Review on Recent Applications of Nitrogen-Doped Carbon Materials in CO2 Capture and Energy Conversion and Storage
Abstract
Carbon materials play an integral role in our everyday lives. Extensive research has been carried out on the applications of carbon materials in its various morphologies and properties. The addition of dopants in carbon materials, whether in situ or post-treatment, has gained significant interest and has driven researchers to explore its benefits and address existing issues. Nitrogen doping, in particular, has been shown to be a highly effective strategy in creating advanced materials for various applications, such as CO2 capture, energy conversion, and energy storage. However, the key factors that contribute to the properties and performance of the material, such as method of synthesis, starting materials, level of doping, and porosity, should be considered and studied at great depths. In this review, the synthesis methods of N-doped carbon materials and their recent progress in CO2 adsorption, energy conversion, and energy storage applications is discussed. These applications represent some of the most important and promising solutions to burgeoning issues in environmental and energy fields. In addition, the remaining issues with N-doped carbon materials and the possible strategies to overcome them will be discussed.
1 Introduction
Doping was first discovered with semiconductors, which involved the unintentional addition of impurities in crystallized silicon and gave rise to positive (p)-type and negative (n)-type junctions.[1] In general, doping refers to the addition of impurities (elements or compounds) into a material to enhance or modify its properties. From here on, doping process has developed and extended into other applications beyond semiconductors. By doping carbon materials with more electronegative atoms, such as nitrogen, boron, phosphorus, and sulfur (Figure 1a), a positive charge density is created on adjacent carbon atoms, which facilitates oxygen adsorption and charge transfer. Heteroatom doping of carbon materials, especially graphene, has been shown to have important applications in energy storage and conversion.[2] Since these atoms are similarly sized to carbon, substitution within the carbon network can result in a stable structure and enhanced properties that contribute to a better electrochemical performance. These include improvements in conductivity, porosity, surface area, and wettability. Nitrogen, in particular, has attracted much attention due to its suitable atomic size and electronegativity.
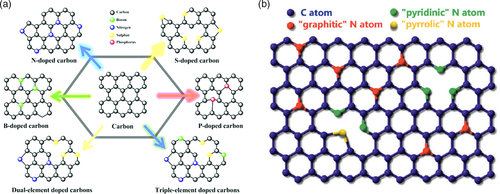
The addition of nitrogen atoms into the carbon framework and subsequent activation by a chemical agent have been shown to be an effective technique in increasing the number of available adsorption sites.[3] Nitrogen atom is considered an ideal choice because it has an atomic size close to carbon and five valence electrons, which is conducive to forming strong valence bonds with carbon atoms, and thus opens the possibility to a wide range of applications. N-doped materials were found to exhibit high performance capabilities as electrode in fuel cells and excellent catalytic activity for the oxygen reduction reaction (ORR) – an important electrochemical reaction in energy conversion.[4] Moreover, it has been reported that N-doped carbon can catalyze not only O2 reduction reaction, but also H2O2 decomposition.[5, 6] In N-doped materials, several classifications of substituted N atoms can be identified. For instance, N-doped graphene will have “graphitic”, “pyridinic”, and “pyrrolic” N atoms, which depend on their location within the graphene structure (Figure 1b).[7] In particular, pyridinic N atoms have been suggested to be the active site responsible for the improved catalytic activity of ORR,[8] as well as enhancing the function of electrode materials.[9]
A wide range of carbon materials exist with different structures, properties, and applications. Some of the most known carbon materials include, but are not limited to, diamond, graphite, graphene, fullerene, activated carbon, carbon black, and carbon nanotubes.[10] The structure, porosity, and surface chemistry are what determines the properties of carbon materials, which also dictate their applications. Carbon is very significant when it comes to renewable energy, environmental science, food, and other research fields. Moreover, carbon materials can be found in portable electronics and medical devices as electrodes and coatings. However, the use of bare carbon materials have been proven to have its limitations, which fail to satisfy the ever-increasing demands, especially in the field of energy. For instance, conventional graphite-based electrodes in Li-ion batteries suffer from cracking when cycled at high rates, which hinders its commercialization for high-performance energy storage systems.[11]
With the rapid development of technology and availability of portable devices, secondary (rechargeable) batteries have greatly increased in demand, especially with the higher energy and faster charging rate requirements. Doping carbon-based electrode materials with nitrogen atoms has been shown to positively affect the level of porosity, size of interlayer space, and composition of the surface, which all contribute in the electrochemical performance of intercalation-type rechargeable batteries.[12] Newer technologies such as laser-assisted and microwave-assisted synthesis of carbon materials have been evaluated as cost-effective, reproducible and controllable, as summarized in several review articles.[13-18] For instance, microwave-assisted synthesis of novel N-doped carbon materials have been studied for electromagnetic interference shielding[19] and fuel cells as a catalyst.[20] Compared to traditional synthesis methods, the use of microwave radiation provides uniform heating and accelerates the synthesis duration to only a few seconds or minutes.[13] In addition, the use of N-doped carbon materials in various applications gives the opportunity to use waste and biomass as precursors, which are sustainable, cost-effective, and environmentally friendly.[21, 22] Additionally, carbon materials have been utilized as adsorbents for CO2 gases to help combat global warming, but its adsorption capacity can still be improved for greater efficacy.[23] Research has shown that the addition of nitrogen atoms as dopants in porous carbon materials have the ability to boost adsorption capacity due to the presence of active nitrogen species, well-formed porous structures, and large surface area.[24] Therefore, significant interest has been generated in the benefits and potential application of doped carbon materials.
This paper will discuss the recent progress on the design of nitrogen-doped carbon materials and their application in the fields of environment and energy. To the best of our knowledge, a combined review of N-doped carbon materials in environmental and energy applications has not been done. These are two of the most important fields of study that involve growing issues which require immediate solutions through material design, including the development of doped materials. A summary of literatures discussed in this review will be provided in Table 1. The various synthesis methods and carbon precursors used will also be discussed briefly. Finally, a conclusion on the progress of N-doped carbon materials and their role in CO2 adsorption, energy conversion, and energy storage will be delivered, along with future considerations from our perspective.
