duration) to evoke action potential (AP) firing. The AP frequency was correlated to the current injected in an input/output (I/O) curve. AP feature analysis was performed for the first AP evoked at rheobase. Voltage-clamp recordings of inward and outward currents were elicited by 5 mV steps (10 ms) from −75 to +85 mV and neurons were held at −65 mV. Currents were amplified, filtered at 5 kHz and digitized at 20 kHz. All the analyses were performed offline with Clampfit 10.1 software (PClamp, Molecular Devices, USA).
3 RESULTS
3.1 Case description and diagnosis
The patient is 19 years old, female, born at 40 weeks of an uneventful pregnancy by Caesarean section. Cytogenetic analysis on amniotic fluid sampling revealed a normal female karyotype. Birth weight was 3.2 kg, length was 51 cm. The proband showed a normal psychomotor development up to the age of 18 months, when she manifested focal febrile seizures and they continued to occur annually usually lasting several days. Brain MRI at 5 years of age was normal. EEG reported “right temporo-occipital irritative abnormalities”. The patient has been on therapy with phenobarbital and later on with topiramate till the age of 7. At 16 years of age, the patient had a recurrence of epileptic symptomatology, for which polypharmacological therapy was reintroduced (topiramate, carbamazepine and levetiracetam). When she was 11, she obtained a full scale IQ of 70 at WISC scale. A quite good performance at school was reported and at the last examination (19 years old) she was attending university. Frequent episodes of headache and some short-term memory difficulties were also reported. At the time of our first evaluation, the patient was 11 years old and already tested negative for mutations in SCN1A and SCN1B, and negative for chromosomal rearrangements. Exome sequencing was performed when the patient was 19 years old and revealed a single heterozygous frameshift mutation at chrX:99661461 (c.2133delG; p.Thr712Profs*8) within the exon 1 of PCDH19 (NM_001184880). The mutation was then confirmed by Sanger sequencing and is not present in parents.
3.2 PCDH19-CE hiPSCs
Patient's skin fibroblasts were analyzed for X-inactivation and a skewed X-inactivation pattern was confirmed by a HUMARA assay and showed a ratio of 75:25 (data not shown). Sanger sequencing of cDNA from fibroblasts confirmed the presence of WT and mutated alleles. Two hiPSC clones obtained from the patient's skin fibroblasts expressed mutant or WT allele (Figure 2). The cells carrying the wild-type allele of PCDH19 gene were used as control.
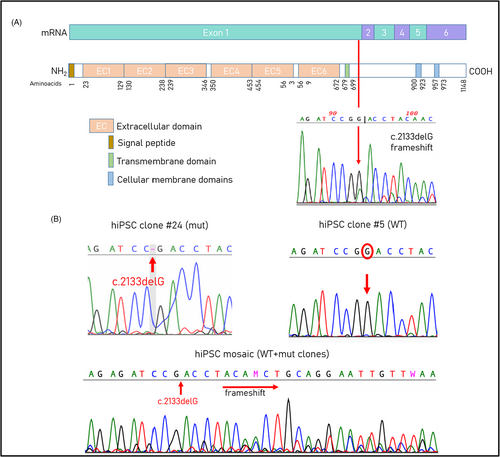
Human induced pluripotent stem cells were fully characterized by immunostaining and tested for self-renewal and pluripotency properties by trilineage differentiation. All clones had a typical hESC-like morphology (Figure 3A) and were able to form embryoid bodies (Figure 3B). HiPSC expressed self-renewal markers NANOG and SOX2 (Figure 3C). In vitro trilineage differentiation confirmed the ability of the clones to spontaneously differentiate into three germ layers (Figure 3D). The staining with anti-PCDH19 antibody showed the localization of the protein in the centrosomes of dividing cells (Figure 3E).

