Advances in the Study of Mitophagy in Renal Ischemia–Reperfusion Injury
Funding: This work was supported by the Zhejiang Provincial Medical and Health Science and Technology Plan, 2024KY1426.
ABSTRACT
Renal ischemia–reperfusion injury (IRI) is a leading etiology of acute kidney injury (AKI), predominantly observed in complex cardiovascular surgeries, severe traumatic shock, and renal transplantation. Oxidative stress, calcium overload, mitochondrial dysfunction, autophagy, and apoptosis are closely associated with renal IRI. Mitochondria not only serve as critical organelles for cellular oxidative respiration and energy production but also play vital roles in various biochemical processes including calcium homeostasis, signal transduction, energy metabolism, cell differentiation, apoptosis, renal ischemia, and reinjury. One particular form of autophagy, mitophagy, can accurately eliminate damaged or defective mitochondria, thereby maintaining mitochondrial homeostasis and playing a key role in renal IRI. To give a new approach to mechanistic research and clinical prevention and therapy of renal ischemia–reperfusion injury, this paper examines the key signaling pathways and novel therapeutic targets of mitophagy in ischemia–reperfusion injury.
1 Introduction
Renal ischemia reperfusion injury (IRI) is a complicated pathophysiological process, which is characterized by aggravated tissue damage when the kidney resumes perfusion after blood flow interruption. In renal ischemia, the tissues of the kidney are affected by insufficient oxygen supply, which leads to the dysfunction of intracellular adenosine triphosphate (ATP) synthesis, the imbalance of calcium homeostasis, and the accumulation of reactive oxygen species (ROS), inducing mitochondrial dysfunction and apoptosis. Then, blood flows back into the kidney through the reperfusion process. This causes the abnormal activation of calcium channels and a large amount of Ca2+ influx that further aggravates the generation of ROS and forms a vicious cycle of oxidative stress and inflammation, which ultimately results in renal parenchymal cell damage and renal dysfunction [1]. Renal IRI is common in complex cardiovascular surgery, severe traumatic shock, and kidney transplantation [2], which is one of the main causes of acute kidney injury (AKI). Acute kidney injury is a global health issue that is associated with a high morbidity and mortality rate [3]. Despite substantial advances achieved in clinical and laboratory studies, current research has revealed significant limitations in treatment strategies for AKI. Moreover, AKI imposes a heavy financial burden on healthcare systems worldwide [4-6]. Therefore, further investigations to better understand the pathophysiology of IRI and to explore novel therapeutic targets remain urgently needed.
Mitochondria, a double-membrane semi-autonomous organelle, are the primary sources of intracellular energy and ATP [7, 8]. The functional state of mitochondria is closely related to mitochondrial membrane potential, mitochondrial membrane channels, mitochondrial Ca2+ concentration, respiratory complex activity, reactive oxygen species generation, and DNA mutation. Mitochondria, as organelles with diverse functions and high dynamism, are widely involved in key physiological processes such as intracellular ATP production, regulation of signal transduction, calcium ion homeostasis maintenance, redox homeostasis, and programmed cell death. Therefore, ensuring the integrity of mitochondrial function and timely removal of damaged mitochondria is crucial for maintaining cellular homeostasis. The kidney is a metabolically important organ with high blood perfusion and is the second most mitochondria-rich organ after the heart, as it requires large amounts of ATP for essential functions such as nutrient reabsorption, acid–base and electrolyte balance, and hemodynamics [9]. Therefore, these energy-intensive cells are particularly vulnerable to mitochondrial dysfunction. In order to maintain effective basal metabolism, the mitochondria in the proximal tubules must have the ability to detect and respond to fluctuations in energy availability since the proximal tubules reabsorb a significant amount of glomerular ultrafiltrate via active transport. Any insult to quality control mechanisms can cause disruption, preventing damaged mitochondria from being cleared and resulting in morphological alterations and tissue dysfunction. Therefore, the maintenance of mitochondrial quantity and function is crucial for the physiology and pathology of the kidney [10, 11]. Mitophagy maintains cellular homeostasis by selectively degrading damaged mitochondria and reducing the release of reactive oxygen species from dysfunctional mitochondria [12]. An increasing number of research studies conducted in recent years have linked abnormal mitophagy to the development and progression of renal IRI. Mitophagy plays a vital role in renal IRI by maintaining mitochondrial quality control and preventing the accumulation of damaged mitochondria [13-16]. This article reviews the related molecular mechanisms of mitophagy and its therapeutic potential in renal ischemia–reperfusion injury.
2 Overview of Autophagy and Mitophagy
In the early 1960s, De Duvet observed double-membrane vesicles containing intracellularly degraded organelles and lysosomal enzymes while studying the structure of lysosomes by electron microscopy and subsequently named them “autophagy” [17]. Under normal physiological conditions, autophagy activity is often maintained at a low basal level. The autophagy pathway is a critical regulatory mechanism for maintaining cellular homeostasis, enabling cells to adapt and survive under stressful conditions. This evolutionarily conserved process is characterized by the formation of autophagosomal double-membrane vesicles that sequester cytoplasmic components such as dysfunctional organelles, protein aggregates, and metabolic byproducts. These autophagosomes subsequently undergo lysosomal fusion, forming autolysosomes where captured cargo is enzymatically degraded through acid hydrolase activity. Through this precisely regulated catabolic process, autophagy mediates the selective elimination of damaged mitochondria, toxic cellular components, and misfolded proteins, thereby protecting cellular integrity and preserving normal physiological function [18].