| N-doped carbon materiala) | Synthesis | Nitrogen content [%] | Electrolyte | Application | Performance | Ref. |
|---|---|---|---|---|---|---|
| Porous carbon from licorice residue | Hydrothermal carbonization at 600 °C, KOH-activated | 7.98 | N/A | CO2 adsorption | 6.43 mmol g−1 at 1 bar (0 °C); 3.89 mmol g−1 at 1 bar (25 °C = RT) | [3] |
| Porous carbon from rGO/melamine resin | Pyrolysis | 6.92 | N/A | CO2 adsorption | 5.21 mmol g−1 at 5 bar (RT) | [36] |
| Porous carbon co-doped with P | Pyrolysis | 7.89 | N/A | CO2 adsorption | 5.68 mmol g−1 at 5 bar (RT) | [38] |
| 6 m KOH | Supercapacitor | C = 172.7 F g−1 at 1 A g−1 | ||||
| N-carbon dots on GO | Hydrothermal | 10.6 | 0.1 m KOH | Electrocatalyst | 18.4 mA cm−2 at −0.70 V | [43] |
| 3D carbon-coated NiCo2S4 nanowires | Annealing | – | 1 m KOH | Electrocatalyst | 10 mA cm−2 at 1.64 V | [44] |
| Supercapacitor | 343.75 mAh g−1 at 1.82 A g−1 | |||||
| MoC quantum dots@NC | Carbonization | 2.02 | – | Photocatalyst | 1.7 mmol h−1 g−1 H2 evolution rate | [46] |
| Cobalt phosphide@NC | Carbonization | – | – | Photocatalyst | 66% CO selectivity | [48] |
| Hollow carbon fiber from duck feathers | Carbonization | 8.9 | 1 m LiPF6 | Li-ion capacitor | 91.1% capacity retention at 1 A g−1 in 10 000 cycles | [52] |
| Porous carbon from chitosan/urea | Sol-gel | 8.6 | 6 m KOH | Supercapacitor | C = 329.2 F g−1 at 0.5 A g−1 in 10 000 cycles | [53] |
| Co-doped carbon from tea waste | Pyrolysis | – | KOH | Supercapacitor | C = 170 F g−1 at 0.5 A g−1; 94.8% retention over 14 000 cycles at 3 A g−1 | [54] |
| rGO wrapped carbon microfibers | Pyrolysis | 13.5 | PVA/H2SO4 gel mixture | Supercapacitor | C = 18.7 F cm−3 at 1 A cm−3 | [55] |
| Na-ion battery | 400 mAh g−1 in 100 cycles | |||||
| BiFeO3-NC | Polymeric precursor method | – | 1 m Na2SO4 | Supercapacitor | C = 811 F g−1 at 50 mV s−1 | [56] |
| Carbon co-doped with F (triazine-based framework) | Carbonization | 12% | 1 m KOH | Supercapacitor | C = 326 F g−1 at 1 A g−1 in 10 000 cycles | [57] |
| Carbon-coated LZTO/TiO2 from chitosan | Calcination under N2 | – | 1 m LiPF6 | Li-ion battery | 266.3 mAh g−1 at 0.2 A g−1 in 100 cycles | [58] |
| Fe3O4@NC nanocapsules | Calcination under Ar | – | 1 m LiPF6 | Li-ion battery | 873 mAh g−1 at 5 A g−1 in 500 cycles; 480 mAh g−1 at 20 A g−1 in 1000 cycles | [59] |
| Core-shell Fe3O4 carbon composite | Carbonization | – | – | Li-ion battery | 1222 mAg h−1 at 0.2 A g−1 in 100 cycles | [60] |
| Hollow Ga2O3@N-CQD nanospheres | Hydrothermal | – | 1 m LiPF6 | Li-ion battery | 700.5 mAh g−1 at 0.5 A g−1 in 500 cycles | [62] |
| Porous carbon from silk cocoon | Activation with KOH | – | LiTFSI/EMIMBF4 | Li-O2 battery | 500 mAh g−1 at 0.2 mA cm−2 over 400 cycles | [63] |
| Porous hollow carbon nanospheres | NH3-assisted carbonization | 6.25 | 6 m KOH | Li–S battery | 1620 mAh g−1 at 0.1C; 789 mAh g−1 at 0.5C in 200 cycles | [64] |
| Carbon material from ZIF-8 precursor | Carbonization | 10.45% | 1 m LiTFSI + 2 wt% LiNO3 | Li–S battery | 529 mAh g−1 at 0.3 A g−1 in 180 cycles | [67] |
| Hollow carbon material from ImIp-encapsulated ZIF-8 | Pyrolysis | 5.53 | 1 m LiTFSI + 1 wt% LiNO3 | Li–S battery | 562 mAh g−1 at 2C in 800 cycles | [66] |
| Multi-channel hollow carbon nanotube/nanofiber co-doped with S (CNT/SNCF) | Calcination | 48.4 (of CNT/SNCF) | 1 m NaClO4 + 5% FEC | Na-ion battery | 274.1 mAh g−1 at 1 A g−1 in 1000 cycles; 150.4 mAh g−1 at 5 A g−1 in 5000 cycles | [68] |
| 0.8 m KPF6 | K-ion battery | 212.51 mAh g−1 at 1 A g−1 in 1000 cycles; 100.1 mAh g−1 at 5 A g−1 in 5000 cycles | ||||
| Carbon nanotube paper (CNTP) | Pyrolysis | 11.7 | 0.85 m KPF6 | K-ion battery | 250.1 mAh g−1 at 0.1 A g−1 in 100 cycles | [69] |
| SeS2@NCNFs | Carbonization | 11.2 | 0.7 m KPF6 | K-SeS2 battery | 703 mAh g−1 at 0.05 A g−1 in 150 cycles; 417 mAh g−1 at 0.5 A g−1 in 1000 cycles | [70] |
| 3D porous carbon | Sintering | – | AlCl3 | Al-ion battery | 33 mAh g−1 at 0.5 A g−1; 8 mAh g−1 at 2 A g−1 in 20 cycles | [72] |
| Graphene-nanotube complex | Annealing | 3.12 | 0.1 m KOH (N2- and O2-saturated) | Zn-air battery | 873 mAh g−1 at 10 mA cm−2 | [73] |
| Honeycomb-like FeZnN-C and Zn/ZnN-C | Pyrolysis | 9.85 | 6 m KOH | Zn-air battery | 785 mAh g−1 at 10 mA cm−2 | [74] |
| 2 m ZnSO4 | Zn-ion battery | 397.7 mAh g−1 at 0.1 A g−1; 279.5 mAh g−1 at 2 A g−1 | ||||
| Porous hollow carbon from MF resin | Calcination | 2.94 | 0.1 m KOH | Metal-air battery; fuel cell | 94% relative current after 400 min. | [75] |
- a) C = specific capacitance, PCA = photocatalytic activity.
2 Synthesis of Nitrogen-Doped Carbon Materials
Due to the advantages brought by nitrogen doping, N-doped carbon materials have emerged as an important topic of research. There are many ways of preparing N-doped materials as reported in literatures, with various sources of nitrogen, working conditions, and equipment used. The synthesis of N-doped carbon materials can be carried out in situ or as a separate process after the preparation of carbon materials (post-treatment). On one hand, the addition of a nitrogen source along with a carbon source or the use of N-containing carbon-based compounds (C and N sources) during the chemical vapor deposition (CVD) method allows for the substitution of N atoms into the carbon network as it is synthesized, which are typically used to prepare carbon nanotubes and graphene.[25] Other common synthesis routes that can integrate in situ doping are solvothermal and arc-discharge methods. Researchers have also explored the use of biomass as precursors, which are often pre-treated to obtain dried particles. The use of biomass as starting material is ideal as it contains both nitrogen and carbon, and allows for an in situ doping process, typically done by pyrolysis (heat treatment at 800 °C).[26, 27] Similarly, metal organic frameworks (MOFs) with nitrogen and carbon atoms have been studied as a precursor for N-doped carbon materials, which are produced from the carbonization of MOFs at 500–800 °C.[28] However, the use of high temperatures (>800 °C) can cause defects and degradation in the structure. In some cases, mechanochemistry has also been utilized to incorporate nitrogen atoms into the carbon matrix without the use of high temperatures.
