Traumatic brain injury induces macrophage subsets in the brain
Abstract
Traumatic brain injury (TBI) elicits innate inflammatory responses that can lead to secondary brain injury. To better understand the mechanisms involved in TBI-induced inflammation, we examined the nature of macrophages responding to TBI in mice. In this model, brain macrophages were increased >20-fold the day after injury and >77-fold 4 days after injury in the ipsilateral hemisphere compared with sham controls. TBI macrophage subsets were identified by using a reporter mouse strain (YARG) that expresses eYFP from an internal ribosome entry site (IRES) inserted at the 3′ end of the gene for arginase-1 (Arg1), a hallmark of alternatively activated (M2) macrophages. One day after TBI, 21 ± 1.5% of ipsilateral brain macrophages expressed relatively high levels of Arg1 as detected by yellow fluorescent protein, and this subpopulation declined thereafter. Arg1+ cells localized with macrophages near the TBI lesion. Gene expression analysis of sorted Arg1+ and Arg1− brain macrophages revealed that both populations had profiles that included features of conventional M2 macrophages and classically activated (M1) macrophages. The Arg1+ cells differed from Arg1− cells in multiple aspects, most notably in their chemokine repertoires. Thus, the macrophage response to TBI initially involves heterogeneous polarization toward at least two major subsets.
Introduction
Traumatic brain injury (TBI) is the leading cause of morbidity and mortality from childhood to age 44 1. Following the initial trauma, inflammatory responses can expand brain damage 1. TBI rapidly leads to activation of microglia, macrophages, and neutrophils, and to local release of inflammatory cytokines 1-5. Understanding the inflammatory events that occur during this critical window is an important step toward developing interventions targeting the immune response 6.
Following brain injury, the host response has the potential for both benefit and harm. While inflammatory mechanisms may be required for wound sterilization, the response can extend neuronal cell death and impair recovery. Macrophages have previously been studied in models of CNS injury including experimental autoimmune encephalitis, ischemic stroke, and spinal cord injury as well as TBI, and there is conflicting evidence as to whether macrophages are overall harmful or beneficial to the brain. A detrimental role for macrophages has been found in most neuroimmunologic studies 7-13. However, the inflammatory response is also important for clearing necrotic debris and for wound repair 14. In support of this, macrophages have also been shown to suppress inflammation and were critical for recovery in one model of spinal cord injury 15. Moreover, in EAE, macrophages that suppress inflammation through the production of IL-10 and TGF-β are beneficial 16. These differing roles for macrophages may reflect different functional states of macrophage activation.
In vitro and in vivo studies have demonstrated that macrophages can be activated into two major subsets: classically activated (M1) and alternatively activated (M2) macrophages 17-19. M1 macrophages directly incite inflammation by releasing IL-12, TNF-α, IL-6, IL-1β, and nitric oxide (NO) in response to microbial pathogens or LPS. In contrast, M2 cells are activated in response to helminths, to allergens, by adipose tissue, and in vitro by IL-4 20, 21. M2 macrophages suppress inflammation and promote wound healing 14. They express increased levels of arginase-1 (Arg1), CD206 (mannose receptor), Clec7a (dectin-1), CD301, resistin-like alpha (RELM-α), and PDL2. Additional macrophage subsets have been identified 17, 18. In vivo studies demonstrate that macrophages may differentiate along a spectrum of phenotypes that do not adhere to well-defined in vitro phenotypes 14, 17, 22, 23. Furthermore, macrophages may shift from one phenotype to another 17.
