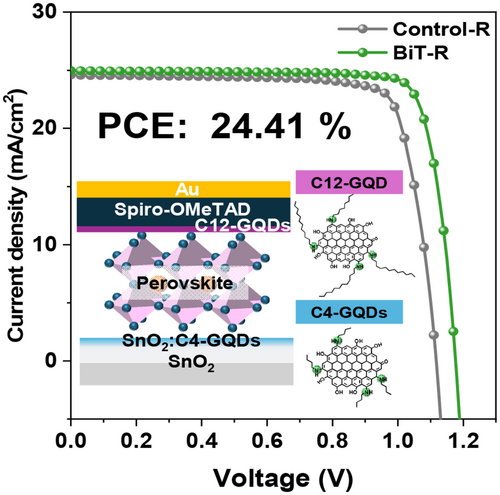
Enhancing FAPbI3 Perovskite Solar Cell Performance and Stability Through Bespoke Graphene Quantum Dots
Funding: This work was supported by Ministry of Trade, Industry and Energy (20012770, High Permeability Thermoplastic Elastome) and Ministry of Science and ICT, South Korea (2022R1A2C3004964, 2022R1C1C2008126), and the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea (No. 20227410100040), as well as a Korea University Grant.
Jin Kyoung Park and Yunmi Song contributed equally to this work.
ABSTRACT
A novel approach to enhancing the efficiency and long-term stability of perovskite solar cells (PSCs) is presented through strategic interfacial modification using bespoke graphene quantum dots (GQDs). GQDs with controlled alkylamine chain lengths, such as butylamine (C4), octylamine (C8), and dodecylamine (C12), were customized to have the proper optical and electronic properties toward specific interfaces within the PSCs. The incorporation of C4-GQDs significantly improved the energy level alignment and conductivity of the SnO2 electron transport layer (ETL), while C12-GQDs effectively reduced trap density on the perovskite surface, leading to enhanced defect passivation. These modifications resulted in a substantial increase in power conversion efficiency of 24.41% in a unit cell and 18.91% in a mini-module, respectively. Notably, the maximum power point tracked perovskite mini-module retained 89% of its initial efficiency during 1000 h of continuous light soaking condition at 25°C under 35% relative humidity. This work highlights the potential of bespoke GQDs to advance both the performance and durability of PSCs, providing a scalable approach for future photovoltaic applications.
1 Introduction
Metal halide perovskites have garnered significant attention in photovoltaic research over the past decades due to their remarkable properties, such as long carrier lifetimes and high defect tolerance [1-5]. In particular, the cost-effective fabrication through low-temperature solution process has placed perovskite solar cells (PSCs) as a leading technology in the field. The low-energy consumption manufacturing process of highly efficient PSCs not only offers economical advantages but also contributes to environmental sustainability, making it a key driver of PSCs development. Since 2009, PSCs have rapidly advanced, achieving power conversion efficiency (PCEs) soaring from 3.8% to 26.7% [6]. However, despite these impressive advancements, most reported PCEs still fall short of their theoretical limits, primarily due to interfacial non-radiative recombination [7].
These efficiency losses are mainly caused by inherent defects in the perovskite crystals, such as vacancies, and interfacial defects like pin-holes and grain boundaries that arise during the formation of polycrystalline films [8-11]. These defects, especially those located at the buried interfaces of perovskites, lead to significant carrier losses and a high voltage deficit, ultimately degrading both the photovoltaic performance and the long-term stability of the devices [12, 13]. As a result, there is a growing demand for a deeper understanding and for a more effective interfacial modification strategy to enhance the performance and long-term stability of PSCs further.
Graphene quantum dots (GQDs) with several nanometers sizes are composed of sp2-bonded carbon in the core and various functional groups attached to their surfaces. GQDs have attracted interest not only due to their non-toxic and biocompatible nature but also because of their excellent electrical and optical properties, which are derived from quantum confinement and edge effects [14-16]. These properties can be easily tuned by modifying the various functional groups and by introducing heteroatom dopants, making GQDs highly bespoke materials for interface modification in PSCs. Additionally, GQDs can be stably attached to the perovskite interface to form a passivation layer that can maintain long-term device stability [17]. For example, Gao et al. [18] achieved a high efficiency of 22.37% by aligning the energy levels between the SnO2 electron transport layer (ETL) and the perovskite layer, thereby reducing interface defects and improving the quality of the FAPbI3 perovskite. Similarly, Wang et al. [19] achieved a high fill factor (FF) of 85.24% and a PCE of 23.75% in FAPbI3-based PSCs by using GQDs with OH and NH2 groups to promote defect passivation and improve the crystal quality at the SnO2/perovskite interface. Khorshidi et al. [20] improved the device stability in an ambient environment by using amide-conjugated GQDs composed of carbonyl and dodecyl amine at the interface of the perovskite layer and the hole transport layer (HTL). These studies confirm that GQDs are beneficial materials for interfacial engineering in PSCs, enhancing both performance and stability.
