Insights into Langerhans cell function from Langerhans cell ablation models†
Abbreviations
BACbacterial artificial chromosome
CHScontact hypersensitivity
CLNcutaneous LN
dDCdermal DC
DNFBdinitrofluorobenzene
DTdiphtheria toxin
DTAdiphtheria toxin subunit A
DTRdiphtheria toxin receptor
HB-EGFheparin-binding EGF-like growth factor
LCLangerhans cell
Abstract
Langerhans cells (LC) are the principal dendritic cell (DC) population in the epidermis of the skin. Owing to their prominent position at the environmental barrier, LC have long been considered to be prototypic sentinel DC. More recently, the precise role of LC in the initiation and control of cutaneous immune responses has become debatable. To elucidate their contribution to immune regulation in the skin, our laboratories have generated genetically modified mice in which LC can be followed in situ by expression of enhanced green fluorescent protein and can be either inducibly or constitutively depleted in vivo. This review highlights the similarities and differences between these mouse models, discusses the discovery and functional significance of Langerin+ dermal DC, and examines some recent data that help to shed light on LC function.
The Langerhans cell paradigm
Langerhans cells (LC) were discovered by Paul Langerhans in 1868 1, but it took more than 100 years before they were first associated with the immune system and recognized as antigen-presenting cells 2. In particular, Ralph Steinman's group 3 dissected the functional characteristics of LC starting from the seminal finding that murine epidermal LC mature into potent immunostimulatory dendritic cells (DC) in vitro. The work that followed shaped the concept of DC as professional inducers of T-cell immune responses largely by studying LC, though the term ‘LC paradigm’ was coined much later by Wilson and Villadangos 4.
This concept assigned three key functions to LC/DC (reviewed in 5). First, immature LC that reside in the periphery in the steady state are highly specialized in antigen uptake. LC form a dense network in the epidermis where they constantly scan the environment by extending and retracting their dendrites and are ideally positioned to detect any pathogen breaching the skin barrier 6. Second, LC transport antigen to skin-draining lymph nodes (LN), which is essential to ensure interaction with rare antigen-specific naive T cells. Stimulation by a number of pathogen products in addition to pro-inflammatory cytokines induces LC activation 7-9. Activated LC downregulate expression of E-cadherin, which is thought to maintain their position in the epidermis, and begin to express CC chemokine receptor 7, which guides the cells to the T-cell areas in the LN.
Third, during migration the LC undergo a process of maturation in which they process antigen acquired in the skin and present it in the context of MHC class I/II. In addition, they dramatically upregulate their surface expression of co-stimulatory molecules and start to produce cytokines required for proper Th1 or Th2 instruction. Thus, by the time LC arrive in the LN, they have acquired the surface phenotype of a ‘functionally’ mature DC capable of activating naive T cells and thereby initiating an adaptive immune response specifically tailored to fight off the invading cutaneous pathogen 5.
The LC paradigm has been expanded to include LC that migrate to skin-draining LN in the steady state in the absence of activating stimuli where they present self-peptides to T cells 10-14. This steady-state presentation of self-antigens has been proposed to eliminate self-reactive T cells and provide a mechanism of peripheral tolerance 15, 16. Hemmi et al. 17 showed that an epidermally restricted self-antigen is transported to draining LN by TGF-β-dependent cells, presumably LC. In addition, targeted delivery of antigen in the immunological steady state, via the endocytic receptor CD205 expressed on immature DC (including LC), failed to induce immunity and instead established CD4 and CD8 T-cell tolerance possibly through the induction of regulatory T cells 18-20. Recent evidence suggests that disruption of E-cadherin interactions used by LC to remain tethered in the epidermis and associated β-catenin signaling may participate in this process 21.
The first suggestion that the LC paradigm does not explain all aspects of skin-adaptive immunity arose from work with herpes simplex virus (HSV) infection models. During murine HSV2 infection of the vaginal epithelium, CD8α+ CD45RA− CD11b+ submucosal DC, thought to be the equivalent of dermal DC (dDC), and not LC were responsible for the induction of a protective CD4+ Th1 response 22. In addition, in a cutaneous HSV1 infection, LC were incapable of priming CD8+ T cells but rather appeared to transfer their antigenic cargo to the priming LN-resident CD8α+ DC population. Importantly, the authors did not exclude a role for antigen uptake and transport to the LN by LC, only direct MHC class I-restricted antigen presentation and CTL priming 23. An important caveat, however, is that HSV infection is lytic, known to induce DC apoptosis 24 and liberates many apoptotic cell particles that may travel as free material into the LN. Antigens from non-lytic viruses such as lentiviruses are directly presented by skin-derived DC and not CD8α+ DC 25.