In considering the role of macrophages in brain injury, it may be important to distinguish between macrophage subsets. Thus, in vitro studies have demonstrated that M1 macrophages are neurotoxic, while M2 macrophages promote regenerative neuronal growth 24. CCL2, which is expressed post-TBI in the brain and cerebrospinal fluid, has been thought to elicit primarily M1 macrophages, and the presence of macrophages/microglia early after TBI by histology is often associated with the expression of TNF, IL-6, and IL-1 1, 13, 25-27. These findings previously suggested that there is a prominent M1 phenotype in early macrophage recruitment following TBI. Characterization of macrophages in TBI by histology has been complicated by difficulty in distinguishing them from microglia; there is no known marker that is expressed by macrophages but not microglia or vice versa. By flow cytometry, however, the two cell populations can be distinguished by the level of CD45 expression. Using this approach, we have examined the nature of macrophages responding to TBI in mice. To facilitate macrophage subset identification, we examined TBI in YARG mice, in which yellow fluorescent protein (YFP) is expressed under the promoter for the M2 marker, Arg1 28, 29, and Yet40 mice, in which YFP is expressed under the promoter for the M1 marker, IL-12p40. We here demonstrate that a subset of brain wound macrophages upregulate Arg1 and home to the site of injury. At day 1 after injury, 21 ± 1.5% of the ipsilateral hemisphere macrophages express high levels of Arg1, but the number of Arg1+ cells falls thereafter and cannot be detected after 1 week. Whole genome expression analysis of Arg1+ and Arg1− macrophages following TBI revealed that these macrophage subsets differ in their expression of over 1300 genes, with notable differences in genes encoding chemokines. The pattern of gene expression in neither population is characteristic of in vitro derived M2 or M1 cells. Our results indicate that the macrophage response to TBI is heterogeneous, and the early response includes at least two distinct subsets. As assessed by expression of Arg1, the ratio of these subsets changes with time.
Results
Macrophages are recruited to the lesion site in large numbers early post-TBI
To assess the immune response following TBI, we used an adult murine controlled cortical impact model. Histological analysis of brain sections following TBI confirmed cortical injury, which extended into the hippocampus (Fig. 1A). Hematoxylin and eosin (H&E) staining revealed increased cellular recruitment to cortical tissues adjacent to the lesion (Fig. 1A). Immunohistochemical staining for F4/80 showed that macrophages/microglia are widely present at the pericontusional site (e.g. in areas of the cortex adjacent to the lesion) (Fig. 1B).

To assess leukocyte subset frequencies present in the brain following TBI, single-cell suspensions from TBI and sham-injured brain contralateral and ipsilateral hemisphere tissues were analyzed by flow cytometry. Flow cytometry permitted discrimination of macrophages from microglia based on levels of CD45 expression; both microglia and macrophages express CD11b, but macrophages express a higher level of CD45 30, 31. In our analyses of macrophages and microglia, neutrophils (which also express CD45 and CD11b) were consistently excluded by using an antibody against Ly6G (Clone 1A8). Blood leukocytes were excluded by perfusing the brain prior to cell recovery.
Flow cytometry plots of cell preparations from brain tissues 4 days following TBI of WT mice showed that macrophages are a major part of the inflammatory response to TBI primarily on the side of injury (Fig. 1C); macrophages comprised 40 ± 2% of all CD45+ leukocytes in the ipsilateral TBI hemisphere compared with 5.7 ± 1.5% of CD45+ cells in sham control tissues (p < 0.001).
Quantification of the kinetics of macrophage numbers that accumulate in brain hemispheres after TBI revealed that macrophage infiltration in ipsilateral hemispheres of TBI mice increased by 21-fold on day 1 (mean ± SEM, 22 115 ± 1732), and by 77-fold on day 4 (46 968 ± 5918) compared with sham controls (1081±151 and 613± 205, respectively) (Fig. 1D). On day 7, WT ipsilateral TBI macrophage numbers declined but were still 25-fold higher than levels in sham controls, and on day 14 macrophage numbers were fourfold higher (Fig. 1D).
On the first day following TBI, there was also a substantial increase in neutrophils (CD45hiCD11b+Ly6G+) in the brain (41 520 ± 4533 compared with 1419 ± 94 in sham controls), with a decline thereafter (Fig. 1D). These findings are similar to the recent findings of Jin et al. 32, although our results add quantification of absolute cell numbers as well as proportions, and we find that macrophage levels are higher on day 4 than on day 1.
Early macrophage response to TBI includes Arg1+ and Arg1− subsets
To examine macrophage polarization post-TBI, we first sought to trace the genetic expression of Arg1, which is highly expressed during M2 polarization, or of Il12b, the gene for IL-12p40, a signature of M1 polarization. To do this, we took advantage of two reporter mouse strains, YARG (YFP-Arginase-1) and Yet40 (YFP-enhanced transcript for IL-12p40) 28, 33. TBI was performed in YARG and Yet40 mice, and YFP expression in brain and peripheral blood leukocytes was compared by flow cytometry to WT animals, which lack YFP expression.
