The thiol redox state of lymphoid organs is modified by immunization: Role of different immune cell populations†
Abbreviations
Cyscysteine
Cysscystine
DCNB1-chloro-2,4-dinitrobenzene
NPSHnon-protein thiols
SACStaphylococcus aureus Cowan I
TBMtingible body macrophages
Trxthioredoxin
xcystine/glutamate transporter
xCTx light chain
Abstract
Resting T lymphocytes can internalize reduced cysteine (Cys) but not cystine, the oxidized form of the amino acid that predominates extracellularly. In vitro studies have shown that DC provide Cys to T cells during antigen presentation, allowing their activation. Here, we show that increased thiol production is a hallmark of immune response in vivo. Indeed, the thiol content of LN increases dramatically after antigen injection. Non-protein thiols co-distribute with DC and are highly abundant in germinal centers. In agreement, activated but not resting B lymphocytes and macrophages release free thiols. Increased thiol release following activation requires thioredoxin and is paralleled by increased thioredoxin expression. The T zones of LN are consistently less stained, and both resting and activated T cells are unable to release thiols. Interestingly, the cystine/glutamate transporter x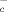 is absent in resting T lymphocytes but is rapidly induced by TCR triggering in vitro, indicating that the release of T cells from the need of exogenous Cys occurs early after activation. These results indicate that a reducing microenvironment is essential to start the immune response but dispensable for its evolution, and support the emerging concept that extracellular redox is implicated in the control of crucial cellular functions.
is absent in resting T lymphocytes but is rapidly induced by TCR triggering in vitro, indicating that the release of T cells from the need of exogenous Cys occurs early after activation. These results indicate that a reducing microenvironment is essential to start the immune response but dispensable for its evolution, and support the emerging concept that extracellular redox is implicated in the control of crucial cellular functions.
Introduction
The free thiol (cysteine, Cys) and the disulfide bonded (cystine, CySS) form of the amino acid Cys exist in an equilibrium defined by redox conditions. In the oxidizing environment of the extracellular space, CySS prevails while Cys is almost absent 1. Conversely, within the cytosol, Cys is present mainly as a free thiol. Interestingly, T lymphocytes can take up Cys but not CySS 2. Since Cys is an essential amino acid for T lymphocytes, required for GSH synthesis 3, its reduced form must be made available in the microenvironment of T cells to sustain their activation and proliferation. In addition, pre-B and resting B lymphocytes require exogenous Cys to grow in vitro, but are released from this requirement upon differentiation 4.
We have previously shown in vitro that, during antigen presentation, DC increase the uptake of CySS and release Cys, allowing T-cell activation. The intracellular conversion of CySS to Cys requires the activity of the redox-active enzyme, thioredoxin (Trx) 5.
Although the interaction of T or B lymphocytes with antigen-pulsed APC or mitogens may recapitulate lymphocyte activation in vitro, the generation of an immune response in vivo is the result of a much more complex cellular choreography. In secondary lymphoid organs, distinct subsets of immune cells are strategically compartmentalized to allow the occurrence of efficient immune responses. T lymphocytes localize mostly in the paracortex (T zone) where they interact with migrating DC carrying antigens 6. Some T cells are also present in the B lymphocyte-enriched follicles of the outer cortex (B zone), especially in the GC where they may provide costimulatory signals to B cells 6, 7. GC develop a few days after arrival of the antigen from afferent lymphatic drainage, when antigen-activated B cells move to the center of B-cell follicles in secondary lymphoid organs and proliferate within the follicular DC network 6. Many of the B cells generated within the GC die in situ and nuclei of dead B cells are identified as tingible bodies clustered inside macrophages (TBM) 8.
The role of extracellular redox in this plastic context in vivo remains to be defined. Here, we show that lymphoid tissues have an environment more reduced than the other tissues and the amount of free thiols increases dramatically during the immune response. Moreover, not only DC, but also macrophages and B lymphocytes contribute to the generation of the reducing microenvironment of the immune response.