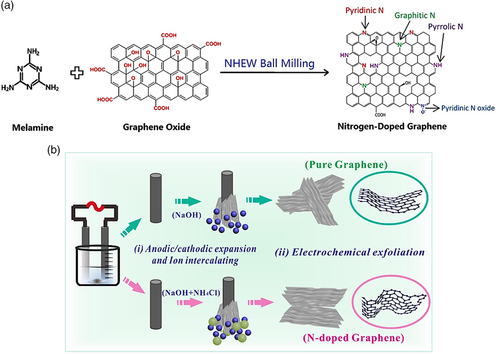
Zhuang et al.[29] reported the mechanochemical synthesis of N-doped graphene as an electrocatalyst via wet ball milling method at room temperature (Figure 2a). The starting materials are activated by the mechanical energy produced from the grinding process, which initiates the reaction. With the ability to alter the time and speed of ball milling, doping levels, N-bonding, and particle size can be controlled. Another method is by electrochemical exfoliation of a carbon material, which can produce N-doped graphene as electrode material. Jing et al.[30] investigated the performance of N-doped graphene as a supercapacitor, which was prepared by electrochemical exfoliation of graphite rods using alternating voltage while immersed in an aqueous mixture of NaOH and NH4Cl (Figure 2b). The N-doped material exhibited better electrochemical performance than pure graphene.
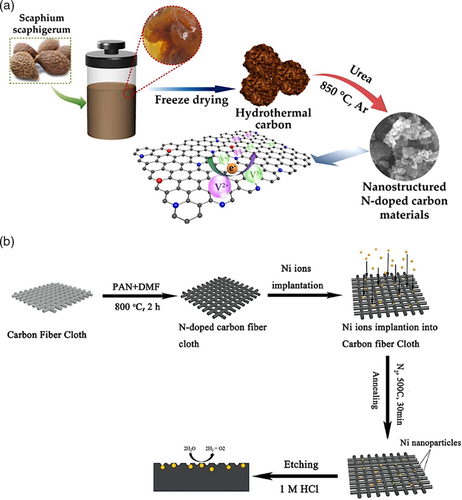
On the other hand, post-treatment processes involve the incorporation of nitrogen atoms in available sites, such as the edges of the substrate and empty sites caused by defects in the structure.[25] Typical post-treatment doping methods include annealing (300–500 °C), hydrothermal method, plasma treatment, and ion implantation.[26] By using biomass, N-doped carbon material can be produced via hydrothermal treatment, followed by freeze-drying and mixing the hydrothermal product with urea (Figure 3a). The resulting mixture then undergoes a heat treatment under inert atmosphere at various temperatures (700–1000 °C).[31] The researchers found that heating the carbon-urea mixture at 850 °C had the highest levels of N-species, which resulted in the highest catalytic activity in a vanadium redox flow battery. In a study by Wu et al.,[32] carbon fiber cloth was doped with nitrogen atoms by carbonization at 800 °C. The N-doped carbon fiber material was then treated further via ion implantation of nickel ions to improve its performance as an electrocatalyst. The preparation method (Figure 3b) is a promising technique that can be modified with other reagents and adapted into a large scale production. However, post-treatment methods may not easily incorporate nitrogen atoms into the carbon matrix as the carbon material holds a stable, highly-ordered structure, especially when synthesized at high temperatures.[33] This is commonly observed in graphitic carbon materials, which can only form bonds in the structure's edges and surface.
Among these in situ and post-treatment methods, CVD is one of the most effective ways of doping carbon materials with nitrogen as the method is highly efficient and easily modifiable, depending on the requirements and application, as well as offering greater purity than other methods. However, it is not a simple procedure and requires high temperatures. On another note, ion implantation has many benefits: uniform doping on carbon materials, regulated doping levels, and production of doped material with less impurities. The drawback with this technique is the cost generated from the equipment and operations due to sophisticated technology and high energy requirements. The use of self-doping biomass as precursor is also a very appealing strategy due to the abundant supply and sustainability. However, harsh chemicals are often used as activating agents in conjunction with biomass utilization to obtain carbon materials. For instance, KOH is a strong alkali and is harmful to the environment.[22] Therefore, careful consideration is required when using activating agents for a greener approach, especially with the use of biomass.
3 Environmental Applications
3.1 CO2 Adsorption
Given that carbon dioxide (CO2) emissions are generally recognized as one of the leading causes of global warming, scientists have been investigating N-doped materials application in the environment and sustainability fields. The process of CO2 adsorption, also known as CO2 capture, relies on solid adsorbent materials which CO2 molecules can adhere to via van der Waals forces.[34, 35] Different porous solid materials, such as carbon materials, metal-organic frameworks (MOFs) and their composite materials, have been examined as CO2 adsorbents. With its low cost, excellent CO2 adsorption performance and their physicochemical stability, porous carbon materials showed great potential applications in CO2 adsorption.
Recently, Ouyang et al.[36] examined N-doped material for CO2 adsorption. The researchers fabricated a novel N-doped material by carbonizing the precursor of melamine-resorcinol-formaldehyde resin/graphene oxide (MR/GO) composites with KOH as the activation agent. Batch adsorption experiments showed that the fabricated MR/GO porous carbon material has an impressive ability in adsorbing CO2 (5.21 mmol g−1) at 298.15 K and 5 bar. In addition, the material has exhibited high selectivity of CO2/N2, fast adsorption kinetics, and good recyclability. During 5 cycles of continuous adsorption and desorption, the amount of CO2 adsorbed by MR/GO decreased slightly, with a final recovery ratio of 98.5% (Figure 4a). Although the results show good stability, the number of cycles is insufficient to determine its stability for practical applications. Hence, further work is required to conclude the long-term stability of MR/GO material in the application of CO2 adsorption. Similarly, Gong et al.[37] reported that the high electron affinity of the nitrogen atom can produce a positive charge density into the adjacent carbon atom, which provides easier oxygen adsorption.
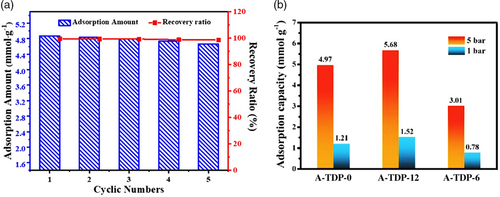
Zhou et al.[3] studied the effects of activation temperature, nitrogen levels, and porosity on the efficacy of CO2 adsorption by porous carbon materials. The N-doped carbon materials were synthesized from licorice residue via hydrothermal carbonization and activation by KOH. The best activating temperature was found to be at 600 °C, which demonstrated remarkable CO2 adsorption capacity at 0 and 25 °C (6.43 and 3.89 mmol g−1, respectively) at 1 bar. Furthermore, the material exhibited high CO2/N2 selectivity (21.3). According to the researchers’ observations, the high selectivity for CO2 adsorption of is related to the highly porous structure and nitrogen dopant level. Likewise, Wang et al.[38] explored the adsorption abilities of porous carbon (dicyandiamide precursor) for CO2 capture using binary co-doping of nitrogen and phosphorus. Compared to N-doped porous carbon, the addition of phosphorus in porous carbon enhanced the nitrogen content in the structure by allowing more defects to form. Different molar ratios of phosphate group was investigated and researchers found that the addition of 1/12 phosphate group (A-TDP-12) delivered the best results. Therefore, the resulting structure is of a micro/meso-porous nature with a large surface area of 1332 m2 g−1, which allowed for an effective adsorption of CO2 at room temperature (1.52 mmol g−1 at 1 bar, 5.68 mmol g−1 at 5 bar for A-TDP-12), as illustrated in Figure 4b. Despite the binary co-doping technique, the results did not improve significantly compared to the studies mentioned previously.[3, 36] This implies that the type of porous carbon used is one of the key factors to consider as some are more effective than others, as opposed to the level of doping and number of heteroatom dopants. It would be worthwhile to determine the effects of co-doping for activated carbons that previously delivered the best CO2 adsorption abilities, including those obtained from graphene oxide and hydrochar precursors.