One day after TBI, 21 ± 1.5% (mean ± SEM, n = 6) of ipsilateral hemisphere brain macrophages in YARG mice expressed YFP (Fig. 2A), but brain macrophages in the contralateral hemisphere and from either hemisphere of sham animals uniformly lacked YFP (data not shown). YFP expression in YARG brain macrophages peaked on day 1 after TBI, fell to 4–7% of the macrophage population by day 4, and was undetectable on days 7 and 14 (data not shown). YFP expression could not be detected in microglia following TBI at any time point. F4/80+ blood monocytes isolated from the same injured YARG animals also lacked expression of YFP (Fig. 2A), suggesting that TBI induces macrophage differentiation after localization in the tissue. Brain macrophages and blood monocytes from TBI animals differed markedly not only in YFP expression but also in their gene expression profiles as assessed by microarray (Fig. 4 and Supporting Information Fig. 1), confirming that macrophages isolated from brains were not significantly contaminated by blood monocytes. Yet40 mice subjected to TBI had little or no upregulation of YFP in macrophages or microglia on days 1, 4, 7, and 14 (day 1 is shown), and this was subsequently confirmed for macrophages by microarray analysis for IL-12p40 on day 1 where all comparison ratios were close to 1, indicating no change in expression in comparison to blood monocytes or between brain macrophage subsets. Thus, TBI rapidly induces a macrophage response that is characterized at early time points by at least two major subsets of cells that differ in Arg1 expression, and these are hereafter called Arg1+ and Arg1− cells.

Analysis of markers for cell activation and for antigen presentation on macrophages from YARG mice revealed that both Arg1+ and Arg1− populations upregulated the activation marker CD86 compared with sham control macrophages (Fig. 2B). Few Arg1+ macrophages, however, expressed MHC class II antigens (MHCII; Fig. 2C), a marker that has been described on both M1 and M2 cells 17, 34. In contrast, 25–30% of Arg1− macrophages expressed MHCII (Fig. 2C). This is similar to the proportion of macrophages that express MHCII in sham brains (Fig. 2C), and it suggests that the Arg1− cells include at least two subpopulations, one lacking and the other expressing MHCII.
Although microglia from TBI brains did not express detectable MHCII (Fig. 2C), virtually all microglia upregulated CD86 following TBI (Fig. 2B). This finding is consistent with previous observations that TBI induces widespread activation of microglia 35, 36.
To examine the spatial localization of YFP+ cells in YARG mice post-TBI, we performed immunofluorescent colabeling for YFP and F4/80 in brain sections 2.2 days post-TBI, when macrophage infiltration of the brain peaks. F4/80+ macrophages/microglia localized in and around the area of injury (Fig. 3, second row). F4/80 expression was below level of detection by immunofluorescence in sham-injured tissues (data not shown). The Arg1+ cells were scattered among the F4/80+ cells in TBI mice (Fig. 3, third row) and were not detectable in the contralateral hemisphere or in sham-treated mice. The majority of the Arg1+ cells costained with F4/80. As suggested from our flow cytometry data in which only a subset of macrophages expresses YFP, the majority of F4/80+ cells were Arg1− (Fig. 3).
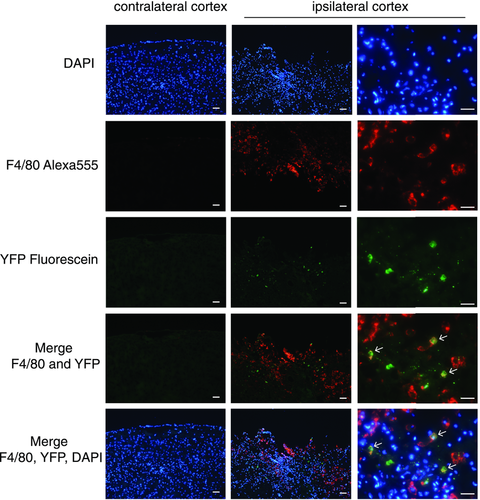
Arg1+ and Arg1− brain wound macrophages represent at least two distinct subsets
To further characterize the nature of brain macrophages following TBI, we sorted the Arg1+ and Arg1− cell populations from the ipsilateral hemisphere of YARG mice 1 day after TBI (when the proportion of Arg1+ cells peaked) and performed gene expression analysis of both cell populations. We also examined gene expression by peripheral blood monocytes from injured animals to assess the expression state of monocytes prior to their infiltration into the brain and differentiation into macrophages. As a control, peripheral blood monocytes from uninjured animals were also analyzed. It was not technically feasible to perform arrays on brain macrophages from sham animals, because there were insufficient cells to generate adequate amounts of RNA. Pairwise analyses of differentially expressed genes showed that Arg1+ and Arg1− brain macrophages differed in the expression of 1360 genes, and both populations showed even greater differences from TBI monocytes (11 799 genes differed between Arg1+ macrophages and TBI monocytes; 9932 genes differed between Arg1− macrophages) (Fig. 4A). TBI monocytes displayed few differences compared with normal monocytes (15 genes) (Fig. 4A). Principal component analysis (PCA), an analytical technique that uses dimensionality reduction to identify dominant patterns within highly multivariate data, was performed. PCA confirmed that distinctions separating macrophages from monocytes were the largest source of variance in the dataset (principal component (PC) 1), and that the monocyte populations had fewer differences that were not represented in either of the top two PCs (Fig. 4B). PCA also confirmed that Arg1+ and Arg1− brain macrophages represented two distinct populations, representing the second most significant PC (PC2) (Fig. 4B).