Macrophage, chaperone-mediated autophagy, and microautophagy are the three primary cellular component types found in mammalian cells, based on the way they fuse with lysosomes. The principal mechanism of autophagy is called macroautophagy (non-selective autophagy). It is classified into two categories based on the substrates that it breaks down selectively. One is non-selective autophagy, which mainly provides nutrition for cells under starvation to maintain the most basic energy and substance metabolism of cells. The other is substrate-specific autophagy, or selective autophagy, which is essential for preserving homeostasis and primarily involves the elimination of certain damaged organelles or cellular structures to stop additional cellular damage. Selective autophagy can specifically degrade different cellular components, such as mitochondria, endoplasmic reticulum, ribosomes, protein aggregates, lipid droplets, and cytoplasmic pathogens [17, 19]. In 2005, Lemasters JJ first proposed “mitophagy” and pointed out that mitochondrial damage is the signal to initiate mitophagy [20]. The main steps of mitophagy are a cellular process that includes the depolarization of damaged mitochondria, the entrapment of mitochondria by autophagosomes to form mitophagosomes, the fusion of mitophagosomes with lysosomes, and the degradation of mitochondrial contents by lysosomes (Figure 1) [21].
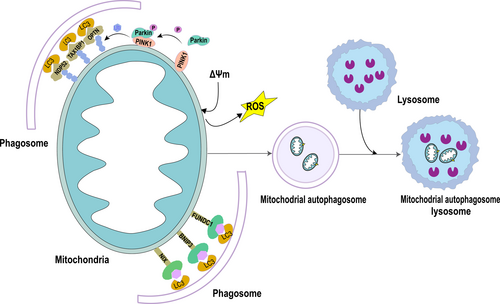
When mitochondria are damaged, their surface is ubiquitylated, and the related receptors are produced to collect some core autophagy-related proteins, such as OPTN, TAX18P1, and NDP52 [21-23]. When these proteins are linked to mATG8, a membrane is formed around the mitochondria, and autophagic vesicles are gradually formed, which are eventually absorbed and degraded by lysosomes [21]. The goal is to selectively remove damaged or excess mitochondria, thereby fine-tuning mitochondrial numbers and maintaining energy metabolism; this mechanism plays a crucial role in maintaining cellular homeostasis [24]. With the deepening of research, it is presently believed that mitophagy has a dual nature and is usually regarded as a protective mechanism of cells under fatal pathological conditions. Mitophagy is activated under oxidative stress injury, such as renal ischemia–reperfusion injury, which will assist in removing damaged mitochondria, reduce the accumulation of reactive oxygen species, and protect cells [25]. However, emerging evidence indicates that overactivation of mitophagy may paradoxically exacerbate cellular damage. This might be attributable to either overwhelming mitochondrial damage surpassing clearance capacity or defective autophagic flux during degradation [26].
3 The Regulatory Mechanism of Mitophagy in Renal Ischemia–Reperfusion Injury
The mechanisms of mitophagy have been extensively studied since its concept was proposed. Main mechanisms were usually classified into two categories: ubiquitin-dependent pathways and non-ubiquitin-dependent pathways [27, 28]. The ubiquitin-dependent pathway represents a sophisticated mitochondrial quality control mechanism that mediates through extensive ubiquitination of mitochondrial surface proteins, which serves as a molecular signal for selective mitophagy. The PTEN-induced putative kinase 1 (PINK1)/Parkin axis has been extensively characterized as a canonical pathway among these regulatory mechanisms. Under conditions of mitochondrial depolarization or damage, PINK1 accumulates on the outer mitochondrial membrane (OMM) and phosphorylates ubiquitin at specific residues (e.g., Ser65), creating a molecular platform for Parkin recruitment. As an E3 ubiquitin ligase, Parkin catalyzes the synthesis of polyubiquitin chains on multiple OMM proteins, which are subsequently recognized by selective autophagy receptors, including OPTN and NDP52. These receptors contain conserved LC3-interacting region (LIR) motifs that facilitate their binding to LC3/GABARAP family proteins on nascent autophagosomal membranes, thereby guiding damaged mitochondria to autophagic degradation [29].
3.1 Ubiquitin-Dependent Mitophagy in Renal IRI
The major proteins in the ubiquitin-dependent pathway are PINK1 and Parkin, thus the name PINK1-Parkin-mediated mitophagy [30]. PINK1 is encoded by the PARK6 gene, a serine/threonine kinase located in the inner mitochondrial membrane [30]. Normally, PINK1 in mitochondria enters the inner mitochondrial membrane through the outer and inner membrane translocase (TOM and TIM) complexes, in which the mitochondrial processing peptidase processes it. Proteases such as MPP and presenilin-associated rhomboid-like protease (PARL) are rapidly degraded; therefore, they are expressed at low levels in mitochondria [31]. Parkin is an enzyme 3 (E3) ubiquitin ligase that catalyzes the transfer of ubiquitin to mitochondrial substrates. When mitochondrial membrane potential (MMP, Am) is impaired, the pathway of PINK1 into the mitochondrial inner membrane is blocked, leading to the stable accumulation of PINK1 on the cytoplasmic surface of the mitochondrial outer membrane. Parkin is simultaneously recruited and activated, resulting in a change in the spatial conformation of the Parkin protease to that of an activated E3 ubiquitin ligase. This subsequently ubiquitinates proteins on the mitochondria, integrating with LC3B and facilitating phagocytosis by autophagosomes that target mitochondria. The poly-ubiquitinated mitochondria are then degraded as a result of the autophagosome-lysosome formation (Figure 1) [32, 33].