Generation of three LC-deficient mouse models
A major obstacle to examining the functional requirement for LC (and other DC) in skin immunity was the absence of mice with a selective LC deficiency. Unlike T or B cells, where ablation of a single gene can lead to the absence of an entire cell type, an equivalent gene for LC had not been identified. Thus the discovery of Langerin, a C-type lectin expressed by LC that is responsible for the generation of Birbeck granules, which are the ultrastructural hallmark of LC, was met with great excitement 26. Although Langerin is dispensable for LC development 27, the identification of a gene that is selectively expressed by LC proved quite valuable and was exploited by our three groups (B.C.: Dutch group; A.K.: French group; D.K.: American group) to direct expression of either the diphtheria toxin (DT) receptor (DTR) or subunit A of DT itself (DTA) to LC in an effort to generate mice with a selective deficit of LC.
DT is a heterodimeric protein. Binding of the B subunit to heparin-binding EGF-like growth factor (HB-EGF) precursor on the surface of mammalian cells facilitates entry of DTA into the cell. Once inside the cell, DTA inhibits protein biosynthesis and induces rapid cell death. The rodent homologue of HB-EGF precursor does not bind to the B subunit of DT, which prevents internalization of DTA and renders mice resistant to DT 28. Transgenic expression of the high-affinity human HB-EGF precursor, henceforth referred to as DTR, in a particular murine cell type such as LC makes these cells sensitive to DT and enables their inducible depletion in vivo after exogenous introduction of DT.
To achieve inducible ablation of LC, two of our groups generated knock-in mice of the human DTR into the langerin locus (Langerin–DTR–EGFP). The Dutch group targeted a DTR–EGFP cassette into the second exon of the langerin gene 29. The French group generated two separate lines in which either an IRES–EGFP or an IRES–DTR–EGFP cassette was knocked-in to the sixth exon, in the 3′-untranslated region of langerin 30. In F1 mice, LC were sensitive to DT and expressed high levels of EGFP. In both the Dutch and French mice, epidermal LC are efficiently depleted within 24–48 h following a single systemic (i.p.) injection of DT. Repopulation of the depleted epidermis is slow with patches of LC starting to reappear only at 2–4 wk post-DT injection. Although langerin was thought to be expressed only by epidermal LC, a subset of CD8α+ DC found throughout the secondary lymphoid tissues (LN, spleen and thymus) also express Langerin and are ablated after DT administration 30.
Our third (American) group used an alternative approach and generated bacterial artificial chromosome (BAC)-transgenic mice by inserting DTA (not DTR) into the 3′-untranslated region of the langerin gene contained within a 70-kb fragment of human genomic BAC DNA 31. Since DTA is expressed coordinately with Langerin, the resulting Langerin-DTA (American) mice have a constitutive and durable absence of LC. Interestingly, unlike in the Langerin–DTR–EGFP mice, only epidermal LC and no other Langerin+ DC are ablated in the DTA mice. This presumably reflects altered regulation of expression of the human langerin promoter compared with the endogenous mouse promoter driving DTR expression in the Dutch and French mice.
Contact hypersensitivity in the absence of LC
Contact hypersensitivity (CHS) to epicutaneously applied hapten is a classic assay for examining cutaneous adaptive immunity. In this assay, mice are sensitized by the application of hapten on shaved abdominal skin and are then challenged 5 days later by painting the same hapten onto the ear (Fig. 1). The degree of inflammation is quantified by the amount of ear swelling that develops. The challenge measures the extent of anti-hapten effector T-cell responses and not memory responses 32. Prevailing views of LC function in CHS would predict that LC-deficient mice would exhibit an absent or greatly diminished CHS response 33, 34.
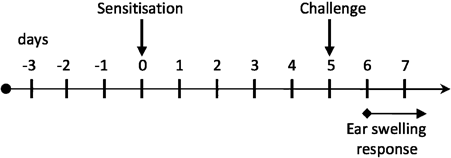
Schematic of the sensitization process.