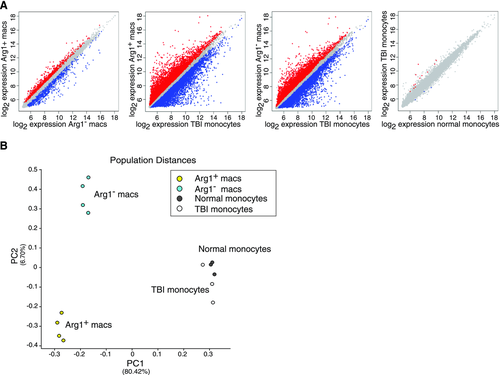
TBI-induced macrophages exhibit transcriptional responses distinct from known macrophage subsets
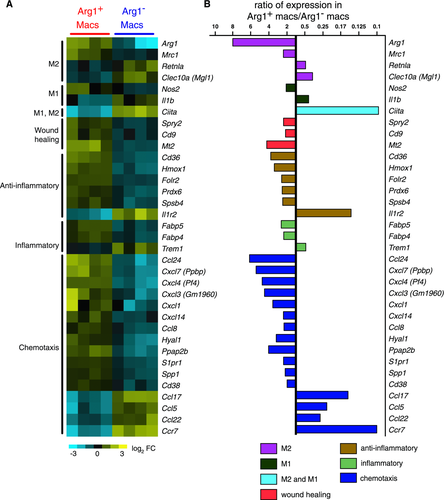
Although robust Arg1 expression is often used as a marker for alternative activation of macrophages, we observed that Arg1+ and Arg1− brain macrophages after TBI did not represent clear M2 and M1 macrophages, respectively, but instead each subset expressed markers of both M1 and M2 cells. Comparison of gene expression between Arg1+ and Arg1− macrophages confirmed that the former expressed much higher levels of Arg1 (eightfold) as well as higher levels of Mrc1 (2.4-fold), which encodes the mannose receptor/CD206 17 (Fig. 5). Increased expression of these two genes is a feature of M2 cells. The expression of other genes, however, indicated that Arg1+ macrophages were not identical to M2 cells. For example, Arg1+ macrophages preferentially expressed Nos2 (2.1-fold), an M1-associated gene 17 (Fig. 5). Similarly, although Arg1− macrophages had increased expression of Il1b (IL-1β) (2.4-fold), they also preferentially expressed signature M2 markers, notably Retnla (resistin-like α) (2.1-fold) and Clec10a (C-type lectin domain family 10, member A)/CD301 (2.9-fold) 17, 37 (Fig. 5). The relative increases in expression levels of Arg1, Mrc1, Nos2, and Il1b were confirmed by real-time PCR, demonstrating that relative to GAPDH, these genes were indeed transcriptionally active (Fig. 6). In accordance with flow cytometry data (Fig. 2C), gene expression analysis of MHCII, a molecule thought to be on both M1 and M2 cells, revealed that the Arg1− macrophage population as a whole expressed much higher levels of MHCII transcripts (not shown) and higher levels of Ciita (class II, MHC, transactivator) than the Arg1+ macrophages (Fig. 5). The MHCII+ Arg1− macrophages may thus have increased capacity to present antigen to CD4+ T cells.

Semiquantitative real-time PCR data of selected M2-, M1-, and chemotaxis-associated genes identified by microarray analysis to have significant differences between the macrophage populations. Data were normalized to GAPDH levels and are shown as mean +SEM of triplicate values. Data shown are from one experiment representative of two experiments performed on separate animals.