3.3 hiPSC-derived PCDH19-CE neurons
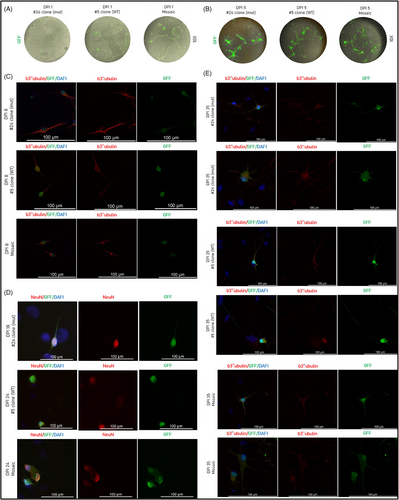
To investigate the role of PCDH19 during neurogenesis, mature neurons were obtained from hiPSC through the forced expression of Ngn2. We aimed to establish a mosaic cell line by mixing WT and mut hiPSC clones in a ratio 1:1, thus creating a cell model of PCDH19-CE where both cell populations co-exist. Neuronal differentiation of the generated hiPSC lines was assessed by immunostaining. Doxycycline-induced GFP expression of differentiated neurons was present from the first day of induction (Figure 4A) and all along the experiment. As expected, we observed neuron-like cells starting from DPI 5 (Figure 4B). All clones were already b3-tubulin positive starting from DPI 8 (Figure 4C) until the DPI 35 (Figure 4E). We stained mature neurons for b3-tubulin to assess the differences in neurite length and ramification between clones (Figure 4E). Mutated clone #24 expressed the mature neuronal marker NeuN at DPI 18 while WT clone #5 and mosaic – at DPI 24. (Figure 4D). HiPSC-derived neurons immunostained with neuronal markers were also GFP positive and easily distinguishable from the DAPI positive rat astrocytes (Figure 4D,E).
3.4 Neuronal excitability
To recreate the mosaic conditions, PCDH19 mut and PCDH19 WT hiPSCs were expanded and mixed in a 1:1 ratio (mosaic neurons). The current-clamp experiments showed a decrease in AP firing frequency upon depolarizing current injection (Figure 5B). We analyzed the features of the AP evoked by the injection of depolarizing current (Figure 5C) and it showed increased threshold and lowest AP amplitude in mut neurons (Figure 5D). We also observed an increased AP width in mosaic and mut clones that suggested alterations in AP kinetic (Figure 5D).
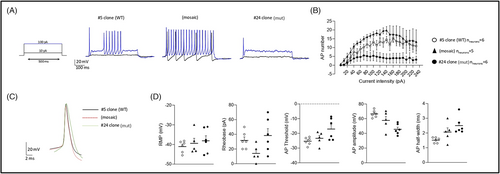
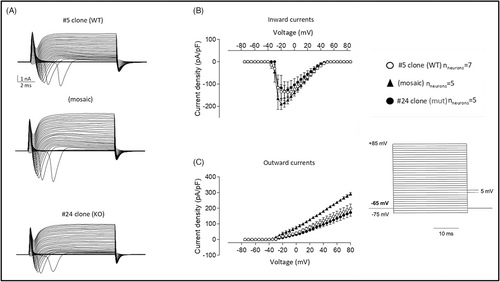
Voltage-clamp analysis helps to measure ion movements as electric currents (Figure 6). The clamp records reveal an inward current followed by an outward current during step depolarizations.18 The currents were recorded with depolarization steps ranging from −75 mV a + 85 mV in 5 mV steps. Current densities (Figure 6B) are expressed in current divided by capacitance and allow to calculate current densities and compare ionic currents in neurons. We observed the increase in inward and outward currents in mosaic neurons, compared to WT and mut neurons (Figure 6B,C).
4 DISCUSSION
Epilepsy in PCDH19-CE is often pharmacoresistant and assessment of the existing AEDs is difficult because of a possible age-dependent spontaneous seizure remission and provocation of seizure clusters by fever and infection.19, 20 Antiepileptic drugs such as clobazam, bromide, or levetiracetam have shown to be efficient against seizures in 40%–70% females for up to 12 months but with the reduction of response during the long-term follow-up.20, 21 It remains extremely difficult to prevent and abort clustering seizures and, despite the trials of multiple AEDs, patients often require recurrent hospitalisations.22 In PCDH19-CE, a significant risk of ASD and ID can emerge during the second year of life.23-25 Some girls may outgrow seizures naturally but they continue to present ID traits. Therefore, it is important to identify novel therapeutic strategies to start a personalized treatment earlier.