In this study, we successfully developed a synthetic method to fabricate interface-tailored GQDs by utilizing amines with various alkyl lengths to enhance the performance of PSCs and compare their suitable optical and electrical properties. We synthesized alkylamine-GQDs (AA-GQDs) with butylamine (C4), octylamine (C8), and dodecylamine (C12) and applied them to the SnO2/perovskite and perovskite/spiro-OMeTAD (2,2′,7,7′-Tetrakis[N,N-di(4 methoxyphenyl)amino]-9,9′-spirobifluorene) interfaces in PSCs with an ITO/SnO2/perovskite/spiro-OMeTAD/Au structure. The C4-GQDs optimized energy alignment and improved the electrical conductivity of the SnO2 ETL, while C12-GQDs reduced trap density, leading to a PCE of 24.41% in the unit cell. Additionally, perovskite solar mini-modules with a 25 cm2 aperture area, treated with bespoke GQDs, achieved a PCE of 18.91% and retained ~89% of their initial performance after 1000 h, demonstrating effective scalability and long-term stability.
2 Results and Discussion
2.1 Synthesis of Bespoke GQDs Tuned by Alkylamines
GQDs functionalized with amines of varying alkyl chain lengths, including butylamine (BA), octylamine (OA), and dodecylamine (DDA), were synthesized through the Amadori reaction with glucose [21, 22]. Figure 1A–C shows the morphology of the synthesized GQDs as observed through transmission electron microscopy (TEM) analysis. As the alkyl chain length of the amine increases, the average size of particles of GQDs tends to decrease: C4 is 6.82 nm, C8 is 2.91 nm, and C12 is 2.68 nm. (Figure S1) The inset images in each TEM image reveal a lattice spacing of 0.21 nm, corresponding to the in-plane (100) facet of graphene, confirming the synthesis of GQDs. The decrease in GQDs particle size with longer alkyl chain lengths is attributed to the steric hindrance caused by the long alkyl chains obstructs the crystal growth of GQDs from the Amadori products, preventing the particles from growing beyond a certain size over time, as shown in Figure S2 [23]. Further confirmation of the synthesis was obtained through UV–Vis and photoluminescence (PL) spectra analyses, as shown in Figure S3, where all three types of GQDs exhibited strong absorption peaks at around 280 nm and 350 nm, typical of the ππ* transition of sp2-bonded carbon and the nπ* transitions of CO and CN groups. The PL spectra also showed excitation-dependent emission, with consistent changes in wavelength, which are characteristic of GQDs [24]. These results confirm that the GQDs were successfully synthesized.
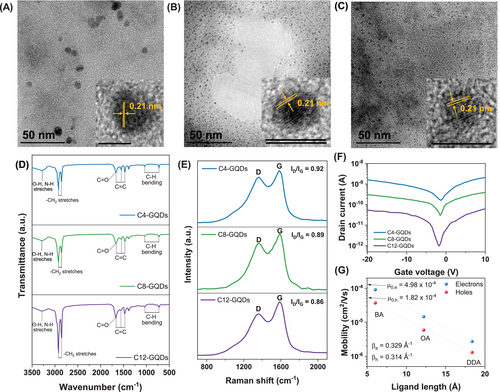
To investigate the effect of alkyl chain length in alkyl amines on the properties of GQDs, we conducted Fourier transform infrared (FT–IR) and Raman analyses, as shown in Figure 1D,E. In the FT–IR spectra, all three samples had characteristic peaks of asymmetric and symmetric CH2 stretches (2922 and 2856 cm−1), OH and NH stretches (3293 cm−1), CC aromatic ring stretches (1473, 1565, and 1666 cm−1), CO stretches (1666 cm−1), overlap with ring stretching region, and in-plane and out-plane CH ring structure bending (726 and 1037 cm−1). As the alkyl chain length of the alkylamine increases, more pronounced peaks are observed in the CH stretches, which suggests that longer alkylamines are progressively binding as functional groups on the surface of the GQDs, consistent with previously reported findings [25]. Additionally, x-ray photoelectron spectroscopy (XPS) analysis was performed on the GQDs. According to Figure S4A, the GQDs are primarily composed of C, N, and O, and as alkyl chain length increases, the N/C ratio decreases from 0.1 for C4 to 0.08 for C8 and 0.05 for C12. Furthermore, the CN peak observed in the C1s spectra in Figure S4B and the peaks present in the N1s spectra in Figure S4C suggest that alkylamines with different alkyl chain lengths are present in each sample.