Numerous studies have discovered that PINK1/Parkin-mediated mitophagy may play a crucial role in renal IRI by eliminating damaged mitochondria while maintaining a healthy mitochondrial population. Ischemic preconditioning (IPC) has been shown to protect against renal IRI by enhancing tissue tolerance to subsequent IRI through transient ischemic stimulation. Livingston MJ et al. observed that PINK1 was activated, mitochondrial lysosome production was increased, and mitophagy was activated in a mouse IPC model, which plays a protective role against renal IRI. In vitro experiments further confirmed that IPC not only promoted mitochondrial production and transport to lysosomes, but also inhibited mitochondrial depolarization, increased ATP production, and reduced reactive oxygen species production. PINK1 knockdown could inhibit the PINK1-Parkin pathway and attenuate mitophagy, thereby eliminating the protective effect of IPC on renal IRI. These results suggest that mitophagy, mediated by IPC via PINK1-Parkin, plays a key role in the protective mechanism of renal IRI [34]. N-myc downstream-regulated gene 2 (Ndrg2) was mainly expressed in HK-2 cells and lowly expressed after IRI. Liu et al. found that systemic knockout of Ndrg2 could activate PINK1/Parkin-mediated mitophagy in vitro and in vivo models of renal IRI, thereby reducing serum creatinine (SCr) and blood urea nitrogen (BUN) levels after R-I/R injury while inhibiting apoptosis, protecting against renal histological damage, and repressing proximal tubular degeneration [35]. The enhancer of liver regeneration (ALR) is an essential cellular protein with anti-apoptotic effects. Zhu et al. discovered that ALR expression was significantly increased in the rat model of IRI injury. However, knocking out ALR of HK-2 cells can inhibit the activation of the PINK1/Parkin signaling pathway and increase the expression of ALR susceptibility of HK-2 cells to ischemia–reperfusion injury. Further studies have shown that overexpression of ALR can reduce mitochondria-derived ROS and NLRP3 inflammasome activation through PINK1/Parkin-mediated mitophagy, which has the therapeutic potential to target mitophagy in ischemia–reperfusion injury [36]. Mitophagy is a dynamic and complex process during renal IRI that may be affected by the metabolic environment in the body. In some cases, it can either play a protective role or aggravate the injury. A recent study found that hyperglycemia inhibited the expression of PINK1 and Parkin in HK2 cells during IRI, promoted apoptosis of HK2 cells, and exacerbated tubular injury and renal insufficiency, suggesting that diabetes exacerbates I/R injury by inhibiting PINK1/Parkin-mediated mitophagy [37]. Using an in vitro and in vivo hyperbilirubinemia renal IR model, Liao et al. discovered that hyperbilirubinemia may promote mitophagy and PINK1 silencing may lessen oxidative stress, apoptosis, and mitochondrial damage in bilirubin-induced H/R injury. These results suggest that hyperbilirubinemia exacerbates oxidative stress, apoptosis, mitochondrial damage, and fibrosis in renal IR injury by aggravating PINK1-Parkin-mediated mitophagy [38]. Apart from the PINK1-Parkin pathway, there are non-Parkin-dependent ubiquitin-dependent pathways, in which PINK1 directly recruits the autophagy receptors OPTN and NDP52 to promote autophagy via ubiquitin phosphorylation. Aside from Parkin, other E3 ubiquitin ligases involved in ubiquitination of mitochondrial proteins and induction of mitophagy include ARIH1 [39], SIAH1 [40], HUWE1 [41], MUL1 [42], and others, although they have not received much attention in the literature on renal IRI. These findings suggest that exploring PINK1/Parkin-mediated mitophagy in renal IRI may be an important avenue for future research and development of innovative therapeutic approaches.
3.2 Non-Ubiquitin-Dependent Mitophagy in Renal IRI
Non-ubiquitin-dependent mitophagy, also known as receptor-mediated mitophagy, is dominated by the mitophagy receptor, which is distinctly different from the ubiquitin-dependent pathway. Mitophagy receptors mediate the non-ubiquitin-dependent pathway through their conserved LC3-interacting region (LIR), enabling them to bind LC3 directly, and initiate autophagy independently of ubiquitination. So far, among mammals, the best-known receptors are BCL2 interaction protein 3 (BNIP3L)/Nip3 protein × (NIX) receptor, BCL2 interaction protein 3 (BNIP3) receptor, and the FUN14 structure domain containing 1 (FUNDC1) receptor, and others (Figure 1) [33]. It is essential for renal IRI non-ubiquitin-dependent mitophagy.