Taken together, we conclude that Arg1+ and Arg1− macrophages each have mixed expression of M2 and M1 properties, and under the conditions of TBI Arg1 cannot be used as a marker for conventional M2 cells. To further compare Arg1+ and Arg1− TBI brain macrophages with M1 and M2 macrophages, we performed a meta-analysis of genes differentially expressed between Arg1+ and Arg1− TBI brain macrophages compared with genes differentially expressed between IFN-γ- or IL-4-stimulated bone marrow derived macrophages (BMDMs) stimulated in vitro with IFN-γ or with IL‑4, representing M1 and M2 cells, respectively 38. Arg1+ and Arg1− macrophages each upregulated a variety of genes that were also expressed by BMDMs in response to either IFN-γ or IL-4 (Fig. 7). Thus, Arg1+ and Arg1− TBI brain macrophage subsets have features of both M1 and M2 phenotypes (Fig. 7). There are at least two explanations for these findings, not mutually exclusive: (i) individual brain macrophages may have features of both M1 and M2 cells (including cells that are incompletely polarized or are in transition from between different states of polarization and (ii) there may be subsets of cells within the Arg1+ and Arg1− cells that have different expression of M1 and M2 markers. Regardless, the gene expression profiles demonstrate that Arg1+ and Arg1− macrophages differ by many genes other than just Arg1.
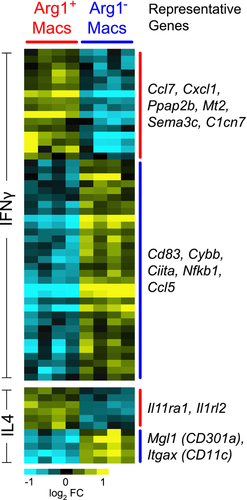
The most striking and novel differences between Arg1+ and Arg1− macrophages were in their unique chemokine profiles. Arg1+ macrophages preferentially expressed a chemokine repertoire that included Ccl24 (which is also secreted by M2 cells; 6.2-fold), Cxcl7 (5.4-fold), Cxcl4 (2.4-fold), Cxcl3 (4.5-fold), Cxcl1 (3.6−fold), Cxcl14 (2.4-fold), and Ccl8 (2.3-fold) (Fig. 5). Arg1− macrophages, in contrast, preferentially upregulated Ccl17 (6.8-fold), Ccl5 (4.4-fold), Ccl22 (3.7-fold), and Ccr7 (tenfold) (Fig. 5).
Although the gene profile of the Arg1+ macrophages suggests that they are not typical or homogeneously polarized M2 cells, they may have a role in promoting wound healing and in suppressing inflammation. Thus, Arg1+ macrophages preferentially expressed Spry2 (sprouty2; 2.4-fold), Cd9 (2.2-fold), Cd38, and Mt2 (metallothionein-2; 4.2-fold, Fig. 5). Sprouty2 and CD9 have protective roles in wound healing in skin injury models 39, 40. Mt2 and Cd38 have been implicated in neuroprotection during brain injury 41, 42. Arg1+ brain macrophages also preferentially expressed several other genes that are associated with protection against tissue injury, including Cd36 (3.8-fold), Hmox1 (heme oxygenase 1; 3.4-fold), Folr2 (folate receptor-2; 2.6-fold), Prdx6 (periredoxin-6; 2.5-fold), and Spsb4 (SPRY domain and SOCS box containing protein 4; 2.5-fold) (Fig. 5) 43-49. If Arg1+ cells do have the potential for neuroprotection following TBI, this may be overwhelmed by Arg1− cells, which are greater in number and are less transient.
Discussion
Our findings demonstrate a heterogeneous macrophage response to TBI that changes over time. Expression profiling of Arg1+ and Arg1− macrophage subpopulations demonstrate that they do not exemplify previously described in vitro derived macrophage subsets 17. They also differ from macrophages that accumulate in skin wound macrophages 50. Skin wound macrophages, such as TBI-induced Arg1+ cells, both express Arg1 and Mrc1. However, skin macrophages additionally upregulated Clec7a, and do not express Nos2, features that distinguish them from TBI-induced Arg1+ cells.
It may not be surprising that the macrophage response to TBI differs from macrophage polarization induced in vitro or in other organs and other in vivo conditions. It is likely that macrophages can assemble their functions and products in a variety of combinations with great diversity. Our findings do demonstrate the heterogeneity of the macrophage response to TBI and they suggest that Arg1 should not in isolation be used as a marker for M2 cells. In this regard, Arg1 expression can be induced by pathways independent of IL-4/STAT6 51.