In the Raman spectra shown in Figure 1E, two distinct peaks at approximately 1364 and 1588 cm−1 correspond to the D band and G band, respectively, arising from the out-of-plane vibration of sp3 carbons and the in-plane vibration of sp2 carbons [26]. The intensity ratio of the D band and to the G band (ID/IG) was measured to be 0.92, 0.89, and 0.86 for C4, C8, and C12, respectively. All three samples generally exhibit low ID/IG ratios, indicating a low level of defects. However, as the alkyl chain length increases, the ID/IG ratio gradually decreases. This suggests, as observed in the TEM analysis, that the degree of defects decreases as the particle size becomes smaller [27]. Based on these results, it was confirmed that all three samples were successfully synthesized and alkylamines of varying lengths effectively incorporated as functional groups.
2.2 Effects of AA-GQDs at the Interface
First, to analyze the changes in the perovskite crystal according to the characteristics of SnO2 treated with AA-GQDs, scanning electron microscopy (SEM), contact angle, and X-ray diffraction (XRD) patterns were analyzed. As shown in Figure S7, we used rutile SnO2 with the (110) plane of 3.06 nm [29]. The AA-GQDs treated SnO2 were uniformly coated on the surface as the hydrophilicity of AA-GQDs increased, and the wettability of SnO₂ was also enhanced (Figure S8).
To investigate the impact of GQDs synthesized with alkylamines of varying chain lengths on charge dynamics at PSC interfaces, we introduced GQDs at each interface, observed the resulting changes in optical and electrical properties, and optimized the system as shown in Figure 2. The perovskite films deposited on SnO2 and AA-GQDs treated SnO2 were analyzed by SEM images and XRD patterns (Figures S9 and S10). It was confirmed that the perovskite deposited on SnO2 and AA-GQDs treated SnO2 had similar grain sizes and film thicknesses, and they were confirmed that highly crystalline FAPbI3 was formed. We also applied 10 wt.% of GQDs with different alkylamines to the SnO2 ETL/perovskite and perovskite surfaces to quickly identify the most suitable GQDs for each layer. As shown in Figure S11, the PL spectra in Figure S11A and the time-resolved photoluminescence (TRPL) spectra in Figure S11B indicate that C4-GQDs provide the most efficient charge extraction, attributed to the increased conductivity of SnO2 (Figure S11C). Thus, C4-GQDs were identified as the best for enhancing charge transfer at the SnO2 ETL/perovskite interface. Conversely, applying C12-GQDs on the perovskite surface reduced traps, as evidenced by the lower VTFL in space-charge-limited current (SCLC) measurements (Figure S11D), leading to the highest PL intensity and longest TRPL decay time, confirming effective defect passivation [20]. (Figure S11E and S11F) Therefore, C4-GQDs and C12-GQDs were found to be suitable for their respective interfaces.
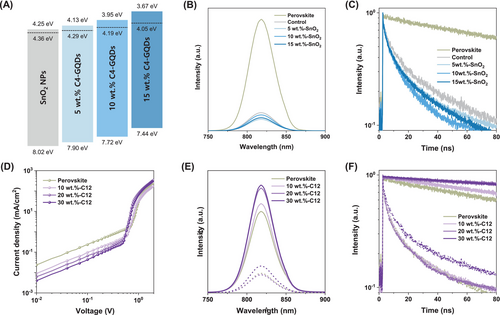
Based on the initial screening experiments, we selected the most suitable AA-GQDs for each interface. To optimize PSCs performance through the introduction of GQDs at each interface, we systemically evaluated the device characteristics by varying the concentrations of the selected GQDs. First, we aimed to optimize the SnO2/perovskite interface by treating SnO2 with C4-GQDs. We conducted UPS analysis on samples with varying concentrations, as shown in Figure S12A, to observe how the band energy structure of SnO2 ETL changes. From this analysis, we calculated the band energy structure of SnO2 ETL and presented the results in Figure 2A. As C4-GQDs, which have a higher Fermi level than SnO2, were applied at increasing concentrations, the Fermi level of SnO2 also shifted upward. Additionally, the electrical conductivity increased due to the higher conductivity of C4-GQDs compared to SnO2, as shown in Figure S12B.