There is a large and expanding spectrum of PCDH19-related epilepsies.26, 27 Based on the “cellular interference” model, the PCDH19-CE phenotype results from a dysfunction of interaction between mosaic (mutated and wild-type) neuronal populations, independently from the type of mutation, but rather depending on the PCDH19 protein domain involved.26 The major part of disease-causing mutations: whole gene deletions, stop codon mutations, indels, missense, and splicing mutations, are located within the first exon, encoding for the extracellular domains of the protein.3, 28 Even though the protein-truncating mutations are spread throughout the length of the gene, missense and non-synonymous mutations are always located within the extracellular domains of the PCDH19 protein.29, 30 The studied missense mutation c.2133delG was not yet reported in literature and is located on the first exon of PCDH19 gene, where pathogenic mutations usually occur. Therefore, we believe that our results obtained with the described cellular model of PCDH19-CE may represent a broad spectrum of the disease-associated variants.
Thanks to the advent of the genetic reprogramming approach it is now possible to generate hiPSCs from patients' primary fibroblasts. This approach has made it possible to model in vitro neurological diseases for which affected cells (neurons) have always been difficult to access. Here, we characterized the neuronal phenotype of PCDH19-CE hiPSC-derived neurons using an overexpression of Neurogenin-2.
The first important point in modeling a neuronal disease is the use of appropriate controls. One option would be those differing from the patient by only studying genetic defect. For instance, it can be achieved by introducing mutations in control cell lines or by restoring the wild-type sequence in a patient cell line.31, 32 Instead, we generated isogenic cell lines taking advantage of changes in X-inactivation pattern during reprogramming of patients' primary cells into hiPSC. This strategy allowed us to generate hiPSC clones carrying the mutant or the wild-type allele of PCDH19 gene. It is known that inactive X undergoes chromatin changes during reprogramming, and expansion of hiPSCs can lead to partial loss of XIST RNA.33 HiPSC clones from patient-derived mosaic fibroblasts can reactivate somatically silenced X-chromosome and undergo random X-inactivation.34 Due to the changing X-inactivation occurring during reprogramming into hiPSC coming from female cell lines, it is possible to generate hiPSC clones carrying the mutant or the wild-type allele of an X-linked affected gene. That strategy has proven its advantages in studies modeling Rett syndrome, using female patients with mutations in the X-linked MeCP2 gene34, 35 and to model PCDH19-CE, using two female patients with mutations and hemizygous transmitting male.36 Therefore, we obtained both WT and mutated hiPSC clones from fibroblasts by reprogramming.
In PCDH19-CE, high or low percentages of mosaicism due to skewed X-inactivation in female patients, have been suggested to lead to a milder phenotype,2, 37 or asymptomatic.38 In later studies, no correlation was found.39, 40 It has also been shown that the prediction of the phenotype, based on the percentage of mosaicism in blood, is not possible, most likely because it does not necessarily represent the percentage of mosaicism in the brain.6 Considering these data, in our study, after mixing WT and mut hiPSC in a ratio of 1:1, we further did not control if the percentage had changed during the experiment.
It is known that PCDH19 protein localizes at the poles of the mitotic spindle in dividing cells,41 altering its orientation during neurogenesis. It interacts with the proteins of centrosome complexes such as y-tubulin and centriolin and its mutations alter the mitotic spindle structures, with a consequent centrosome hyperamplification.42 We observed multiple dividing cells with 4 and more centrosomes in hiPSC clone with a mutation c.2133delG while in isogenic WT clone derived from the same patient were present only normal metaphases. It confirms that reprogramming of patient dermal fibroblasts into hiPSC can be used for in vitro studies of PCDH19-CE as soon as they represent the involvement of the mutated gene in cellular processes and development.
We studied the neurogenesis in PCDH19-CE hiPSC using a rapid and efficient method of direct differentiation by the overexpression of transcription factor Neurogenin 2. This method provides mature neurons in 3 weeks and is characterized by low variability and high reproducibility compared to classical differentiation protocols.43, 44 In previous studies, it was shown that mutated and mosaic hiPSC-neurons mature earlier than WT and are being born earlier in these cultures – mut and mosaic cultures had a four-fold increase in the number of neurons relative to WT at the stage of neural rosettes (b3-tubulin positive) and also the neural rosettes appear earlier in mut hiPSCs (day 5 in comparison with day 15 in control and mosaic hPSC).36, 42 With the application of Neurogenin-2 forced differentiation of hiPSC, all hiPSC (mut, WT and mosaic) demonstrated neuron-like morphology at the day 5 post infection. They were also b3-tubulin positive at the DPI 8. In our study, we demonstrated that mut neurons maturate earlier than WT. Mutated neurons started to express mature neuronal marker NeuN at DPI 18, while WT neurons – at DPI 24. The average primary neurite length in mut and mosaic hiPSC-neurons have been shown to be significantly longer than in WT.36 By visual examination, we conclude that mut cells demonstrate more ramified dendrites than WT neurons. Interestingly, in our experiments mosaic neurons had the same maturation rate as WT neurons. However, these results may depend on mutation present in the neurons or on the changing ratio of mut and WT cells during cell expansion.