Results and discussion
Lymphoid tissues are embedded with large amount of non-protein thiols (NPSH)
Tissue extract analyses revealed that the thiol content of spleen from germ-free mice is slightly higher than that of non-lymphoid organs, such as kidney and liver, and increases dramatically after immunization (Fig. 1A). To gain information about the intra-tissue distribution of thiols, frozen tissue sections from non-lymphoid and secondary lymphoid organs were stained with mercury orange, a dye specific for non-protein thiols (NPSH) 9-11 and analyzed by confocal microscopy. Liver and kidney displayed a diffuse but faint staining (Fig. 1B, panels 1f and 1g), while LN and spleen from non-injected mice were mildly positive (Fig. 1B, panels a, c and h). After Ag injection, both LN and spleen increased the positivity to NPSH staining (Fig. 1B, panels b, d and i). The mercury orange signal did not appear restricted inside cells but was also present in the intercellular space, suggesting that NPSH are externalized. Pretreatment of cryostat sections with the thiol-specific blocking agent n-ethylmaleimide completely abrogated the mercury orange staining (Fig. 1B, panels e and m), confirming the thiol-selectivity of the dye 10, 12. Following immunization, the MFI of inguinal LN increased from 1.5- to 2-fold and that of mesenteric LN up to 10-fold (Fig. 1C). Portal LN resulted 2-fold more intensely stained by mercury orange in mice with a Con A-induced liver injury 13 than in untreated mice, indicating that NPSH are induced also in intra-visceral LN upon activation (Fig. 1C). In contrast, no fluorescence increase was observed in the injured liver (not shown). Together, these results indicate that lymphoid tissues are significantly more “reduced” than the other tissues, and that the immune response is paralleled by a conspicuous increase in the NPSH production. GSH is the major antioxidant NPSH inside cells, but irrelevant outside. At variance, even if usually in very low concentrations in the extracellular space, the Cys/CySS pool is the major low-molecular-weight thiol/disulfide couple in plasma, and serves important antioxidant functions 14. Therefore, although mercury orange staining does not discriminate between GSH and Cys, it is conceivable that the intracellular staining is due mainly to GSH, while the extracellular to Cys.
Free sulfhydryls in lymphoid and non-lymphoid organs. (A) Total-SH content of kidney, liver and spleen from non-immunized or immunized mice. Results are expressed in nanomoles of total thiols/mg of protein. The mean of three different analyses±SD is shown. (B) Sections from inguinal LN (a, b), mesenteric LN (c, d, e), liver (f), kidney (g) and spleen (h, i, m) from non-immunized (N: a, c, f, g, h) or immunized (I: b, d, e, i, m) mice, stained in mercury orange solution were analyzed by confocal microscope at 400×magnification. Sections shown in (e) and (m) were pretreated with n-etylmaleimide (NEM) before staining. (C) Quantification of the mercury orange fluorescence levels in the different tissues. Results are expressed as MFI of 3–5 chosen fields (300 000 μm2)+SD.
Gut-associated Peyer's patches were the only lymphoid organs almost negative to mercury orange staining (Fig. 1C, GALT) and non-activated mesenteric LN were significantly (p<0.001) less stained than inguinal LN. This feature may be related to the physiological unresponsiveness to antigen exposure in the intestinal microenvironment 15. In this regard, lamina propria macrophages have been found unable to release Cys, and thereby responsible for the low proliferative potential of lamina propria T lymphocytes 16. Moreover, mesenteric LN have been identified as the key site for tolerance induction to food antigens, mediated by DC 17. The dramatic increase of NPSH in mesenteric LN after Ag injection indicates that this deficit is not permanent but is overcome by a powerful immunization, such as i.p. injection. This suggests that a dysregulation of GALT redox may be involved in inflammatory bowel disease 18, 19. However, it cannot be ruled out that in non-immunized mice the higher staining of superficial LN with respect to LN associated with the gastrointestinal tract is due in part to a higher basal activation, related to an easier accessibility of superficially located LN to external noxia.