The changes in charge dynamics were analyzed through PL and TRPL measurements of the SnO2 ETL/perovskite interface treated with varying concentrations of C4-GQDs, as shown in Figure 2B,C. In Figure 2B, the PL intensity significantly decreases when the SnO2/perovskite interface is formed, with the lowest intensity observed at 10 wt.% C4-GQDs in the SnO2 ETL. This reduction is attributed to minimized energy offset between the conduction bands of the SnO2 ETL and the perovskite layer, along with improved conductivity that enhances charge extraction. However, at 15 wt.%, the PL intensity slightly increases as the conduction band of the SnO2 ETL rises above that of the perovskite layer. This trend is further confirmed in the TRPL spectra shown in Figure 2C, where the fastest PL decay was observed at a C4-GQDs concentration of 10 wt.%. Therefore, the introduction of 10 wt.% C4-GQDs between the SnO2 ETL and perovskite layer is expected to minimize the energy offset and increase conductivity, thereby improving charge transfer properties at the SnO2/perovskite interface, reducing electron losses in PSCs, and ultimately contributing to enhanced open-circuit voltage (Voc).
The differences in the perovskite films according to the C12-GQD treatment were analyzed by the top SEM images and XRD. As shown in Figure S13A, the XRD patterns showed that both samples have similar crystallinity. In contrast, the SEM images showed that the C12-GQDs sample has relatively larger crystal grains than the control sample as we reported in the previous paper [30] (Figure S13B,C). The perovskite grain boundaries grew smoothly with the treatment of C12-GQDs, resulting in larger grains compared to samples without C12-GQDs [31]. This is because the C12-GQDs coated on the perovskite healed the grains and bulk of the perovskite during the annealing process [30]. The trap passivation effect of perovskite surface treatment with varying concentrations of C12-GQDs was analyzed through SCLC measurements using hole-only devices (ITO/PTAA/perovskite/C12-GQDs/spiro-OMeTAD/Au), as shown in Figure 2D. The SCLC results indicate that as the concentration of C12-GQDs increases, the VTFL shifts to lower voltages, saturating at 20 wt.%, indicating effective defect passivation and reduced trap density. As shown in Figure 2E, the PL intensity of the perovskite film increases noticeably up to 20 wt.% C12-GQDs, with a slight increase at 30 wt.%. This trend is consistent with the TRPL results in Figure 2F and aligns with the SCLC measurements. In contrast, when the perovskite forms a junction with spiro-OMeTAD HTL, the PL intensity decreases sharply, remaining stable up to 20 wt.% C12-GQDs before increasing at higher concentrations. Similarly, TRPL measurements show rapid PL decay when forming a junction with spiro-OMeTAD, with consistent decay rates up to 20 wt.% C12-GQDs and a slight increase at 30 wt.%. These findings suggest that up to 20 wt.%, C12-GQDs enable effective trap passivation and efficient hole transfer to spiro-OMeTAD HTL, but higher concentrations impede hole transfer due to the long alkyl chains of C12-GQDs. Thus, introducing 20 wt.% C12-GQDs between the perovskite and spiro-OMeTAD effectively passivates defects by sharing lone pair electrons from nitrogen and oxygen atoms on GQDs edges with undercoordinated Pb2+ ions in the perovskite layer, without hindering hole transfer [32].