Although we were able to identify macrophage subsets by using Arg1 as a marker in YARG mice, we could not detect robust expression of IL-12p40 by flow cytometry on days 1, 4, 7, or 14 in any macrophages or microglia by using Yet40 mice or by gene expression profiling comparing Arg1+ and Arg1− macrophages, as assessed by gene profiling. This suggests that IL-12p40 may not be a major effector cytokine promoted by brain macrophages or microglia in TBI, and that early in TBI, IL-12p40 is not inversely proportional to Arg1 expression. Other M1 genes are detected, however, both in Arg1+ and Arg1− cells. Thus, the use of a single marker to define M1 and M2 cells in TBI appears not to be sufficient, and the functional consequences of the Arg1+ and Arg1− cell populations on the course of TBI remain unknown.
Our findings do not exclude the possibility that there are more than two subsets of responding macrophages, and this is clearly supported by the bimodal expression of MHCII in Arg1− macrophages. Also, despite the extensive differences in gene expression between these cell subsets, particularly, in the expression of chemokines, it is also possible that Arg1+ and Arg1− macrophages may have a shared lineage and/or be partially polarized and that one subtype could become or becoming the other.
Before conducting the microarrays, we initially considered that the Arg1+ cells might be M2 macrophages, whose formation relies on the transcription factors, PPAR-γ and PPAR-δ (peroxisome proliferator-activated receptor γ and δ) 17. We therefore treated YARG mice both before and after TBI with PPAR agonists, rosiglitazone, and GW0742, but we observed no increase in generation of YFP+ cells. This may reflect our subsequent demonstration that the Arg1+ cells are not, in fact, typical homogeneous M2 cells. Other studies of TBI have shown a beneficial effect of rosiglitazone during TBI, which was associated with reduced presence of myeloid cells, although mechanisms directly involving macrophages were not established 52.
Our findings expand our knowledge on chemokines expressed during TBI. Prior gene expression arrays analyzing cortical brain tissue found that IL-8, CCL2, CCL3, CCL4, CCL6, CCL9, CCL12, CXCL10, and CXCL16 were upregulated 5. Our results identify macrophage subsets as a source of several additional chemokines (Fig. 5) that differ from those that have been previously described, in addition to showing that production of chemokines varies between macrophage subsets.
Macrophages and microglia have distinct roles during homeostasis and pathogenic diseases 11, 53. Our studies took advantage of flow cytometry to distinguish macrophages from microglia 30. It is difficult to make this separation by immunohistology, because microglia and macrophages share many markers. Using YARG and Yet40 reporter mice, we did not detect arginase-1, IL-12p40, or MHCII expression in microglia before or after TBI. Thus, microglial activation in TBI was dissimilar from macrophages, despite a broad increase in CD86 expression in both cell types.
In summary, our studies demonstrate that TBI induces a robust infiltration of macrophages that differentiate into at least two subpopulations in the brain. The two subsets colocalize near the site of injury. They express distinct repertoires of chemotactic molecules, including some that were not previously associated with TBI. In studying the effect of macrophages on the consequences of TBI and in designing strategies to alter these effects, it may be important to consider the role of different macrophage subsets in shaping protective versus pathological responses.
Materials and methods
Animals
C57BL/6 WT males (age 10–16 weeks) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). YARG and Yet40 knockin mice were generated from C57BL/6 mice as previously described 28, 33 and bred in the AALAC-approved transgenic animal facility of the San Francisco VA Medical Center. YARG mice express enhanced YFP from an internal ribosome entry site (IRES) inserted at the 3′ end of the Arg1 gene, leaving the gene and regulatory regions intact, and Yet40 mice express enhanced YFP from an IRES inserted at the 3′ end of the IL-12p40 promoter. Where indicated, mice were administered LPS at 10 mg/kg i.p. and euthanized 4 days later.
Surgery
Controlled cortical impact surgery or sham surgery was performed on anesthetized animals under a protocol approved by the San Francisco VA Medical Center Animal Care Committee. Briefly, bupivacaine was administered subcutaneously above the skull, and an incision was made followed by a 2.5 mm circular craniectomy. TBI was inflicted by a 2 mm circular, flat pneumatic piston traveling at 3 m/s, penetrating 1.5 mm, for 150 ms (Amscien Instruments, Richmond, VA, USA with extensive modifications by H&R Machine, Capay, CA, USA). Target brain coordinates for the center of injury were 1.5 mm lateral, 2.3 mm posterior to the bregma point. After minor bleeding had ceased, the skin was clipped together and animals were monitored for recovery. Sham animals received all surgical procedures without piston impact. As needed, animals were given rehydration therapy for the first 3 days.