3.2 Energy Conversion and Storage Applications
The growing worldwide energy demands and the problem of CO2 emissions have caused a rush to develop renewable energy conversion technologies to satisfy demands, whilst combatting climate change. In this setting, high-performance electrochemical energy conversion techniques, such as electrolysis and photocatalysis, have been investigated as promising solutions to address these difficulties. The same applies when addressing the issues involved with current energy storage technologies. The development of new materials with extraordinary electrochemical characteristics is one of the most important concerns in developing these energy conversion and storage devices.[39, 40] Over the recent decades, researchers have investigated N-doped carbon-based materials for energy conversion and storage applications.
3.3 Energy Conversion
3.3.1 Electrocatalyst
Energy conversion is typically a catalytic reaction involving a catalyst that is spread on a supporting material for optimal use.[41] One of the important reactions involved in electrolytic energy conversion is ORR, which occurs at the cathode. In comparison with the oxidation reaction at the anode, the kinetics of ORR is sluggish and hinders the electrochemical performance of fuel cells.[42] Research has shown that N-doped carbon material as an electrocatalyst is highly favorable due to the material's intrinsic properties as a result of doping. Research carried out by Maldonado and Stevenson revealed that N-doped carbon fiber showed an improved catalytic activity by shifting the ORR potential up by 70 mV, compared to its non-doped counterpart.[4] Furthermore, N-doped carbon dots on graphene oxide hybrid was successfully synthesized via hydrothermal method for the first time and applied as an electrocatalyst for ORR.[43] The performance delivered by the hybrid material was comparable to that of conventional Pt/C catalyst with remarkably greater efficiency as an electrocatalyst compared to non-doped materials. Besides being an effective metal-free electrocatalyst, this material was synthesized through a simple and economical method, which are important aspects to consider for industrial scale applications.
In a study by Zhang et al.,[44] the effects of N-doping and carbon-coating on NiCO2S4 nanowires were explored. The 3D morphology and greater number of catalytic sites in the structure made the prepared N-doped carbon-coated material (N–C@NiCO2S4 NWs) as a suitable electrocatalyst in water splitting processes. To determine the effects of adding N dopant and carbon coating, the catalytic activity of N–C@NiCO2S4 NWs was compared with that of pristine (NiCO2S4 NWs), carbon-coated (C@NiCO2S4 NWs), and N-doped NiCO2S4 nanowires (N-NiCO2S4 NWs). Results have shown that the addition of a carbon layer, heteroatom dopant, and the combination of the two led to larger current densities than NiCO2S4 NWs. Therefore, higher electrocatalytic activity towards water splitting reaction was achieved with the modified NiCO2S4 nanowires (N–C@NiCO2S4, C@NiCO2S4, N-NiCO2S4). Furthermore, N–C@NiCO2S4 NWs functioned as an electrocatalyst with lower onset potential and overpotential requirements, as well as greater stability. This study emphasizes the benefits of modifying catalysts via coating and doping strategies, including the synergy achieved from combining these strategies.
3.3.2 Photocatalyst
Besides electrolysis, the water splitting process can also be carried out via photocatalysis which utilizes visible light to drive the reaction. Although graphitic carbon nitride (g-C3N4) is cost-effective, it's role as a photocatalyst for water splitting process has been unsuccessful due to slow reaction kinetics, rapid recombination rate, small surface area, and inadequate visible light absorption.[45] To address these issues, g-C3N4 was combined with molybdenum (Mo) and N-doped carbon material.[46] This material was capable of delivering superior H2 evolution rate of 1.7 mmol h−1 g−1, which is over 200 times greater than that of pristine g-C3N4. The impressive performance of the hybrid material as a photocatalyst highlights the importance of N-doped carbon materials and the synergistic effects involved when combined with existing materials. Additionally, N-doped carbon quantum dots were found to have an effective role as a photosensitizer in combination with TiO2 photocatalyst upon exposure to visible light.[47] The development of a metal-free photosensitizer in solar cells is beneficial and necessary as it negates the use of rare metal complexes, which are expensive and require rigorous processes prior to application.
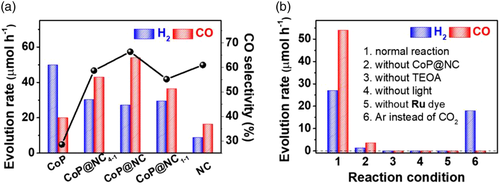
Moreover, N-doped carbon materials have been considered as a protective layer for a cobalt phosphide (CoP) catalyst involved in the photoreduction of CO2.[48] The photocatalytic conversion of CO2 to CO has gained significant interest due to the dual benefit of reducing CO2 emissions and sustaining energy demands. With the porous structure of the N-doped carbon material layer surrounding the catalyst, exposure to water molecules is averted, which prevents the release of hydrogen from the resulting side reaction. Based on the experiments conducted in this study, the rate of CO evolution had nearly tripled with the encapsulated catalyst design, whilst that of H2 significantly decreased by half (Figure 5a). Control experiments were included in the evaluation, which further showed that the photoreduction of CO2 had peak performance with the hybrid catalyst and system design (Figure 5b). Therefore, the added protective layer has been shown to increase the photocatalytic activity and selectivity for CO2.
3.4 Energy Storage
3.4.1 Supercapacitors
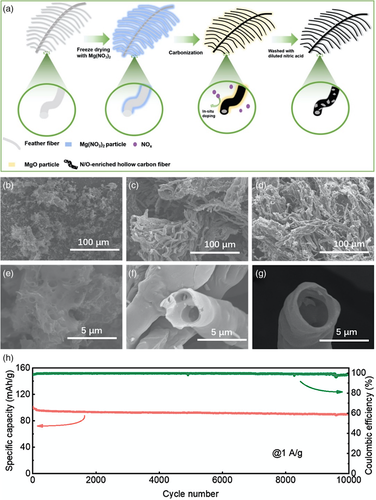
The application of carbon materials as electrodes for energy storage devices, such as supercapacitors and rechargeable batteries, is an important field of research with ever-growing demands for capacity, rate and long-term performance. Capacitors, in principle, consists of two conductors with an insulator sandwiched in between.[49] In particular, double-layer capacitors possess unparalleled specific capacitance values, which allows for quicker charge-discharge processes than batteries. Therefore, they can be suitable as an alternative to batteries, in some cases, or used in conjunction with batteries for hybrid/electric vehicle applications. One of the most attractive research themes is utilizing biomass as a carbon material precursor due to abundance, sustainability, and structural stability.[50, 51] Huang et al.[52] synthesized N/O-doped porous carbon derived from duck feathers. The method of synthesis is unique, which utilized Mg(NO3)2 as a “trifunctional template” due to its function as a supporting material, in the creation of pores within the structure, and as a source of dopants (Figure 6a). These functions are enabled through the decomposition of Mg(NO3)2 into MgO (support, pore formation) and NOx (in situ doping) during the carbonization process, which also results in a hollowed carbon fiber structure (Figure 6f–g). As a cathode in Li-ion capacitor system, the doped carbon material exhibited high energy and power densities with excellent cycling performance at 1 A g−1 after 10 000 cycles (Figure 6h).