NPSH colocalize with DC and increase in B-cell areas of activated LN
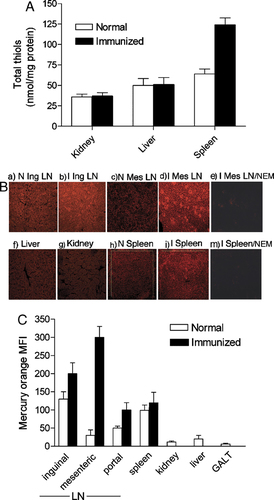
Double-staining with mercury orange and B, T or DC markers (Fig. 2) showed that NPSH staining of non-immunized LN (panel A) was generally diffuse in the cortex and paracortex zones with no clear compartmentalization between B zone (upper left panels, CD45R+) and T zone (middle left panels, CD3+). However, a stronger staining was found to cluster with DC distributed in the T zones as indicated by the co-localization of CD205 and mercury orange highly positive cells (lower left panels). This observation suggests that, like in vitro 5, also in vivo during Ag presentation DC produce Cys in the extracellular space, making it available for T-cell activation and proliferation. In immunized LN (Fig. 2B), the dramatic increase in NPSH staining was highly intense in the B zone of the outer cortex, especially in the GC. This finding suggests that a reducing environment is needed by B cells activation in vivo, confirming the long standing evidence that B lymphocyte cultures require reductants such as 2-ME 4. Mercury orange staining condensed in highly fluorescent large spots within the GC. Hematoxylin/eosin staining revealed the presence of abundant nuclear and cell debris (Fig. 2C, upper panel), indicating that these spots contain remains from dying B cells. This was further confirmed by the presence of MFG-E8+ TBM in the duty to engulf apoptotic B cell (Fig. 2C, lower panel) 20. The GC spots containing cell debris and highly stained by mercury orange are surrounded by T lymphocytes (Fig. 2B middle panels, arrowheads) that may be engaged in interactions with blebs from apoptotic B cells, a process that has been proposed to limit the availability of T-cell help 7.
NPSH colocalize with DC and increase in concentration in the GC of activated LN. Double-staining of serial sections of mouse normal LN (A) and immunized LN (B) with mercury orange and anti-CD45R (upper), CD3ε (middle), CD205 (lower) mAb. Left: immunofluorescence. Right: merged images (magnification 400×). (C) Hematoxylin/eosin (upper) and mercury orange plus anti-MFG-E8 staining of serial sections from immunized inguinal LN (magnification 1000×). Arrows show spots containing remains from dying B cells.
B cells and macrophages release NPSH after activation
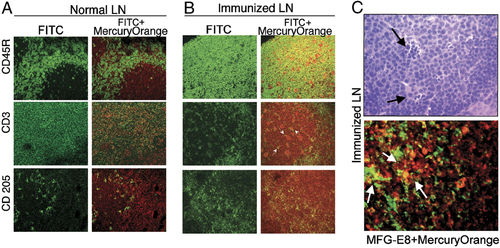
The strong NPSH content in GC of activated LN suggested the involvement of activated B cells in NPSH generation. Indeed, while resting B cells isolated from human tonsils B lymphocytes by Percoll gradient (high-density B cells) 21 did not release detectable NPSH over 12 h of culture, in vivo activated B lymphocytes (low-density B cells) released levels of NPSH varying from 10 to 20 μM in the different subjects (Fig. 3A). A similar amount of released thiols was detected in supernatants from high-density B cells after 12 h of exposure to the B cell mitogen Staphylococcus aureus Cowan I (SAC). That activated B lymphocytes secrete Cys is coherent with their APC function 22 and indicate that they can provide T cells with exogenous Cys during antigen presentation. On the other hand, recent evidence indicates that B cells undergo oxidative stress during differentiation 23 and elicit an adaptive response with increased expression of peroxiredoxins and other anti-antioxidant genes. Therefore, it is possible that the large amounts of released thiols in GC represent an anti-oxidant response that allows differentiating B lymphocytes to resist otherwise fatal stress. Unlike activated B lymphocytes and DC, both resting and PHA-activated T lymphocytes did not release detectable thiols. In contrast, in vitro derived macrophages were found to secrete the highest levels of NPSH. These data are in agreement with previous findings of high levels of Cys secretion by peripheral blood monocytes 16 but is in contrast with other observations that activated macrophages produce a burst of superoxide anion 24. This apparent discrepancy may be related to an adaptive anti-oxidative response, with increased expression of Trx and NPSH, triggered by the oxidative stress as observed in different cell systems 11, 23. The high level of NPSH released by activated monocytes in vitro supports the observation of the strong positivity in the LN areas occupied by TBM. The major function of TBM so far documented is the removal of apoptotic B cells, a process implicated in avoiding autoimmunity 20. On these basis, it is tempting to speculate that beside a role in promoting the immune response, the reduced environment may also help macrophages to clear out dead B lymphocytes and thereby to prevent autoimmune diseases.