3.2.1 BNIP3/NIX-Mediated Mitophagy and Renal IRI
BNIP3 was originally recognized as a pro-apoptotic protein. It is an OMM protein from the Bcl-2 proteins family with a single Bcl-2 homology 3 (BH3) structural domain and a COOH-terminal transmembrane (TM) structural domain that is primarily found in the outer mitochondrial membrane, with the N-terminus in the cytoplasm and the C-terminus in the mitochondria [43]. Physiologically, BNIP3 is lowly expressed in most cells, including the heart, skeletal muscle, and proximal renal tubular epithelium. Under hypoxia or oxidative stress, BNIP3 protein is upregulated and anchored to the OMM via its C-terminal transmembrane structural domain, exposing the N-terminal structural domain to the cytoplasm [44]. BNIP3, like other mitophagy receptor proteins, has a LIR motif at its N-terminus. Phosphorylation of two tandem serine residues, Ser 34 and Ser 35, near the LIR motif stabilizes the NIX-LC3 interaction, thereby promoting mitophagy [45]. NIX is highly homologous to BNIP3, also known as BNIP3L, and belongs to the BH3-only family of Bcl-2 proteins, which contain a C-terminal TM structural domain and a LIR motif. Homodimerization of NIX, especially phosphorylation of its C-terminal region, is critical for mitophagy. Mutations in Ser212 at the C-TM terminus of NIX hinder the homodimer formation and lower the affinity between LC3A and NIX [46]. On the other hand, phosphorylation of the LIR motif Ser34/35 site of NIX raises the autophagosome recruitment to mitochondria and increases the affinity of the motif [45]. Interestingly, the LC3-independent pathway can also undergo NIX-mediated mitophagy [47]. In addition to playing a significant role in the differentiation of retinal ganglion cells and the reprogramming of somatic cells to produce pluripotent stem cells, NIX-mediated mitophagy has the potential to contribute to the maturation of reticulocytes through mitochondrial clearance. In contrast to Parkin, BNIP3 or NIX are also crucial for the removal of depolarized mitochondria in cells. BNIP3 inhibits the proteolytic cleavage of PINK1 kinase by interacting with PINK1, leading to the accumulation of PINK1 on the OMM, which promotes the recruitment of Parkin to mitochondria and activates mitophagy to remove damaged mitochondria [48]. NIX is a substrate of Parkin. After Parkin ubiquitination, NIX recruits NBR1 to mitochondria to promote mitophagy [49].
Recent research has shown that BNIP3 is a regulator of mitophagy and is essential for renal tubular autophagy. Tang and colleagues [50] discovered that BNIP3 expression was raised and mitophagy was enhanced in both in vitro and in vivo renal IRI models. The reduction in mitophagy caused by BNIP3 knockout increased cell apoptosis, damaged mitochondria, and the production of ROS, aggravating the inflammatory response to renal IRI. These findings highlight the critical role of BNIP3-mediated mitophagy in tubular cell survival, mitochondrial quality control, and renal function during renal IRI. It has been discovered that, in hypoxic conditions, hypoxia-inducible factor-1α (Hif-1α) controls the transcription of BNIP3. The BNIP3 promoter contains a HIF-1a response element (HRE) with a core sequence of 5′-CGTG-3′, which can be activated under hypoxia or by the overexpression of Hif-1α. The NF-κB response element present in the BNIP3 promoter, on the other hand, can activate the NF-κB signaling pathway and suppress BNIP3 transcription [51]. By using an I/R mouse model, Fu et al. discovered that the knockdown of renal tubular HIF-1α significantly inhibited I/R-induced mitophagy and aggravated I/R-induced renal tubular apoptosis and renal injury. This suggests that HIF-1α-BNIP3-mediated mitophagy in renal tubular epithelial cells could play a protective role in I/R injury by inhibiting apoptosis and ROS production [52]. Acyl-coa synthetase family member 2 (ACSF2) is a protein-coding gene that is highly specifically expressed in the mitochondria of renal tubular epithelial cells. ACSF2 knockdown significantly enhanced IR-induced mitophagy and improved renal function in mice with IR injury. BNIP3 knockdown inhibited mitophagy and exacerbated renal damage in ACSF2-knockdown mice with IR injury, suggesting that ACSF2 could aggravate renal IRI through BNIP3-mediated mitophagy [53].
Human antigen R (HuR) is an RNA-binding protein. Yu et al. found that under hypoxia, HuR could significantly bind to PARKIN and BNIP3L mRNA, increase the stability of its mRNA, and promote the expression of PARKIN/BNIP3L, thereby regulating mitophagy in renal tubular epithelial cells [54]. These studies suggest that BNIP3/NIX-mediated mitophagy regulate the removal of damaged mitochondria, ROS production, and apoptosis of renal tubular epithelial cells, thus playing an important role in renal IRI.
3.2.2 FUNDC1-Mediated Mitophagy and Renal IRI
FUNDC1 is a complete mitochondrial OMM protein consisting of 155 amino acids, which contains three highly hydrophilic conserved α-helical segments, including the cytoplasmic N-terminus, OMM transmembrane region, and C-terminal region [55]. After the induction of hypoxia or loss of mitochondrial membrane potential, FUNDC1 is dephosphorylated at Tyr 18 and Ser13, leading to conformational changes that promote the interaction with LC3B and trigger mitophagy. The phosphorylation of FUNDC1 at Tyr18 may be a key switch for FUNDC1-mediated mitophagy [56]. AMPK is a key protein that senses changes in cellular energy. During energy depletion, it induces ULK1 to recruit to mitochondria, phosphorylating FUNDC1 at Ser17, thereby promoting the interaction between FUNDC1 and Lys49 of LC3B, and subsequently initiating mitophagy. FUNDC1, a mitophagy receptor, plays an essential role in renal IRI. A study found that the knockdown of endogenous FUNDC1 significantly prevented hypoxia-induced mitophagy [57, 58].