Supercapacitors, which can supply energy in a short time and is beneficial for applications that include braking systems, peak power management and backup memory devices, have been examined extensively with N-doped carbon material. Recently, Liu et al.[53] examined N-doped porous carbon as electrode materials for supercapacitors. KNO3 was used as an activating agent for the pore formation as it disperses evenly in the chitosan gel precursor. The pore size formed by carbonized dry gel appear to increase the specific surface area of the carbon material. The research revealed that the prepared porous carbon material has a unique pore structure, large specific surface area and high nitrogen/oxygen content of 8.6% and 13.5%, respectively. In 6 m KOH electrolyte, the material can achieve a high specific capacitance of 329.2 F g−1 at 0.5 A g−1 and good long-term cycling performance with approximately 200 F g−1 at 5 A g−1 (only 8.4% capacitance loss after 10 000 cycles), which has a good prospect in the field of supercapacitors (Figure 7a).
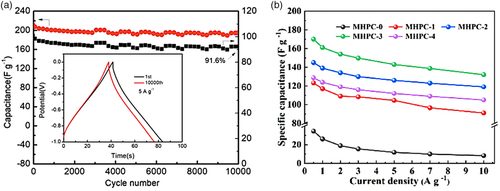
By utilizing tea waste, a group of researchers prepared a porous carbon material co-doped with multiple heteroatoms (N, O, P).[54] The method of synthesis is through pyrolysis and tea waste activation, in which tea waste functions as the “self-template” for the carbon network and dopants. The defects formed from the addition of heteroatom dopants can improve conductivity and provide additional pseudocapacitance, whilst repressing the hydrophobicity of carbon-based materials. In terms of electrochemical performance, the prepared carbon material delivered capacitance values of 170 and 132 F g−1 at 0.5 and 10 A g−1, respectively (Figure 7b). Therefore, the porous carbon material is ideal as an electrode for supercapacitors. Extensive research has looked at nitrogen doping materials to improve the performance of supercapacitors. In a recent study, researchers examined N-doped reduced graphene oxide wrapped carbon fibers for flexible fiber supercapacitors.[55] N-doped carbon microfibers (CFs) and reduced graphene oxide (rGO) composite was synthesized via pyrolysis in the application of flexible supercapacitors. Based on the results, the addition of N-doped graphene on the surface of CF exhibited high capacitance. Moreover, researchers worldwide are focusing on developing hierarchical porous hollow carbon spheres composed of macro-porous core and microporous and/or mesoporous shell due to their potential electrochemical applications. Similarly, Zhang et al.[44] investigated 3D carbon coated NiCo2S4 nanowires structures doped with nitrogen (N–C@NiCo2S4 NWs) for energy storage applications. The researchers used a simple heat treatment by annealing the C@NiCo2S4 NWs under inert atmosphere at 500 °C for an hour with thiourea and ethylene glycol to decorate the carbon layer and nanowires with nitrogen-doping. In this study, however, the enhanced nanowires were applied in both supercapacitors and water splitting. The N–C@NiCo2S4 NWs electrodes show much enhanced electrochemical performance as a supercapacitor (97.5% capacity retention after 10 000 cycles). The improvement in performance was ascribed to the synergistic effect of N-doping and carbon coating on the nanowire substrate.
In a new study, researchers synthesized bismuth ferritic embedded with N-doped carbon (BiFeO3-NC) by polymeric precursor method. It shows that the BiFeO3-NC nanocomposite shows superior electrochemical performance over the reported electrode materials.[56] The specific surface area of BiFeO3-NC nanocomposite was found to be 339 m2 g−1. The porous BiFeO3-NC electrode offered bigger surface for significantly larger SO42− ion diffusion. This resulted in high specific capacitance (C) value of about 811 F g−1 in 1.0 m Na2SO4 electrolyte. Moreover, the porous BiFeO3-NC electrode was found to be remarkably stable with up to 5000 cycles with excellent retention capacity. On the same note, Gao et al.[57] synthesized F/N co-doped porous carbons (FNCs) with a large specific surface area of 1849.1 m2 g−1 through an in situ doping process using F- and N-rich covalent triazine-based frameworks (FCTFs) as the starting material. As an aqueous-based supercapacitor electrode, the as-prepared high-content F/N-doped FNC-700 achieved a specific capacitance of 326 F g−1 at 1 A g−1, remarkable cyclability with negligible capacitance decay after 10 000 cycles in three-electrode systems, and a maximum energy density of 31.4 Wh kg−1 in two- electrode systems. This study, however, used the in situ doping process. Thus, it provided an insightful hint in exploring multifunctional heteroatom-doped carbons using the said method.
3.4.2 Batteries
Li-Ion/Li-Metal
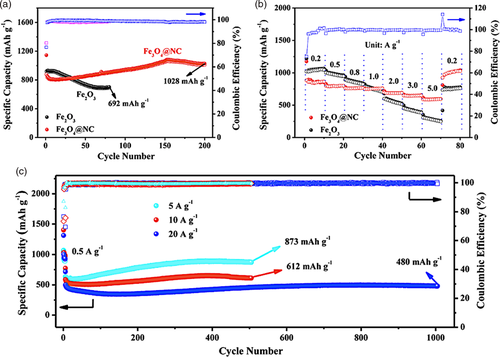
In a research conducted by Sim et al.[58] N-doped carbon coating layer was produced with chitosan as the precursor. The synthesis of a chitosan-derived N-doped carbon coating layer on LZTO/TiO2 composite utilizing a hydrothermal method, following that a calcination step, with the resulting material being used as an anode for lithium-ion batteries (LIBs). The prepared N-doped carbon coated LZTO/TiO2 (N–C@LZTO/TiO2) composite electrode was subject to electrochemical tests to determine its performance when utilized as an anode material for LIBs. Characterization analyses (XRD and EDS mapping) confirmed the formation of N-doped carbon layer, which coated the surface of the LZTO/TiO2 composite material. The addition of the coating has shown improvements in the surface area, pore size, and pore volume of the material. The specific discharge capacity delivered by the N–C@LZTO/TiO2 composite anode material was 266.3 mAh g−1 at a current density of 0.2 A g−1 after 100 cycles, which is 80% greater than pristine LZTO. In addition, the researchers reported that the anode material exhibited excellent rate capability and large specific discharge capacity, even at a high current density (140.7 mAh g−1 at 1 A g−1), due to its highly porous structure. Therefore, the results from this study revealed the synergistic effects of N-doped carbon-coated layer on LZTO/TiO2. In another research conducted by Duan et al.[59] Fe3O4 was incorporated in N-doped carbon with self-formed channels in a nanocapsule structure. Compared to a pristine Fe2O3 electrode, Fe3O4@NC delivered a better performance in both cycling and rate performances, with higher capacities achieved and greater stability when cycled at higher current densities (Figure 8a,b). In terms of its long-term performance as an anode in LIBs, a reversible capacity of 480 mAh g−1 was sustained at a high current density of 20 A g−1 within 1000 cycles and nearly 100% Coulombic efficiency on average. At a lower current density of 5 A g−1, the reversible capacity achieved was 873 mAh g−1 (Figure 8c). Furthermore, the Fe3O4@NC anode was able to satisfy demands in high-rate applications with the ability to complete charging in about 2 min.