Protocadherin 19 downregulation reduces the rheobase and increases the frequency of AP firing, thus indicating neuronal hyperexcitability.45 Homozygous null mice also do not show increased electrical brain activity, as do heterozygous mice compared to WT controls.30 With the current-clamp experiment, we observed the highest frequency of AP firing in mosaic neurons, compared to mut and WT neurons (Figure 5B). In addition, mosaic neurons had the lowest rheobase—they required less current injection to trigger action potentials, which also confirms their hyperexcitability (Figure 5D). In a voltage-clamp experiment, an increasing inward current (Na++ flux into a cell) happens due to the depolarization of a cell membrane. As more sodium channels open, more inward current flows into a cell, depolarizing the membrane even more, and finally leading to an action potential. Thus, the highest values of inward current observed in mosaic neurons means that they are excited more quickly (Figure 6B). The following increase in outward currents (K+ flux out of a cell and Cl- flux into a cell) in mosaic neurons suggests that mosaic neurons are going back to a resting state more quickly because outward currents are necessary to depolarize neurons after AP (Figure 6C). In this way, they can be excited again in a short time. Taken together, these preliminary results in the developed in vitro model of PCDH19-CE are in accordance with neuronal hyperexcitability of mosaic neurons present in PCDH19-CE and in murine disease model but need a confirmation on a bigger number of neurons.
5 CONCLUSIONS
In conclusion, our findings provide evidence that PCDH19 c.2133delG mutation affects proper metaphases with increased numbers of centrosomes in stem cells and accelerates neuronal maturation in premature cells. PCDH19 mosaic neurons showed elevated excitability, representing the situation in PCDH19-CE brain. Due to these results, Ngn-2 hiPSC-derived PCDH19 neurons represent an informative experimental tool for understanding the pathogenesis of PCDH19-CE and may be a suitable approach for use in targeted drug screening strategies.
AUTHOR CONTRIBUTIONS
Conceptualization and design: DA, EF, AC and BS; experimental assays, data analysis, and interpretation: DA, AC, and IM; collection and assembly of data: DA, JB, LM, MP, IM, and EF; methodologies: AC, FB, BS, LM, MP, and IM; collection and provision of study material: IM, FM, and AR; manuscript writing: DA and EF; supervision: AC, IM, EF, and FB; funding acquisition: EF. All authors contributed to the drafting and revision of the article and gave definitive approval to the version to be published.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Italian Ministry of University and Research, PRIN 2017 (20172C9HLW to EF and MP) and Insieme per la Ricerca PCDH19—ONLUS (http://www.pcdh19research.org/it/home-2/). We thank Marina Cardano, Cell Technology Facility (CIBIO—University of Trento) for the generation and characterization of hiPSC clones and Anna Margherita Corradi, Department of Experimental Medicine, University of Genoa, for the provision of Ngn2 and rtTA plasmids.
FUNDING INFORMATION
This research was funded by the Italian Ministry of Education, Universities and Research (MIUR) within the PRIN (Research Projects of National Relevance) 2017 (PRIN 20172C9HLW) to MP and EF. The funder had no role in the design or conduct of the study, in the collection, analysis, or interpretation of the data, or in the preparation, review, or approval of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
INFORMED CONSENT STATEMENT
Informed consent was obtained from all subjects involved in the study (n. 12362).
REFERENCES
Test yourself
- What is the cause of PCDH19 clustering epilepsy?
- Males carrying PCDH19 mutations usually have a normal phenotype. In which case males can be affected?
- Where is localized the major part of mutations causing PCDH19 clustering epilepsy?
Answers may be found in the supporting information.