Renal ischemia preconditioning (IPC) typically triggers mitophagy and is a practical and effective strategy to preserve kidney function and lower the incidence of AKI [59]. Following exposure of IRI mouse models to IPC, Wang J et al. observed that IPC attenuated IRI-mediated renal injury, inflammation, and tubule cell death, while proximal tubule-specific FUNDC1 knockout mice eliminated the benefits of IPC-offered renoprotection. In IRI kidneys, IPC stimulates FUNDC1-related mitophagy by encouraging FUNDC1 phosphorylation at Ser17. IPC requires FUNDC1-activated mitophagy to break down the mitochondria-localized Drp1, hence reducing IRI-activated fatal mitochondrial fission. Ulk1 is essential for IPC-mediated FUNDC1 mitophagy, and Ulk1 deletion hinders IPC-mediated maintenance of mitochondrial and renal function. These data imply that FUNDC1 mitophagy is the major molecular messenger for IPC-mediated kidney protection, and IPC can maintain mitochondrial homeostasis, minimize renal tubular damage, and protect kidney function through the Ulk1-Fundc1-Drp1 axis [25]. In addition, Zhang et al. demonstrated that FUNDC1 is a downstream regulator of HIF-1α in mitophagy in renal tubular cells, and that HIF-1α/FUNDC1 signaling reduced apoptosis and ROS generation by promoting mitophagy in both in vitro and in vivo renal IRI models [60]. Overall, FUNDC1-mediated mitophagy can alleviate renal IRI by enhancing mitophagy in renal tubular epithelial cells.
3.3 Other Pathways in Renal IRI Mitophagy
In addition to the previously reported mitophagy pathways, several new mitophagy regulatory molecules play an essential role in renal IRI. For example, the Pannexin 1 (PANX1) channel transmembrane protein can drive inflammation and ATP release during I/R injury. Su et al. used an in vivo renal I/R injury mouse model and an in vitro cell H/R model to clarify that PANX1 inhibits mitophagy by affecting the ATP-P2Y-mTOR signaling pathway. Moreover, PANX1 deletion could alleviate renal tubular cell death, oxidative stress, and mitochondrial damage after I/R injury through enhancing mitophagy. Therefore, targeting PANX1 may be a therapeutic target to alleviate I/R injury [61]. Suppressor of cytokine signaling 3 (SOCS3) is highly expressed in renal IRI mice, and knockout of SOCS3 in IRI mice and H/R cell models can reduce renal mitophagy. Among them, activating transcription factor 3 (ATF3) can bind to the SOCS3 promoter, indicating that ATF3 promotes the activation of mitophagy by mediating SOCS3 expression, thereby aggravating renal IRI [26]. Transcription factor forkhead box O1 (FOXO1) is a key regulator of mitochondrial homeostasis. The expression of FOXO1 is increased in renal tubular epithelial cells with IRI. In vivo and in vitro studies have shown that inhibition of FOXO1 can reduce mitochondria-mediated apoptosis, mitochondrial ROS production, and accelerate ATP recovery. Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) is a master regulator of mitochondrial biogenesis. FOXO1 repressed PGC-1α transcription by competing with cAMP response element-binding protein (CREB) for binding to the transcriptional co-activator CREBBP/EP300, indicating that FOXO1 activates mitophagy in IRI through CREB/PGC-1α-mediated mitochondrial biogenesis [62]. Dynamin-related protein 1 (DRP1) is recruited to mitochondria during mitochondrial failure and plays a role in mitophagic clearance of damaged mitochondria, protecting cells from IRI-induced apoptosis [16]. Sirtuin 3 (SIRT3) is a mitochondrion-localized member of a conserved family of proteins with NAD+-dependent deacetylase activity [63, 64]. Zhao et al. demonstrated that Sirtuin 3 (SIRT3) can activate mitophagy through the DRP1 pathway to reduce renal tubular cell injury, apoptosis, mitochondrial alterations, and ROS production, thus protecting renal function in a murine model of IRI in mice [65]. Liu et al. observed that in both in vivo and in vitro IRI models, long noncoding RNA MEG3 is upregulated, competitively bound with miR-145-5p to upregulate RTKN, thereby triggering the Wnt/β-catenin pathway. The downstream effector of the Wnt/β-catenin pathway, c-MYC, further activated MEG3 at the transcription level. MEG3 activated by c-MYC aggravates renal IRI through activating mitophagy [66]. PHB2 is a protein found in the inner membrane of mitochondria. Wang et al. discovered that Bax inhibitor-1 (BI1) maintains mitochondrial genetic integrity by retaining PHB2, which promotes mitochondrial respiration and reduces mitochondrial oxidative stress, thereby inhibiting excessive mitochondrial fission and improving mitophagy to prevent apoptosis of renal tubular epithelial cells [67]. In conclusion, mitophagy can be indirectly regulated by other signaling molecules such as PANX1, SOCS3, and MEG3 during renal IRI. At the same time, it also works with regulatory mechanisms such as mitochondrial biogenesis, mitochondrial fission, and fusion to maintain mitochondrial homeostasis and protect cells and tissues from damage.