Wang et al.[60] designed iron oxide/carbon nanocomposites with a hollow core–shell structure as an electrode for LIBs. Fe2O3@polydopamine nanocomposite was prepared using an Fe2O3 nanocube and dopamine hydrochloride as precursors. Then, Fe3O4@N-doped C composite was obtained by means of further carbonization treatment. Finally, Fe3O4@void@N-doped C-x composites with core–shell structures of different void sizes were obtained by means of Fe3O4 etching. The effect of the etching time on the void size was studied. The electrochemical properties of the composites when used in LIBs showed that the sample that demonstrated the best electrochemical performance was obtained via etching for 5 h using 2 mol L−1 HCl solution at 30 °C. The discharge capacity of the Fe3O4@void@N-Doped C-5 was able to reach up to 1222 mAg h−1 under 200 mA g−1 after 100 cycles. Furthermore, Ga was studied as a self-healing anode by several researchers[61, 62] as they proposed a Ga–Sn liquid metal alloy, which showed inherent self-healing property as well as superior electrochemical performance for alkali-ion batteries. The hollow Ga2O3@nitrogen-doped carbon quantum dot (H-Ga2O3@N-CQD) nanospheres were synthesized via a facile approach as an anode material for LIBs.[62] As a result, the anode material delivered an initial discharge capacity of 1348.5 mAh g−1 at 0.1 A g−1 and a reversible capacity of 700.5 mAh g−1 under 0.5 A g−1 after 500 cycles. Similarly, N-doped porous carbon obtained from silk cocoon (NCFC) was first used as cathodes for Li-O2 batteries with ionic liquid.[63] NCFC is made up of irregular bending slices and has a high specific discharge product of most Li-O2 surface area which means numerous active sites for electrochemical reactions. The results show that NCFC based Li-O2 battery has good cycle stability and high energy efficiency under different current density. In particular, NCFC based Li-O2 batteries can work more than 400 cycles with the maximum overpotential less than 0.92 V, which is much better than carbon black super P-based Li-O2 batteries (188 cycles with overpotential up to 1.88 V) and many reported Li-O2 batteries. The results show that NCFC is a very promising material for Li-O2 batteries. A recent study designed a highly porous, N-doped hollow carbon nanospheres (PN-HCNs) through an “interfacial copolymerization strategy” using N-containing polymer precursors and a subsequent NH3-assisted carbonization process.[64] This study further demonstrates the significance and effectiveness of N-doping in enhancing the electrochemical performances. The results have shown that the improved porosity and surface area were achieved without the elimination of N moieties during the NH3-assisted carbonization. The prepared PN-HCN demonstrates outstanding electrochemical performances as a cathode host in lithium–sulfur (Li–S) batteries, including a near-to-theoretical capacity of 1620 mAh g−1, high-rate capability and good cycling stability (789 mAh g−1 at 0.5C after 200 cycles). Furthermore, significant improvements were demonstrated by PN-HCN, compared to the non-doped HCN material (Figure 9a–c). PN-HCN was also cycled at different current densities (0.05, 0.1, 0.5, 0.8, and 1.0 g−1), which revealed a symmetric profile that is indicative of high reversibility (Figure 9d). In long-term cycling, PN-HCN demonstrated excellent stability during 10 000 cycles and maintained a high specific capacitance value of about 170 F g−1 (Figure 9e).
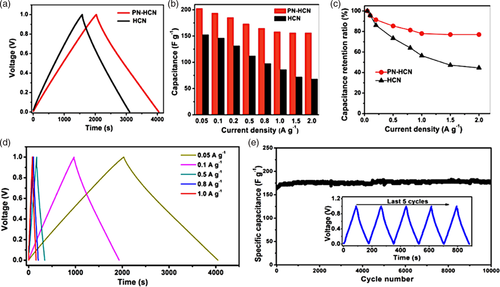
With such a rational structural integration of the significantly enhanced porous structure, well-retained N functionality, and well-defined nanosized hollow spherical structure, the PN-HCN showed their significance and effectiveness in enhancing the electrochemical performances in the Li–S batteries and supercapacitors, as compared with the HCN without the NH3 treatment. Moreover, N-doped carbons were found to be able to anchor Li-polysulfide complexes, which resulted in the suppression of their shuttling between anode and cathode, and the improvement of the cycling performance of the Li–S batteries.[65] On this regard, the shortcomings of hollow heteroatom-doped materials have been discussed by Li et al.[66] The hollow heteroatom-doped material's structure causes ineffective contact between sulfur and electrolyte, which leads to an overall poor conductivity and low capability. Due to these issues, researchers have been prompted to investigate new methods to synthesize infused hollow carbon materials for high performance Li-S batteries. Researchers have found that utilizing close-packed hollow carbon spheres based on SiO2 hard template methods could improve rate performance of cathodes by improving transfer of electrons. However, given the unfavorable procedures required, researchers investigated the importance of metal organic frameworks (MOFs) to synthesize interfused hollow carbon materials. Zhang et al.[67] prepared N-doped carbon materials using zeolitic imidazolate framework-8 (ZIF-8) as the precursor and the systematic investigations of the effects of nitrogen doping level, graphitization, and surface area on the electrochemical performance of Li–S batteries. As a result, the carbon material is inheriting the rhombic dodecahedral morphology of the ZIF-8. This suggests that conventional MOFs precursors are mostly discrete and solid particles, the carbon-based composites derived from them will usually exhibit discontinuous electrical conductivity among particles. A research conducted by Li et al.[66] involved the preparation of a hollow N-doped carbon (HNPC) material, derived from “imidazolium-based ionic polymer (ImIP)-encapsulated zeolitic imidazolate framework-8 (ZIF-8)”. The carbon precursor was prepared by creating an enclosed layer of ImIP around ZIF-8, resulting in a core-shell structure. After carbonization, a unique “interfused” structure was observed. This structure gave rise to the superior cycling stability and rate capability (562 mAh g−1 at 2C), as well as a large energy density.
Other Monovalent Metal-Ion
As a solution to carbon fibers’ inadequate capacitance and conductivity, carbon fibers (CFs) wrapped with N-doped reduced graphene oxide (N-rGO) was prepared and electrochemically tested for its possible application as an anode for sodium-ion batteries.[55] The anode material exhibited a high reversible specific capacity (400 mAh g−1) with complete capacity retention at 0.1C after 100 cycles, which demonstrates the enhanced electrochemical properties of CFs compared to bare CFs from other studies. Figure 10a illustrates the structure of N-rGO wrapped CF and the intercalation process of Na+, which was proposed to occur on the CF surface, whilst the addition of N-doped graphene helped improve electrochemical performance.
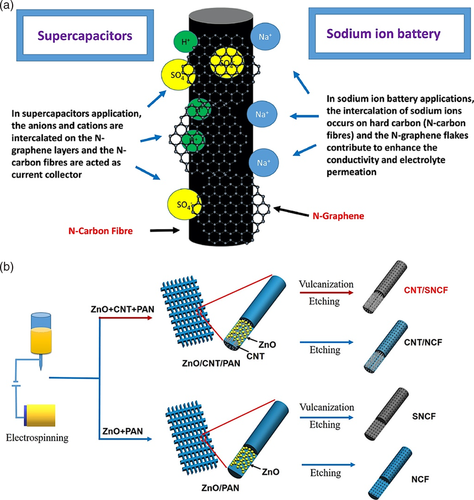
In another study, Chen et al.[68] investigated sulfur and nitrogen doping (S/N doping) impact on sodium-ion batteries (NIBs) and potassium-ion batteries (KIBs). The researchers designed a morphology with unique multi-channel hollow 1D/1D carbon nanotube/carbon nanofiber network and lattice structure of carbon with S/N co-doping (Figure 10b). The S/N doped carbon nanotube/carbon nanofiber composites (CNT/SNCF) possess not only high conductivity but also good structural stability during charge/discharge processes. The synthesized material electrodes were tested in NIBs and KIBs and delivered high discharge capacities (274.1 and 212.5 mAh g−1 at 1 A g−1 after 1000 cycles, respectively) with excellent cycling stabilities (150.4 and 100.1 mAh g−1 at 5 A g−1 after 5000 cycles, respectively) and superior rate performances (109.3 mAh g−1 at 10 A g−1 and 108.7 mAh g−1 at 5 A g−1, respectively). With its flexibility and impressive electrochemical performance, the synthesized S/N-doped carbon material shows promising applications in flexible energy storage systems.