2.3 Optimizing Performance Enhancement Using Bespoke GQDs
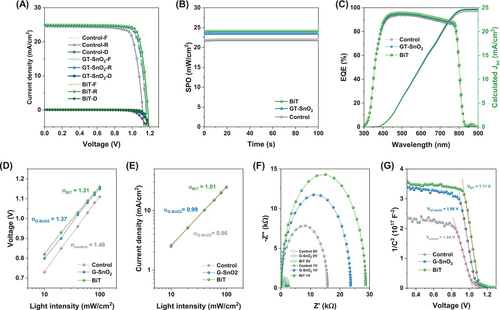
To systematically investigate the effect of tailored GQDs application at different interfaces, specifically the ETL/perovskite and perovskite/HTL interfaces, on device efficiency, we fabricated PSCs with the structure of ITO/SnO2/perovskite/spiro-OMeTAD/Au, which serves as the basic structure for the control device, as shown in Figure S14. Figure 3A presents the forward and reverse J–V curves for three types of devices: the control device with the basic structure, the GT-SnO2 device with GQDs-embedded ETL/perovskite interface structure, and the BiT device with GQDs-embedded ETL/perovskite and GQDs applied to perovskite/HTL interfaces. Notably, the modification of these interfaces resulted in a significant increase in Voc and FF. The control device exhibited a Voc of 1.11 V, a short-circuit current density (Jsc) of 24.60 mA/cm2, an FF of 80.98%, and a PCE of 21.91%. After interface modification by embedding GQDs at the ETL/perovskite interface, the PCE increased to 23.78%, primarily due to a significant increase in Voc and FF, which improved to 1.15 V and 83.05%, respectively. This enhancement in Voc and FF, as previously observed in perovskite thin films, is attributed to the reduction in energy level offset between the conduction band energies of SnO2 ETL and perovskite layer, as well as the increased conductivity resulting from GQDs embedded at the ETL/perovskite interface. Further with the dual modification at the perovskite/HTL interface, all photovoltaic parameters showed additional improvements, leading to an outstanding PCE of 24.41% in the BiT device. This enhancement is attributed to the reduction in trap density at the perovskite/HTL interfaces. The trap density reduction is primarily due to the effective passivation of defects by GQDs, which interact with undercoordinated Pb2+ ions at the surface and grain boundaries of the perovskite film, neutralizing these defects and leading to improved device performance [20, 30]. This PCE is particularly promising when compared to other n-i-p structured perovskite devices using carbon-based materials (Table S1). These changes in photovoltaic parameters are further illustrated by the photovoltaic parameters distribution of 50 devices shown in Figure S15. The best-performing photovoltaic parameters for each device and the average photovoltaic parameters across 50 samples are summarized in Table S2.
The changes in PCE related to the optimization of GQDs applied at each interface are shown in Figures S16 and S17. For the SnO2 ETL/perovskite interface, the optimal GQDs amount was determined to be 10 wt.%. While increasing the GQDs content improved the conductivity of the SnO2 ETL, the efficiency decreased at 15 wt.% because the conduction band of the SnO2 ETL rose above that of the perovskite layer. At the perovskite/HTL interface, the highest efficiency was achieved at 20 wt.%, where defect passivation increased with GQDs concentration but reached saturation between 20 wt.% and 30 wt.%. However, the long alkyl chain with 12 carbon atoms hinders charge transfer between the interfaces, creating a trade-off between the positive effects of trap passivation and the negative effects of long alkyl chains on inhibited charge transfer [30]. Therefore, 10 wt.% at the ETL interface and 20 wt.% at the HTL interface were determined to be the most effective amounts.
Figure 3B shows the stabilized power output (SPO) at the maximum power point (MPP) for the control, GT-SnO2, and BiT devices. Under continuous illumination, the stabilized PCE significantly increased from 21.90% in the control device to 24.08% in the BiT device, following interface treatment. This value closely aligns with the forward scan efficiency of each device.
The external quantum efficiency (EQE) spectra and the corresponding integrated Jsc are displayed in Figure 3C. The integrated Jsc values are 24.22, 24.55, and 24.71 mA/cm2 for the devices with the structure of control, GT-SnO2, and BiT, respectively. The minimal discrepancy between the Jsc values determined from the EQE spectra and the J–V curves indicates that the J–V curve measurements were performed accurately.
To gain deeper insights into the reasons behind the enhanced performance, we investigated the electrical properties of the control, GT-SnO2, and BiT devices. The light intensity-dependent Voc and Jsc measurements for these devices are presented in Figure 3D,E. The ideality factor (n) was determined from the slope of Voc measured under varying light intensities ranging from 10 to 100 mW/cm2. The ideality factor is indicative of the recombination process, where values closer to 1 suggest a dominance of trap-assisted recombination. The ideality factors obtained were 1.48 for the control device, 1.37 for the GT-SnO2 device, and 1.31 for the BiT device, indicating that non-radiative recombination was effectively suppressed in the presence of GQDs, correlating with the observed increase in Voc. Similarly, the alpha coefficient (α) was derived from the light intensity-dependent Jsc measurements, reflecting the charge recombination characteristics. An α value near unity suggests that bimolecular recombination is the prevailing mechanism for electron–hole pairs. As GQDs were incorporated at each interface, the α values progressively approached unity, suggesting an increasing dominance of radiative recombination.