4 The Therapeutic Potential of Mitophagy in Renal IRI
Currently, a growing body of research indicates that mitophagy targeting has significant therapeutic potential for renal IRI. For example, one hormone secreted by the pineal gland is called melatonin, which has a strong antioxidant impact. Wang et al. demonstrated that melatonin pretreatment administered to HK-2 cells and live rats before hypoxia reoxygenation or IRI can increase the level of P-AMPK levels, thereby inhibiting mitochondrial fission, promoting mitochondrial fusion, and attenuating autophagy levels to shield the kidneys from IRI [68]. Another study found that melatonin could improve oxidative stress, mitochondrial dysfunction, apoptosis, mitochondrial dynamics, and mitophagy in renal IRI in obese rats by activating the AMPK-PGC-1α-SIRT3-SOD2 axis, thereby maintaining mitochondrial homeostasis and integrity, and reducing the aggravation of renal IRI in obese rats. It is promising to be a therapeutic drug to improve the prognosis of renal IRI [69]. Dapagliflozin (Dapa) is a sodium-glucose cotransporter 2 (SGLT2) inhibitor, and its renoprotective effect in patients with chronic kidney disease has been widely recognized in clinical practice. Martinez-Rojas et al. found that 10 days of Dapa treatment after the IRI rat model can effectively reverse mitochondrial abnormalities (increased mitochondrial fission, altered mitophagy, metabolic dysfunction, and pro-apoptotic signaling), rapidly restore creatinine clearance (CrCl), and significantly reduce renal vascular resistance. In an independent cohort monitored for 5 months after IRI, 10-day treatment with Dapa improved proteinuria, CrCl, glomerulosclerosis, and fibrosis and delayed the transition from AKI to CKD in rats. This suggested that early Dapa treatment has potential therapeutic value in preventing maladaptive repair after IRI and improving the long-term prognosis of renal IRI [70]. Lasmiditan is a selective 5-hydroxytryptamine 1F (5-HT1) receptor agonist. Rick et al. found that lasmiditan treatment of an I/R mice model can lead to an initial increase in the phosphorylation of mitophagy-related proteins in the renal cortex of mice, followed by an increase in fusion-related proteins upon I/R injury. Mitophagy after AKI is accelerated in a time-dependent manner, resulting in an increase in ATP and a decrease in serum creatinine, thus promoting renal recovery [71]. Roxadustat, an oral HIF-1α inhibitor, increases the levels of HIF-1α, FUNDC1, and LC3BII and decreases the levels of VDAC in the renal cortex of renal IRI rats. Roxadustat may alleviate renal IRI-related AKI by promoting mitophagy and reducing apoptosis in vivo [60]. In addition, urolycitin A (UA) is a specific mitophagy activator that induces the improvement of mitochondrial health by stimulating mitophagy in humans [72]. HIF-1α has two transcriptional activation domains [TAD, N-terminal (NTAD), and C-terminal (CTAD)]. Ma RX et al. demonstrated that HIF-1α CTAD protects against hypoxia-induced kidney injury through hexokinase 2 (HK2)-mediated mitophagy, while mitophagy activation using UA could significantly protect hypoxia-induced kidney injury under the conditions of HIF-1α CTAD knockout [73]. 8-oxoguanine DNA glycosylase (OGG1), a DNA glycosylase with purine/pyrimidine (AP) site cleavage activity, is involved in the excision and repair of nuclear and mitochondrial DNA. Zhao et al. discovered that OGG1 is up-regulated in renal tubular epithelial cells of the IRI mouse model. In IRI, OGG1 binds to PINK1, regulates the transfer of PINK1 into mitochondria, inhibits mitophagy, and thus reduces the clearance rate of damaged mitochondria and aggravates mitochondrial functional damage, including increased mitochondrial ROS production, decreased mitochondrial membrane potential, and decreased SOD and ATP levels. Their study provides substantial evidence that OGG1 activation contributes to ischemic renal damage and that OGG1 inhibition or gene KO can alleviate renal IRI [74]. Prostaglandin E2 receptor 4 (EP4) is one of the important G protein-coupled receptors and a downstream molecule of COX-2. It plays a significant role in renal IRI. Ding et al. observed that when renal I/R occurs, EP4 is significantly down-regulated in rats with IRI. In addition, CAY10598, a commonly used EP4 agonist, can inhibit excessive mitophagy by activating the cAMP/PKA signaling pathway, thereby inhibiting the decrease in mitochondrial membrane potential, cytochrome c release, and cell apoptosis after renal IRI and playing a protective role in renal injury caused by IRI [75]. By reviewing the results of previous studies, the anti-renal IRI mechanisms of these drugs or autophagy agonists were re-recognized; thus, it is possible to induce or improve mitophagy as a therapeutic strategy for renal IRI (Table 1).