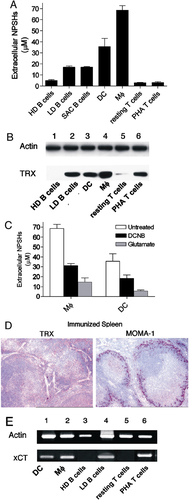
Modulation of NPSH release, Trx and xCT transporter expression in immune cells in different activation states. (A) NPSH concentration in supernatants from high-density (HD) and low-density (LD) tonsillar B cells, SAC-activated (15 h) HD B cells, DC, MΦ, resting and PHA-activated (15 h) T cells. The means of three independent experiments ±SD are shown. (B) Western blot analysis of cell lysates (50 μg) from the same cells as in (A) (with the exception of SAC B cells), as indicated. Filters were hybridized with anti-actin (top panel) and anti-Trx mAb (bottom panel). (C) Spontaneous NPSH release by DC or MΦ during 12 h of incubation without or with glutamate or DCNB as indicated. The means of three independent experiments ±SD are shown. (D) Anti-Trx and anti-MOMA-1 immunohistochemistry of immunized spleen sections (magnification 400×). (E) RT-PCR amplification of xCT from RNA from the same cells as in (B). T and B lymphocytes were activated for 3 h (lower panel). RT-PCR of human β actin on the same samples is shown (upper panel).
Trx expression parallels thiol release
Trx is one of the main intracellular oxido-reductases and plays critical roles in the regulation of the cellular redox environment 25. As shown in Fig. 3B, Trx expression, like thiol release, is undetectable in resting but induced in activated B cells. Mature DC and macrophages, that release the highest amounts of NPSH, also contain abundant Trx. Exceptions are PHA-activated T cells that do not release NPSH in spite of expressing more Trx than resting T lymphocytes. The implication of Trx in the generation of NPSH was supported by the inhibition of NPSH release observed after cell exposure to 1-chloro-2,4-dinitrobenzene (DCNB), a compound that blocks Trx function 5 (Fig. 3C). While non-immunized spleen and LN do not stain detectably with anti-Trx (not shown), lymphoid tissues from immunized mice (Fig. 3D) showed a diffuse positivity for Trx, more intense in macrophage areas around B-cell areas (see for comparison the positivity for MOMA-1+ macrophages in the right panel) and in GC.
T and B Lymphocytes modulate the expression of the cys-SH transporter
The cystine/glutamate antiporter x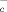 is the main transport system that mediates the cellular uptake of Cys in exchange for intracellular glutamate 26. As shown in Fig. 3C, glutamate inhibits the generation of extracellular NPSH by macrophages and DC, suggesting that CySS is indeed internalized through x
is the main transport system that mediates the cellular uptake of Cys in exchange for intracellular glutamate 26. As shown in Fig. 3C, glutamate inhibits the generation of extracellular NPSH by macrophages and DC, suggesting that CySS is indeed internalized through x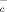 . Accordingly, DC and monocytes expressed the x
. Accordingly, DC and monocytes expressed the x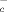 light chain xCT (Fig. 3E). At variance, neither resting B lymphocytes nor resting T cells express xCT. However, a short (3 h) stimulation with SAC or PHA of B and T cell, respectively, induces the expression of xCT (Fig. 3E). Therefore, both resting B and T cells require exogenous Cys for their activation, but this requirement is essential only during the first phases of the immune response, and it is rapidly overcome by the expression of x
light chain xCT (Fig. 3E). At variance, neither resting B lymphocytes nor resting T cells express xCT. However, a short (3 h) stimulation with SAC or PHA of B and T cell, respectively, induces the expression of xCT (Fig. 3E). Therefore, both resting B and T cells require exogenous Cys for their activation, but this requirement is essential only during the first phases of the immune response, and it is rapidly overcome by the expression of x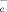 once cells are activated.
once cells are activated.
Concluding remarks
This study uncovers some aspects concerning the involvement of the extracellular redox in the immune system. Collectively, our data provide evidence that the generation of an efficient immune response is paralleled by the generation of a local reduced microenvironment and suggest that extracellular NPSH participate in the regulation of the process. The molecular targets of Trx and redox modifiers in lymphoid tissues remain to be defined. Finally, our observations support the evolving concept that redox in the intercellular space is implicated in the control of many cell functions, through structural or functional alterations in key signaling molecules 27, 28.
Materials and methods
Chemicals and animals
All chemicals were from Sigma (Milano, Italy) unless differently specified.