Brain and blood leukocyte isolation
Brain leukocytes were harvested according to previously published methods 30. Briefly, following perfusion brain tissues were obtained and mechanically disassociated through a 100 μm cell strainer. Washed cells were treated with 400 U/mL DNase I (Sigma-Aldrich) and 0.5 mg/mL collagenase type I (Worthington) at 37°C for 30 min. Leukocytes were isolated by separation on a Percoll gradient (Amersham Biosciences). For PBL isolation, mononuclear cells were separated from peripheral blood using ficoll-hypaque (GE Healthcare).
Flow cytometry and antibodies
Fc receptors were blocked with 10% rat serum (Sigma) and cells were stained with fluorescent antibodies. Leukocyte analysis used a combination of the following antibodies: anti-CD45 (clone Ly5) allophycocyanin (eBioscience), anti-CD11b (clone M1/70) PE (Invitrogen) or PE-Cy5 (eBioscience), anti-Ly6G (clone 1A8) PE-Cy7 (BD Biosciences), F4/80 (clone BM8) FITC or PE-Cy5 (eBioscience), MHCII (clone M5/114.15.2) PE (eBioscience), CD86 (clone GL1) PE (eBioscience). SYTOX Blue (Invitrogen) was used to gate out dead cells. Cells were sorted on a FACSAria (BD Biosciences) and data were analyzed using FlowJo Software (Treestar). All data represent mean ± SEM.
Histology
Brains were perfused with saline followed by 3.7% formaldehyde. After a 2-h fixation, brains were incubated in 30% sucrose overnight and frozen in tissue-freezing medium (Sakura, Inc.). For H&E staining, brains were sectioned 10 μm thick onto glass slides, heat-dried, and stained (at least three animals per group were analyzed, five sections per animal). For F4/80 staining, 5 μm sections that were quenched for endogenous peroxidases and blocked with streptavidin and biotin (VectorLabs) were immunostained with an anti-F480 antibody (Clone BM8, eBioscience), followed by goat anti-rabbit biotinylated antibody and visualized using a Vectastain ABC elite kit (VectorLabs) (three animals per group and at least five sections per animal were analyzed).
For immunofluorescent labeling of YFP and F4/80, a biotinylated goat anti-YFP antibody (Abcam) and streptavidin-HRP (Perkin Elmer) were used and amplified by fluoresceinated tyramide (Perkin Elmer). After an additional round of quenching and blocking, mounted sections were further stained with a biotin-conjugated anti-F4/80 (Clone BM8, eBioscience) antibody followed by streptavidin-HRP and Alexa fluor-555 conjugated tyramide (Invitrogen). DAPI (Invitrogen) was used at 300 nM to identify cellular nuclei. Sections were mounted by using Fluorogel (Electron Microscopy Services). All sections were imaged using either a Nikon Eclipse 80i microscope or an Olympus BX-51 microscope. Three TBI animals were analyzed and at least five sections per animal were analyzed.