| Therapeutic agents or autophagy agonists | Experimental Model | Daily dose, treatment period | Target | Result | Protective effect | References |
|---|---|---|---|---|---|---|
| Melatonin | Male Wistar rats after IRI | a 10 mg/kg, i.p., 24 h before IRI, followed by an additional dose 1 h before the IRI operation. | AMPK/DRP1 | Inhibited mitochondrial fission, promoted mitochondrial fusion, attenuated autophagy levels, and shielded the kidneys from IRI. | Attenuated mitophagy | [68, 69] |
| HFD-fed male Wistar rats after IRI | 10 mg/kg/d, i.p., for 4 weeks before the IRI operation. | AMPK-PGC-1α-SIRT3-SOD2 | Maintained mitochondrial redox balance, preserved mitochondrial homeostasis and integrity, and reduced cell apoptosis. | Attenuated renal IR-induced mitochondrial dynamics and mitophagy imbalance. | ||
| Dapa | Male Wistar Rats after IRI | 1 mg/kg/d,gastric gavage, for 5 days after injury | — | Increased mitochondrial fission, altered mitophagy, metabolic dysfunction, and pro-apoptotic signaling. | Accelerated mitophagy | [70] |
| Lasmiditan | Male C57BL/6NCrl mice after IRI | 0.3 mg/kg/d, i.p, for 2 or 5 consecutive days starting 24 h after I/R injury | — | Restored normal mitochondrial membrane and cristae morphology, decreased excessive mitochondrial fission, and accelerated mitophagy. | Accelerated mitophagy | [71] |
| Roxadustat | Male Wistar rats after IRI | 10 mg/kg/d, i.p., for 7 days,before the IRI operation | HIF-1α/FUNDC1 | Increased the levels of HIF-1α, FUNDC1, and LC3BII in the renal cortex of renal IRI rats while decreased the levels of VDAC | Promoted mitophagy and reduced apoptosis | [60] |
| UA | HIF-1α CTAD−/− mice with IRI | 50 mg/kg, i.p., for 2 days, before the IRI operation | HIF-1α CTAD-HK2 | The expression of inflammatory cytokine mRNAs (TNF-α, IL-1β, and MCP-1 mRNA) was also down-regulated; educed macrophage infiltration; the necrosis and detachment of tubules, accumulation of cellular debris, and formation of casts were markedly attenuated; decreased levels of SCr and BUN. | Activated mitophagy | [72] |
| OGG1 specific inhibitor Th5487 | Male C57/BL6 mice with IRI | 30 mg/kg, i.p., for 3 days, before the IRI operation | PINK1/Parkin | Suppressed cell apoptosis and aggravated mitochondrial dysfunction. | Activated mitophagy | [74] |
| EP4 agonist | Male Sprague Dawley rats after IRI | 1 mg/kg i.p. at 1.5 h prior to IRI | cAMP/PKA | Inhibited mitochondrial membrane potential decline, cytochrome c release, and cell apoptosis. | Inhibited excessive mitophagy | [75] |
5 The Link Between Mitophagy in Renal IRI, Exosomes, and Mitochondria-Associated Endoplasmic Reticulum Membranes (MAMs)
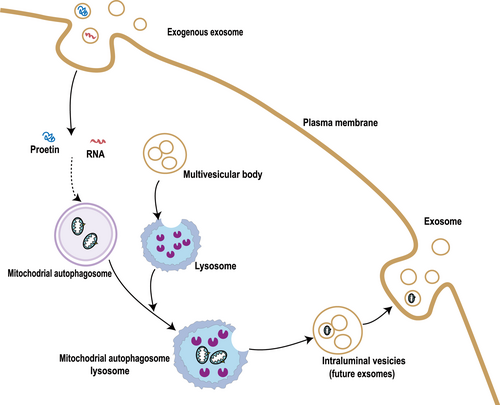
Recent research has revealed the presence of damaged mitochondrial proteins and mitochondrial mtDNA fragments in astrocyte exosomes. The currently recognized mechanism is related to the mutual interference of the mitochondria-lysosome-extracellular vesicle axis. Under stress conditions, mitochondrial dysfunction and mitochondrial debris emerge from cells, which further leads to lysosomal dysfunction, thereby inhibiting autophagic flux and enhancing exosome release. This can serve as an alternative cell quality control pathway to protect cells from harmful protein aggregations and the accumulation of abnormal mitochondrial material [76, 77](Figure 2). Mesenchymal stem cells (MSCS) undergo mitophagy under oxidative stress and use arrestin domain-containing protein 1-mediated MVs (ARMMs) to unload mitochondria. This results in the release of large extracellular vesicles (70–150 nm) containing mitochondrial particles, which are engulfed by macrophages and re-utilized to increase bioenergetics [78]. Many studies have recently discovered that exosomes generated from mesenchymal stem cells can be phagocytosed by other cells and activate mitophagy, which protects the heart [79, 80] brain [81, 82], bone [83], liver [84], and other related diseases. Sun Z et al. have revealed that MSC-derived exosomal miR-223-3p could reduce renal IRI injury by directly targeting NLRP3, attenuating inflammasome activation, and promoting mitophagy through in vitro and in vivo experiments [85]. The CD5L-enriched FRC-Exo modified by LTH (LTHVVWL peptide) could selectively bind primary kidney tubular cells (PKTCs) and transport CD5L to damaged PKTCs to promote kinase PINK-ubiquitin ligase Parkin-mediated mitophagy, prevent pyroptosis, and improve kidney function by hindering the NLRP3 inflammasome. Consequently, FRC-Exos could be a new approach to creating treatments for kidney injury [86]. The above studies show that there is an interaction between mitophagy and exosomes. Under oxidative stress, exosomes can serve as an alternative way to remove damaged mitochondria during mitophagy, and signaling molecules encapsulated by exosomes can also mediate mitophagy.