Recently, researchers designed a free-standing flexible anode, that is, N-doped carbon nanotube paper (NCTP) by pyrolysis of organic polypyrrole materials.[69] The correlations between the material structure and electrochemical properties have been investigated by a series of material analysis and characterizations, as well as electrochemical tests. The research results show that the annealing temperature dramatically affects the N-doping content, the carbon defects, and the graphitization degree. Electrochemical tests indicate that the NCTP annealed at 700 °C displays the best performances with a high reversible capacity of 250.1 mAh g−1 at 0.1 A g−1 and excellent rate capability with 133 mAh g−1 of specific capacity retained at 5 A g−1. The superior electrochemical properties are derived from the synergistic contribution from the moderate N-doping, carbon defect, and high electronic conductivity of the materials. The facile pyrolysis strategy and the appealing performances involved in this work could provide some hints to modify existing anode materials for high-performance applications in KIBs.
Similarly, N-doped materials were used to improve the performance of K-SeS2 batteries. In an attempt to produce solutions for the typical problems associated with Li–S batteries (large volume change, formation of sulfides, shuttling effect of polysulfides), Yao et al.[70] investigated potassium anode with SeS2 batteries to provide an energy storage system with a low-cost and high energy density. The N-doped anode material was synthesized via electrospinning, followed by carbonization, activation by KOH, and “melt diffusion method” to insert SeS2 into the pores (Figure 11a). The study aimed to prove that K-SeS2 battery could deliver a superior long cycle life and high energy density by encapsulating SeS2 into a high-content N-doped free-standing porous carbon nanofiber film (SeS2@NCNFs). The researchers compared the rate performance and energy densities of SeS2@NCNFs, Se@NCNFs, and S@NCNFs at different rates, which showed that SeS2@NCNFs is far more superior than the other two materials (Figure 11c,d).
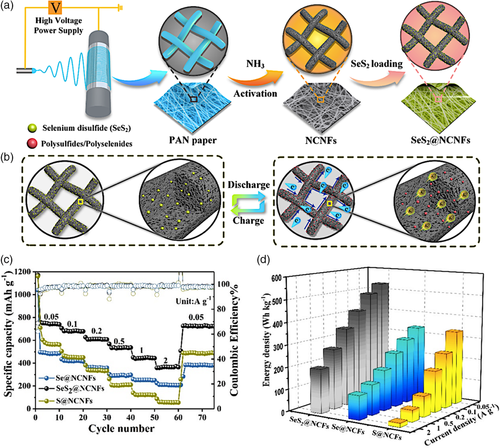
The energy density of the electrode is obtained from the integral area of voltage against specific capacity. The SeS2@NCNFs could deliver a gravimetric energy density of 533 Wh kg−1 at 0.05 A g−1, which is much higher than those of the Se@ NCNFs (337 Wh kg−1) and the S@NCNFs (335 Wh kg−1). Even at higher rates from 0.1 to 2 A g−1, the SeS2@ NCNFs still exhibit enhanced gravimetric energy densities than those of the Se@NCNFs and the S@NCNFs cathodes, indicating the advantages of the SeS2-based electrode for K-ion storage. In summary, when used for K-SeS2 batteries, the SeS2@NCNFs could deliver excellent electrochemical properties in terms of high reversible capacity (703 mAh g−1 after 150 cycles at 0.05 A g−1), superior rate performance (372 mAh g−1 at 2 A g−1), and high energy density (533 Wh kg−1 at 0.05 A g−1). More impressively, the electrode could deliver a high specific capacity of 417 mAh g−1 after 1000 cycles with 85% capacity retention at 0.5 A g−1 with a Coulombic efficiency near 100% for K-SeS2 batteries. By rational and delicate design, the present study may provide an effective and feasible route to construct stable hybrid SeS2 host materials for achieving high-performance K-SeS2 batteries.
Multivalent Metal-Ion
Beyond Li-ion technology, multivalent ions have been considered as promising alternatives due to higher valency and multiple oxidation states, which can contribute to theoretically greater energy densities.[71] Xu et al.[72] synthesized N-doped three-dimensional porous carbon material cathode (N-3PC) via a simple heating reaction in oil bath combined with sintering method to utilize it as a cathode for Al-ion batteries (AIBs). Zn(NO3)2 was used as a pore-forming agent, which is an effective way to control the level of porosity and can support a large surface area. Besides the ease of diffusion of Al3+ ions, the highly disordered structure can also increase the reversible capacity of AIBs. However, the synthesized material as a cathode delivered low specific capacities, which are not comparable to that of other AIBs. Nevertheless, the synthesis method of N-3PC cathode material is attractive due to the low cost involved, environmental-friendly reagents and simplicity, which could potentially be developed further to enhance capacities for AIBs. In another literature, N-doped graphene–nanotube complexes (N-GNT) with hierarchical networks were synthesized with a nitrogen source (urea) and a splicing agent (H3BO3).[73] The researchers concluded that the N-GNT complex delivered an excellent performance for catalyzing ORR and energy storage in Zn-air batteries (ZABs). As a cathode in ZABs, a specific capacity of 873 mAh g−1 was achieved with a high energy density (1056 Wh g−1), based on the weight of Zn consumed, at a current density of 10 mA cm−2. Compared with other ZABs, the N-GNT cathode material has been shown to be more superior with its excellent electrochemical performance.
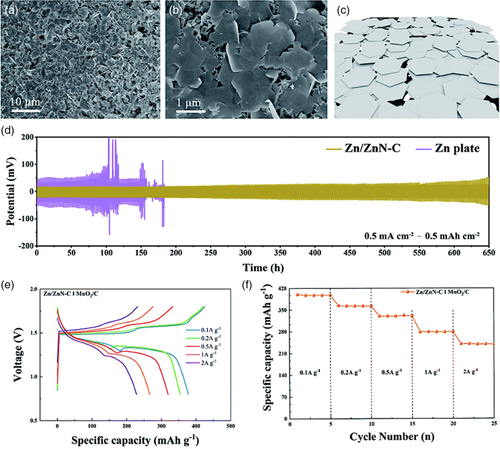
Huang et al.[74] prepared FeZnN-doped carbon material (FeZnN-C) with a honeycomb-like structure via pyrolysis as a potential substitute for precious metal-based catalysts in ZABs. The co-doped carbon material exhibited excellent kinetics of ORR and had a significantly lower formation of H2O2, as opposed to the slow kinetics and higher average H2O2 formation of conventional Pt-based catalysts. Furthermore, the integration of FeZnN-C electrocatalyst in a ZAB resulted in a high specific capacity (785 mAh g−1) with a high voltage discharge of 1.36 V at a current density of 10 mA cm−2. In addition, a Zn-containing anode (Zn/ZnN-C) for Zn-ion battery (ZIB) was also prepared as a probable solution to the dendrite formation that plague Zn anodes. Since the structure of ZnN-C is porous with a large surface area, mass transfer was enabled and prevented the formation of dendrites. Zn was electrodeposited on the synthesized ZnN-C in a solution of ZnSO4 (Figure 12a–c). Symmetrical cells of Zn/ZnN-C and Zn plate (i.e., identical electrodes in a cell) were assembled with 2 m H2SO4 electrolyte and compared in terms of the reversibility of Zn plating and stripping processes. Results show that the Zn/ZnN-C cell was much more consistent with the potential values as shown in Figure 12d. Meanwhile, the cell consisting of Zn plates exhibited significant voltage fluctuations and short-circuits, which highlights the impressive stability of Zn/ZnN-C cell. The prepared Zn/ZnN-C material was also evaluated as an anode in a Zn-ion battery with MnO2/C as cathode. Galvanostatic charge-discharge (GCD) tests were carried out at various current densities (0.1, 0.2, 0.5, 1.0, and 2.0 A g−1), which revealed charge and discharge plateaus at around 1.3 and 1.5 V, respectively (Figure 12e). The specific capacities achieved were impressive, ranging from about 280 mAh g−1 (2.0 A g−1) to almost 400 mAh g−1 (0.1 A g−1). Furthermore, the rate performance of the cell shows highly stable cycling, even at the highest current density, with minor reduction in specific capacity as current density is increased (Figure 12f). Therefore, the co-doping of transition metal(s) and heteroatom has shown promising results as an alternative to Pt-based catalysts in Zn-air batteries, as well as an enhanced performance and stability of Zn-based anodes in ZIBs without the formation of dendrites. This was largely due to the porous structure as a result of material doping, which has been a constant observation in studies involving N-doped carbon materials.