Additionally, electrochemical impedance spectroscopy (EIS) was performed to investigate carrier dynamics in the working devices. The Nyquist plot, recorded in the dark under short-circuit condition (0 V), and the near-open-circuit condition (1.1 V) for the control, GT-SnO2, and BiT devices, are shown in Figure 3F. The low-frequency response, which correlates with carrier recombination at the interface, reveals that larger semicircles correspond to stronger charge recombination resistance (Rrec). The extracted Rrec values obtained were 1.36, 2.07, and 2.56 kΩ at 0 V for the control, GT-SnO2, and BiT devices, respectively, and 28.64, 23.55, and 15.73 kΩ at 1.1 V for the control, GT-SnO2, and BiT devices, respectively. The gradual increase in Rrec with the introduction GQDs for interface modification confirms enhanced resistance to interfacial recombination.
Furthermore, Mott–Schottky measurements were conducted to determine the built-in potential (Vbi). The capacitance–voltage (C–V) curves are shown in Figure 3G, with the Vbi being inferred from the intersection with the x-axis. It is evident that the Vbi significantly increases (from 1.04 in the control to 1.08 and 1.11 V for GT-SnO2 and BiT, respectively), indicating that interface modification effectively enhances the separation, transport, and extraction of photogenerated charge carriers.
Overall, the strategic incorporation of GQDs at the ETL/perovskite and perovskite/HTL interfaces improved charge carrier dynamics and reduced recombination losses, resulting in enhanced photovoltaic parameters across the board. (Scheme S1) Among these, the most notable improvements were in the Voc, which significantly contributed to the overall enhancement in the photovoltaic performance of the PSCs.
To further investigate whether the device modification approach using tailored GQDs can effectively be applied to large-area modules, perovskite solar mini-modules were fabricated with a 25 cm2 aperture area, consisting of five serially connected sub-cells. Figure 4A illustrates the I–V characteristics of top-performing module, with an inset showing photographic image of the module. Under 1 sun illumination, the module achieved a PCE of 18.91%, characterized by a Voc of 5.33 V, an Isc of 116.28 mA, and a FF of 76.27%.
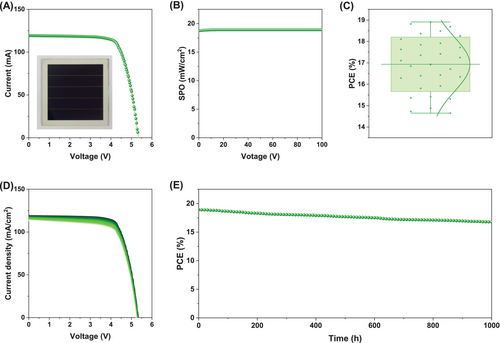
As displayed in Figure 4B, the SPO measured at 4.27 V for 100 s delivered a stable PCE of 18.91%, closely aligning with the I–V scan results, thereby affirming the accuracy of the operational performance. Notably, Figure 4C highlights the high reproducibility observed in the module, demonstrated by a narrow PCE distribution averaging 16.93% ± 1.27% across 30 samples, with no observed device failures. This indicates that GQDs treatment can be effectively applied to module fabrication without additional processes. The PCE of the best-performing perovskite solar mini-module, along with the average photovoltaic parameters of 30 samples, is summarized in Table S3, with the distribution visualized in Figure S18.
To assess the long-term stability of the BiT module, the BiT perovskite solar mini-module was encapsulated and subjected to continuous 1 sun illumination under 25°C/35% relative humidity for 1000 h. The I–V curves recorded during this stability test are presented in Figure 4D, and the corresponding PCE changes over time are shown in Figure 4E. Detailed photovoltaic parameters before and after the stability test are provided in Table S3. The module maintained approximately ~89% of its initial PCE of 18.91%, indicating good photo-stability and reliable performance under prolonged light exposure.
3 Conclusion
In this work, we have demonstrated the efficacy of bespoke GQDs treatments to enhance the performance and long-term stability of PSCs. By strategically incorporating C4-GQDs into the SnO2 ETL and C12-GQDs onto the perovskite surface, we achieved significant improvements in charge transfer efficiency and defect passivation. The C4-GQDs facilitated favorable energy level alignment for charge extraction and boosted the conductivity of the SnO2 ETL, while C12-GQDs effectively reduced surface trap densities, contributing to the stabilization of the PSCs. The resulting enhancements led to a PCE of 24.41% in the best-performing devices. Moreover, large-area perovskite solar mini-module displayed a PCE of 18.91% and demonstrated sustained performance, retaining about ~89% of their initial efficiency after 1000 h of operation. These findings underscore the potential of GQDs as versatile tools for fine-tuning PSC interfaces, paving the way for the development of more robust and commercially viable, and environmentally sustainable solar cell technologies.