BALB/c mice from Charles River (Lecco, Italy) were maintained in pathogen-free facilities. Twelve-week-old animals (three per group) were injected weekly twice with 100 μL of a 1:1 BSA (1 mg/mL)/CFA emulsion ratio, s.c. or i.p. to stimulate superficial and mesenteric LN, respectively. Liver toxicity and portal LN activation were induced by i.v. injection of 400 μg of Con A 13. Spleens were immunized by a single i.v. injection or four times weekly i.p. injections of 200×106 SRBC. One week after the last injection, mice were euthanized and the different organs were removed and analyzed. All protocols were approved by the Institutional Review Board of the Istituto Nazionale per la Ricerca sul Cancro (Genova, Italy).
Thiol measurement
Liver, kidney, spleen and LN were homogenized and processed as described 29. Thiols in clarified homogenates and in cell culture supernatants were quantified using the 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) method as described 5, 29. L-Cys was used as standard. Mean and SD were calculated using GraphPad Software. For NPSH quantification in supernatants of cells treated with DCNB or glutamate, standard curves with L-Cys were supplemented with DCNB or glutamate at the same doses used in culture. No significant differences were detected between the standard curves without or with the two compounds.
Immunohistochemical and immunofluorescence procedures
Serial cryostat sections from frozen organs were reacted with 25 μM mercury orange 9-11 and co-stained with the following Ab: rat anti-mouse mAb to CD45R/B220 (B cell specific), CD205 (DC specific), CD3ε (T-cell specific) from Southern Biotech (Birmingham, AL) and rabbit-anti-mouse MFG-E8 Ab (TBM specific) from DBA (Milan, Italy) followed by the relevant anti-IgG-FITC-conjugates (Southern Biotech), and analyzed by confocal microscopy 11. FITC and mercury orange were excited with 488 and 543 nm laser, respectively. The emission signal was filtered at 505–530 and 540 nm. Excitation lasers were used successively, to avoid overlapping signals. Images were acquired and quantified by the Fluoview FV500 software. When indicated, sections were pretreated 10 min with 100 μM n-ethylmaleimide before mercury orange staining 12.
For immunohistochemistry, mouse mAb to Trx (2B1, kind gift of Dr. F. Clarke, Brisbane, Australia) and rat mAb to mouse MOMA-1 (MΦ specific, Southern Biotech) were used. Immunostaining was performed using a streptavidin–biotin–alkaline-phosphatase-complex staining kit 11 (Bio-SPA Division, Milan, Italy). For each group of organs three representative zones have been sampled.
Cell preparation and cell cultures
T cells were isolated from PBMC from healthy donors as described 5 and stimulated 3 or 15 h with 10 μg/mL of PHA-P as indicated. Macrophages and DC were obtained by culturing adherent cells from PBMC as described 5.
Tonsils were obtained from children undergoing tonsillectomies, after informed consent. B cells were purified and fractionated by Percoll density gradients as described 20 and activated 3 or 15 h with SAC (Calbiochem, Milano, Italy).
For NPSH-release assay, a different cell number was plated according to the cell dimension (DC: 0.25×106, MΦ: 0.4×106, B and T lymphocytes: 4×106).
Western blot analysis
Western blot analysis was performed on cell lysates as described 5. Filters were hybridized with anti-Trx mAb 2B1 or anti-actin mAb (Sigma).
RT-PCR
Total RNA was isolated from 1×107 cells using TriPure Isolation Reagent (Roche, Indianapolis, USA). For RT-PCR, the first-strand of cDNA was synthesized from 1-μg of the total RNA using random examers and M-MuLV RT (Roche). PCR amplification of cDNA was performed using the primer set: 5′-ATG GTC AGA AAG CCT GTT G-3′/5′-TCT TCT GGT ACA ACT TCC AGT ATT A-3′, designed from the nucleotide sequences of human xCT 30 and the primer set 5′-CCT GCT TGC TGA TCC A-3′/5′-TCC GGT GAC GGG GTC A-3′ to amplify human β-actin gene, as internal control.
Acknowledgements
We thank Drs. Frank Clarke (Brisbane, Australia) for advices and reagents, Roberto Sitia (Milano, Italy) for helpful discussion, Enrica Balza, Patrizio Castagnola and Alessandro Poggi (Genova) for helping in mice injections and confocal analyses. This work was supported by grants from Ministero Salute, ISS, Associazione Italiana per la Ricerca sul Cancro and Telethon to Anna Rubartelli.
Conflict of interest: The authors declared no financial or commercial conflict of interest.