Microarrays
For gene expression profiling of macrophages from YARG mice, Arg1+ (YFP+ CD45hi CD11b+ Ly6G− SYTOX Blue−) and Arg1− macrophages (YFP− CD45hi CD11b+ Ly6G− SYTOX Blue−) were isolated by flow cytometry from ipsilateral brain hemispheres at day 1 following TBI (n = 4 for each cell sample). Monocytes (CD11b+ F4/80+) from peripheral blood were also collected. Sorted cells were immediately lysed in denaturation buffer and frozen. RNA was isolated by using an RNAqueous Micro kit (Ambion). Further sample preparation, labeling, and array hybridizations were performed according to standard protocols from the UCSF Shared Microarray Core Facilities and Agilent Technologies. RNA quality was assessed using a Pico Chip on an Agilent 2100 Bioanalyzer (Agilent Technologies), and RNA was amplified by use of a whole transcriptome amplification kit (Sigma-Aldrich). Subsequent Cy3-CTP labeling was performed by using a NimbleGen one-color labeling kit (Roche-NimbleGen, Inc.). The quality of the amplified products was assessed by using an Agilent 2100 Bioanalyzer and Nanodrop ND-8000 (Nanodrop Technologies, Inc.). The products were hybridized to Agilent whole mouse genome 4×44K microarrays according to the manufacturer's protocol. Arrays were scanned with an Agilent microarray scanner, and raw signal intensities were extracted with Feature Extraction v10.5 software. Data were normalized by using the quantile normalization method 54. No background subtraction was performed, and the median feature pixel intensity was used as the raw signal before normalization. A one-way ANOVA linear model was fitted to the comparison to estimate the false discovery rate for each gene for the comparison of interest, and genes with a false discovery rate < 0.05 were considered significant. Scatter plots compared averaged log2 gene expression from each group. PCA was performed using the top 15% of genes exhibiting the most variance across all samples, using the PopulationDistances module of GenePattern (PMID: 16642009). For heatmaps, data were log2 transformed and median centered across genes. Replicates were hierarchically clustered (PMID: 16939791). Heatmaps of genes selected from the top 15% most variable genes that exhibited interesting pairwise comparisons were visualized using Java Treeview (http://sourceforge.net/projects/jtreeview/files/) (PMID: 15180930).
Meta-analysis of transcriptional responses of brain wound macrophages to BMDMs stimulated by either IFN-γ or IL-4 was performed using previously published tables 38. Macrophage genes with significant changes in expression upon IFN-γ or IL-4 stimulation were compared with genes with significant expression differences between Arg1+ brain macrophages versus Arg1− brain macrophages.
Accession number
Microarray data were deposited in Gene Expression Omnibus (GEO) under accession number GSE39759.
Semiquantitative real-time PCR
Total RNA was isolated from sorted cell populations, including macrophages from injured brain hemispheres and monocytes from peripheral blood, by using an RNAqueous micro kit (Ambion). RT was performed using oligo dT primers and Superscript II reverse transcriptase (Invitrogen). Amplicons were amplified using SYBR green (New England Biolabs) and the rate of amplification was measured using a 7500 real-time PCR machine (Applied Biosystems). Relative transcript levels for each gene were normalized to GAPDH controls by calculating delta cycle of threshold values. The following primers were used for: Arg1 5′-CTCCAAGCCAAAGTCCTTAGAG-3′, 5′-GGAGCTGTCATTAGGGACATCA-3′; Mrc1 5′-CTCTGTTCAGCTATTGGACGC-3′, 5′-TGGCACTCCCAAACATAATTTGA-3′; Nos2 5′-TGTGGCTGTGCTCCATAGTT-3′, 5′-CCAGGGCTCGATCTGGTAGT-3′; Il1b 5′-GCAACTGTTCCTGAACTCAACT-3′, 5′-ATCTTTTGGGGTCCGTCAACT-3′; Ccl24 5′-TCTTGCTGCACGTCCTTTATT-3′, 5′-CTAACCACTCGGTTTTCTGGAAT-3′; Cxcl4 5′-CCTGGGTTTCCGGACTGGGC-3′, 5′-CCGCAGCGACGCTCATGTCA-3′; Cxcl3 5′-CAGAGCTTGACGGTGACGCCC-3′, 5′-CCAGACACCGTTGGGATGGA-3′; Spp1 5′-ATCTCACCATTCGGATGAGTCT-3′, 5′-CTTGTGTACTAGCAGTGACGG-3′; GAPDH 5′-ATTCAACGGCACAGTCAAGG-3′, 5′-TGGTTCACACCCATCACAAA-3′.
Acknowledgements
The authors thank Ruby Gribi of the San Francisco VA Flow Cytometry core, Dr. David Erle, Andrea Barczak, Rebecca Barbeau, and Joshua Pollack at the Sandler Asthma Basic Research (SABRE) Center Functional Genomics Core Facility (NIH/NCRR UCSF-CTSI grant number UL1 RR024131), and Ivy Hsieh of the San Francisco VA Cell Imaging core for their contributions. This work was supported by the Department of Veterans Affairs and by grants from the Department of Defense to WES and CLH, which were administered by the Northern California Institute for Research and Education.
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
Abbreviations
-
- BMDM
-
- bone marrow-derived macrophage
-
- MHCII
-
- MHC class II
-
- PC
-
- principal component
-
- PCA
-
- principal component analysis
-
- TBI
-
- traumatic brain injury
-
- YFP
-
- yellow fluorescent protein