4 Experimental Section
4.1 Chemicals and Materials
For synthesizing GQDs, 1-hexanol (99%) was purchased from JUNSEI. D-(+)-Glucose, acetic acid (AC, ≥ 99.7%), BA, 99.5%, OA, 99%, and DDA, 98% were purchased from Sigma–Aldrich. Methanol (ME, 99.5%) was purchased from SAMCHUN CHEMICALS. For fabricating PSCs, the patterned indium-doped tin oxide (ITO)-coated conductive glass and encapsulation cover glass were purchased from 3H.
Ethanol (EA, 99.5%) and isopropanol (IPA, 99.5%) for substrate cleaning were purchased from SAMCHUN CHEMICALS. Tin (II) chloride dihydrate (SnCl22H2O, ≥ 98%), N,N-dimethylformamide (DMF, 99.8%), dimethyl sulfoxide (DMSO, 99.8%) diethyl ether (DE, ≥ 99.0%), bis(trifluoromethane)sulfonamide lithium salt (Li-TFSI, 99.95%), 4-tert-butylpyridine (tBP, 96%), chlorobenzene (CB, 99.8%), and acetonitrile (ACN, 99.8%) were purchased from Sigma–Aldrich. Formamidinium iodide (FAI, 99.5%) and methylammonium chloride (MACl, 99.5%) were purchased from Yingkou Advanced Election Technology Co. Ltd. Lead (II) iodide (PbI2, 99.9985%) was purchased from Alfa Aesar. 2,2′,7,7′-Tetrakis(N,N-di-pmethoxyphenylamino)-9,9′-spirobifluorene (Spiro-OMeTAD, 99.8%) was purchased from Lumtec. All chemicals were used as received without further purification.
4.2 Synthesis of Bespoke GQDs for PSCs
To synthesize GQDs, we first added 10 mL of 1-hexanol as the oil phase to a 25 mL 3-neck round-bottom flask and bubbled N2 through it at an 80 cc/min flow rate for 1 h. Next, we introduced 1 mL of 10 wt.% aqueous glucose solution as the carbon source and 200 μL of AC as the catalyst for the Amadori rearrangement. The mixture was then aged at 80°C, with stirring at 1500 rpm in a closed system for 1 h. To tailor the properties of the GQDs, we added 3.3 mM of alkyl amine with various alkyl lengths, such as OA and DDA, and heated the reactor to 150°C under an 80 cc/min N2 purged condition for 1 h at 1500 rpm. For short-length BA, solvothermal method was used because of its high volatility. The mixture was reacted at 80°C and 3.3 mM BA was placed in a 15 mL Teflon-lined stainless-steel autoclave, heated to 150°C, and stirred at 800 rpm for 1 h. To terminate the reaction, we rapidly combine the reaction mixture with 290 mL of ME. After mixing with ME, we subjected it to bath sonication for 1 h. To separate and purify the solution from residual amines, different methods were employed depending on the alkyl length of the amine. In the case of BA, a thermal evaporator was used to remove the residual amine, followed by redispersing in ME and repeating the process. Following this, the dried GQDs by heating oven were dispersed in the target solvent. For OA and DDA, a simple aggregation technique was adopted. The product mixture was stored in a fridge at temperatures ranging from −25°C to −35°C for over 12 h to promote the aggregation of aliphatic amines. Subsequently, the precipitate was discarded using a simple vacuum filtration process through a polyvinylidene fluoride (PVDF) filter. Afterward, dialysis (3.5 kDa) purification was performed with the sample dispersed in EA for 7 days. The samples were refrigerated until use. The supernatant was then redispersed in the target solvent via a distillation process for additional application.