In a recent study, researchers produced N-doped materials, which can be applied as a cathodic electrocatalyst for both metal-air batteries and low-temperature fuel cells.[75] The researchers utilized melamine-formaldehyde (MF) resin as both carbon and nitrogen sources. The structure of the microspheres consist of sulfonated polystyrene microspheres (SPS) core with MF/SiO2 composite as the outer shell (SPS@MF/SiO2). The synthesized material was then calcined at 800 °C and acid-treated to obtain the N-doped material, N-HPHCS (Figure 13).
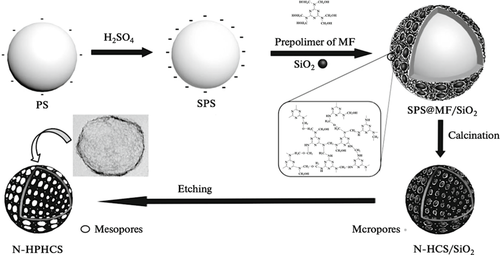
Throughout the heat treatment, the MF resin decomposes, which removes the SPS core and induces the formation of micropores on the shell. The mesopores are then created on the shell via etching of SiO2, which results in the N-HPHCS with high surface area and increased pore volume with high catalytic activity towards ORR. Due to the doped nitrogen atoms and the hierarchical porous structure, the as-prepared N-HPHCS exhibits both good electrocatalytic activity and stability toward ORR, as well as a promising application in metal-air batteries and low-temperature fuel cells as the cathodic electrocatalyst.
4 Conclusions and outlook
The process of nitrogen doping can be as simple as heating carbon materials with a nitrogen source, but the effects on its functionality can be profound. Based on the published reports, N-doped carbon materials has been proven to improve performance as an adsorbent for CO2 capture, as well as in energy conversion and energy storage applications. The combination of nitrogen and carbon materials generated a synergistic effect due to the resulting disordered and highly porous structure. In particular, biomass-derived carbon materials (licorice residue, duck feathers, tea waste, etc.) have shown promising results as a CO2 adsorbent and capacitor, exhibiting superior performance and long cycling stability. Furthermore, these carbon precursors have been selected due to their naturally high nitrogen content, which influenced the high doping levels. In addition, hollow carbon nanospheres have exhibited a promising performance as electrode material for energy storage applications, which can be improved with further modifications. Similarly, MOF-derived carbon materials delivered high specific capacities as electrode material for Li–S batteries. The use of N-containing MOFs as carbon precursor is convenient due to self-doping abilities, which eliminates the need for external chemical reagents and harmful nitrogen sources, such as ammonia. Researchers have also explored MOF-derived carbon materials to solve the issue of insufficient contact associated with hollow structures. As one of the emerging new materials, N-doped carbon dots have gained significant research interests which have led to their realization as promising catalytic materials in energy conversion applications. With its early development stages, N-doped carbon dots require more investigation for other applications, including energy storage. In other studies, the co-doping of nitrogen with other heteroatoms and transition metals were investigated. However, multi-heteroatom co-doping did not achieve significant improvements compared to N-doped carbon materials. In contrast, co-doping of nitrogen and transition metals have shown greater enhancements in performance as electrode materials in energy storage systems, such as LIBs and ZIBs. Therefore, there is great potential for the co-doping of nitrogen and transition metals in carbon materials and the vast majority of transition metals should be explored in the future.
Overall, biomass utilization for carbon material synthesis is the most attractive route to take, especially in terms of large scale production. The simple pyrolysis procedure for biomass is scalable and can be carried out at moderate temperatures. The use of biomass for carbon materials should be explored further due to its cost-effectiveness, sustainability, and environmentally friendly aspect. However, it is important to consider a synthesis route with the least amount of greenhouse gas emissions to maintain the greener approach. Additionally, alternative activating agents to KOH should be considered to reduce or eliminate the use of harmful chemicals. Further research is required to determine the best combination of parameters (doping level, temperature, activating agent), which have been shown to affect the porosity and morphology of the material; thereby, affecting the overall performance. Until now, we do not have a full understanding whether nitrogen content or types of bonding have a direct effect on the resulting performance of N-doped carbon materials. Therefore, more research is required to understand how parameters affect the properties and the respective mechanisms involved in various applications. Most importantly, future studies should consider the scalability of synthesis methods as many research focuses within the laboratory scale. Besides important aspects such as environmental safety, operating costs and simplicity of method should be at the forefront. With these strategies, a significant shift towards sustainable sources and green energy can be achieved to alleviate the effects of environmental pollution and global warming, whilst seeking to create the perfect balance for advanced materials with superior performance.
Acknowledgements
This work was funded by a grant from the Qatar University under its Collaborative Grant number QUCG-CAS-22/23-602. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of Qatar University.
Conflict of Interest
The authors declare no conflict of interest.
Biographies
Wadha Al-Hajri obtained her MA in English Linguistics from Hamad bin Khalifa University in 2017. She joined governmental research and development at the state of Qatar. She is currently pursuing a BSc degree in Chemistry where her research interests lies. (Image not shown due to author request).

Yannis De Luna is a Research Assistant in the Department of Chemistry and Earth Sciences at Qatar University. She has completed her BSc degree in Chemistry at Qatar University in 2021 and has joined Prof. Nasr Bensalah's research team after graduation. Her research interests include the chemistry of materials and their application as electrodes in energy conversion devices.

Nasr Bensalah is a Professor of Chemistry with the department of Chemistry and Earth Sciences, at College of Arts and Sciences, Qatar University. Before that, he held positions of Professor, Associate Professor, and Assistant Professor at University of Gabes in Tunisia. He received his PhD in Chemistry in 2002 from University of Monastir, Tunisia. His research interests are focused on the applications of electrochemical technologies for energy storage, water treatment, and chemical analysis. Prof. Bensalah received several internal and external grants to develop research in the field of electrochemistry. He supervised 12 MSc-students and 9 PhD- students.