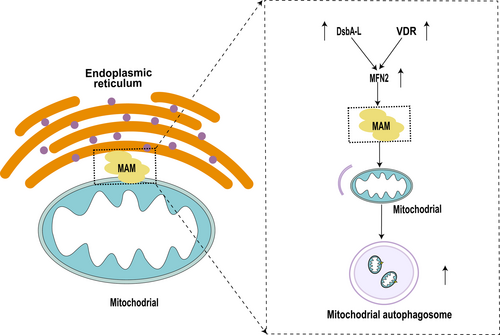
The mitochondria-associated endoplasmic reticulum membrane (MAM) was initially discovered by electron microscopy in the 1950s [87, 88]. In the 1990s, Csordas et al. performed limited proteolysis to perform biochemical identification [89], confirming for the first time that the endoplasmic reticulum and mitochondria are linked by protein tethers [87]. So far, proteins (about 1000 species) have been identified in MAM that can be involved in regulated downstream signaling pathways, mainly including mitochondrial quality control, oxidative stress, ER-mitochondrial Ca2+ and other interference, apoptosis, inflammasome activation, and ER stress [90]. The proteomic analysis of mitochondria-associated membranes in renal IRI suggests that mitochondria and the endoplasmic reticulum (ER) communicate through contact sites of MAM. Many important cellular functions in renal IRI, such as bioenergetics, mitophagy, apoptosis, and calcium signaling, are regulated by MAM. A total of 4489 proteins were identified by 4D mark-based proteomic analysis of MAM from human kidney HK-2 cells under normal (N) and H/R conditions. GO/KEGG analysis showed that MAM proteins were mainly related to mitochondrial function and energy metabolism. Among them, Mitofusion 2 (MFN2) and BNIP3 were the most critical. The in vitro and in vivo validation results were consistent with the proteomics results. MFN2 showed protective effects in maintaining the integrity of MAM and attenuating mitochondrial damage following H/R injury [91]. Chen et al. found that activated vitamin D receptor (VDR) restored the integrity of mitochondrial ATP and MAMs, inhibited mitochondrial fission and mitochondrial ROS, and further found that VDR activation restored mitophagy through the Mfn2-MAMs-Fundc1 pathway in renal tubular cells [92]. Disulphide-bond A oxidoreductase-like protein (DsbA-L), expressed in the MAM, serves as an antioxidant and reduces ER stress. Yang et al. confirmed that DsbA-L ameliorates high glucose induced tubular damage by maintaining MAM integrity [93]. In vitro, overexpression of DsbA-L restored MAM integrity and increased mitophagy in HK-2 cells, a human proximal tubular cell line, after exposure to high-glucose (HG) conditions. Helicase and zinc finger 2 (HELZ2) is a transcription factor that works alongside PPARα to enhance MFN-2 expression. HG substantially reduced the expression of HELZ2 and MFN-2 and inhibited mitophagy. Furthermore, overexpression of DsbA-L partially reduced these effects, implying that DsbA-L stimulates mitophagy to prevent diabetic tubular damage by preserving MAM integrity via the HELZ2/MFN-2 pathway [94]. Therefore, maintaining the integrity of MAM in renal IRI can reduce mitochondrial damage and apoptosis by enhancing mitophagy, thus reducing renal tubular injury. This regulation mechanism deserves further study in the future (Figure 3).
6 Conclusion
Mitophagy, as a core mechanism of mitochondrial quality control, plays a dual role in renal IRI. Moderate mitophagy exerts cytoprotective effects through the clearance of damaged mitochondria, maintenance of mitochondrial homeostasis, and attenuation of oxidative stress, apoptosis, and inflammatory responses. Conversely, excessive activation of mitophagy may result in pathological mitochondrial depletion, thereby exacerbating cellular damage. This review systematically delineates the molecular mechanisms underlying mitophagy regulation in renal IRI pathogenesis, while highlighting its multifaceted roles in renal pathophysiology. Notably, mitophagy activity is modulated by systemic factors including hyperglycemia and hyperbilirubinemia, and is dynamically coordinated with mitochondrial biogenesis, fusion, and fission to preserve mitochondrial integrity. Emerging evidence demonstrates that pharmacological targeting of mitophagy holds therapeutic promise for renal IRI management. Intriguingly, recent discoveries regarding exosomes and MAMs have unveiled novel regulatory dimensions. Specifically, exosomes mediate mitophagy modulation via miRNA cargo delivery and may serve as alternative vehicles for mitochondrial quality control, whereas MAMs orchestrate organelle crosstalk to maintain cellular homeostasis, a mechanism that warrants further investigation. In summary, mitophagy has a complex regulatory network and important pathophysiological implications in renal IRI. Further understanding its molecular mechanism and developing precise regulation strategies are expected to provide new breakthroughs in the treatment of renal IRI and improve the prognosis of patients. Future research should prioritize three key aspects: (1) the dynamic control of mitophagy flux, (2) metabolic regulation of its activity, and (3) the functional interplay between exosomes, MAMs, and mitochondrial homeostasis in renal pathophysiology.
Author Contributions
Rumeng Li: writing – original draft (lead), writing – review and editing (lead). Xiaofeng Zhu: writing – review and editing (equal). Baiyang Lou: writing – review and editing (equal). Xingxia Wang: resources (supporting), supervision (equal).
Acknowledgments
We thank Carson Xing for editorial handling and two anonymous reviewers for helpful comments. This work was partially supported by the Zhejiang Provincial Medical and Health Science and Technology Plan (2024KY1426).
Conflicts of Interest
The authors declare no conflicts of interest.