4.3 Fabrication of PSCs With Tailored GQDs
To fabricate the PSCs with tailored GQDs, patterned ITO glasses were cleaned sequentially using detergent, deionized water, EA, and IPA in an ultrasonic bath. The substrates were then exposed to ultraviolet ozone for 15 min. The SnO2 NPs solution, used for ETL deposition, was prepared following existing literature [29]. The solution was spin-coated onto the substrate at 5000 rpm for 60 s and annealed at 100°C for 30 min. To tailor the ETL properties, SnO2 solutions with different GQDs wt.% were individually prepared. Specifically, a 20 wt.% GQDs stock solution was prepared by dispersing GQDs in water. The SnO2 solution, which had a concentration of approximately 15 wt.%, was dispersed in a 1:2 water-to-IPA mixture. To achieve SnO2 solutions containing 5wt.%, 10wt.%, and 15 wt.% GQDs, approximately 7,5, 15, and 23 μL of the GQDs solution were added to the 3 mL of SnO2 solution, respectively. These mixtures were then spin-coated onto the SnO2/ITO substrate using the same procedure as the initial SnO2 layer. For the control device, the SnO2 NPs solution was spin-coated again with the same process. The perovskite solution was prepared by dissolving 224 mg/mL of FAI, 599 mg/mL of PbI2, and 17.6 mg of MACl in a DMF and DMSO mixture with an 8.5: 1.5 volume-to-volume solvent ratio. The perovskite layer was deposited onto the ETL using a two-step spin-coating process at 1000 rpm for 10 s, followed by 5000 rpm for 30 s. Approximately 10 s before the end of the spin-coating process, a toluene solution containing GQDs at various wt.% was dropped onto the substrate to induce antisolvent dripping crystallization [33] and surface passivation [30]. The GQDs stock solution used in this process was prepared by dispersing 30 wt.% GQDs in toluene. To prepare 10 wt.% and 20 wt.% GQDs toluene solutions, 16.7 and 33.6 μL of the 30 wt.% GQDs stock solution were added to 5 mL of toluene, respectively. After spin-coating, the substrate was annealed at 150°C for 30 min. The spiro-OMeTAD solution was prepared by dissolving 72.3 mg of spiro-OMeTAD in 1 mL of CB and adding 17.5 μL of Li-TFSI (520 mg dissolved in 1 mL of ACN) and 30 μL of tBP. The solution was spin-coated at 4000 rpm for 30 s. For the top electrode, a 60 nm Au layer was thermally deposited at a 2.0 Å/s evaporation rate.
4.4 Characterization of GQDs
TEM images were acquired using a TEM (Tecnai 20, FEI) operating at an accelerating voltage of 200 kV. TEM samples were prepared by placing ME-dispersed GQDs onto a copper grid (01800-F, Ted Pella). Raman spectra were acquired using a Raman spectrometer (LabRam ARAMIS IR2, HORIBA JOBIN YVON) with a 532 nm laser on a sample dropped onto glass. FT–IR spectra were collected using a FT–IR spectrometers with sample dispersed in KBr pellets. UV–Vis spectra were obtained using a UV–Vis spectrometer (UV-3600 Plus, Shimadzu) with the GQDs suspended in ME. PL and TRPL spectra were obtained using fluorospectrometer and photon counting spectrometers integrated within a single system (PC1, ISS). For the TRPL measurement, a 373 nm pulsed laser was utilized for excitation. UPS analysis was conducted by UPS system (Nexsa, Thermo Fisher) with He (21.22 eV) source.
4.5 Characterization of PSCs
The J-V curves were acquired using a source meter (Keithley 2420) under 1 sun illumination (100 mW/cm2, AM 1.5G) from a solar simulator (Sun 3000, Abet technologies) equipped with a 1400 W Xenon lamp (#11044A, Abet technologies). The light intensity was calibrated using an NREL-certified Si solar cell (#15150, Abet technologies). EQE measurements were conducted using a power source featuring a 150 W Xenon lamp (#13014, Abet technologies), a monochromator (MonoRa-500i, DONGWOO OPTRON Co. Ltd.), and a potentiostat (IviumStat, IVIUM).
Author Contributions
Jin Kyoung Park: writing of the original draft and investigation. Yunmi Song: writing of the original draft and investigation. Hyong Joon Lee: investigation. Kyung Ho Kim: investigation. Jin Hyuck Heo: supervision. Sang Hyuk Im: supervision.
Acknowledgments
This study was supported by the National Research Foundation of Korea (NRF) under the Ministry of Science, ICT & Future Planning (no. 2022R1A2C3004964 and no. 2022R1C1C2008126) and the Technology Innovation Program (20012770, High Permeability Thermoplastic Elastomer for Solar Module) funded By the Ministry of Trade, Industry & Energy (MOTIE, Korea), and the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea (No. 20227410100040), as well as a Korea University Grant.
Conflicts of Interest
The authors declare no conflicts of interest.