To determine the functional requirement for LC, each of our three groups examined CHS responses in the absence of LC (Table 1). When the French Langerin–DTR–EGFP mice were sensitized and challenged using a standard dose of dinitrofluorobenzene (DNFB), there was no difference in ear swelling between mice treated with DT 3 days prior to and 1 day after sensitization and controls 30. This suggested that LC are dispensable for the induction of CHS responses. In contrast, Dutch Langerin–DTR–EGFP mice treated with DT 3 days prior to sensitization developed diminished but not absent CHS in response to a standard dose of another hapten, trinitrochlorobenzene, suggesting that LC are required for optimal initiation of CHS responses 29. Finally, the American Langerin–DTA mice, constitutively lacking LC, developed enhanced CHS responses compared with littermate controls to both DNFB and a third hapten, oxazalone 31. This was interpreted as showing that LC have a regulatory role and limit CHS responses. The altered CHS responses observed in the Dutch and American mice were antigen-specific and required the absence of LC during the sensitization and not challenge phases 30, 31, 35. Thus, rather than establishing a precise role for LC, there were divergent results for CHS (no change, decreased and increased) in three independent models of LC deficiency.
| DT (day)b) | Mouse line | LCc) | Langerin+ dDCc) | Hapten | CHS response |
|---|---|---|---|---|---|
| n.a. | Langerin–DTA [31] | − | +++ | DNFB | Enhanced [31] |
| oxazalone | |||||
| FITC | |||||
| −4 and −1 | Langerin–DTR–EGFP (F) [30] | − | − | DNFB | Reduced [36, 37] |
| −3 and 1 | Langerin–DTR–EGFP (F) [30] | − | ± | DNFB | Normal [30, 37] |
| −3 | Langerin–DTR–EGFP (NL) [29] | − | ± | Trinitrochlorobenzene | Reduced [29, 35] |
| oxazalone, low dose | |||||
| −14, −10 or −7 | Langerin–DTR–EGFP (F) [30] | − | + | DNFB | Normal [36] |
| FITC | Normal (C. Hauser, unpublished data) | ||||
| −28 | Langerin–DTR–EGFP (NL) [29] | ± | ++ | Oxazalone, low dose | Reduced [35] |
- a) a)The flanks of mice were sensitized to hapten at day 0 and challenged with hapten at day 5. CHS response (ear swelling) was monitored 24–48 h following challenge.
- b) b)Dutch (NL) and French (F) inducible ablation models were treated with DT at varying time points (days) prior to and after sensitization. Langerin–DTA mice exhibit constitutive LC ablation and therefore do not require DT-induced ablation; n.a., not administered.
- c) c)Number of cells at the time of sensitization.
Since the French and Dutch mice were generated using an almost identical strategy, it is particularly surprising that CHS responses differ between the two. It is likely that rather than intrinsic differences between the two mouse lines, differences in CHS experimental technique account for the different results. The choice of hapten does not seem to be critical since the Dutch mice also have reduced CHS to oxazalone 35. Rather, it appears that the timing of DT administration can affect the outcome since French mice treated with DT only on day −1 develop diminished CHS responses similar to the Dutch mice 36, 37. Thus, even though the mice have not been compared side-by-side in the same experiment and minor differences may still exist, it appears that the two Langerin–DTR lines behave similarly when examined using a comparable protocol.
Unlike the French and Dutch mice, the American mice were developed using a significantly different approach that could explain the dissimilar CHS results. Owing to the constitutive ablation of LC throughout ontogeny, enhanced CHS may result from the chronic absence of LC, which may potentially affect the development of peripheral tolerance as predicted by the LC paradigm. Another difference is that only epidermal LC and not other Langerin+ cells are ablated in the American mice, a finding that is further discussed below.
Langerin is expressed by DC other than LC
Over the last 3 years it has become increasingly clear that Langerin is not a specific marker for LC and that it is expressed by other non-epidermal DC subsets. Utilizing Langerin–EGFP knock-in mice it became apparent that the majority of blood-derived CD8+ DC in the thymus, spleen, cutaneous LN (CLN) and mesenteric LN also express lower levels of Langerin, depending on the genetic background (Table 2) 30, 38, 39. Langerin+ CD8− (CD103+CD11b−) DC have also been described in the lung specifically localized at the basal lamina of the bronchial epithelia and arterioles of the lung 40.
| Epidermal LC | Langerin+ dDC | Langerin− dDC | CD8+ Langerin+ DC | |
|---|---|---|---|---|
| Tissue of residence | skin, epidermis | skin, dermis | skin, dermis | LN, spleen, thymus |
| Langerin expression | +++ | +++ | − | + |
| CD8 | − | − | − | + |
| CD103 | − | + | − | n.d. |
| CD11b | + | − | + | n.d. |
| Ep-Cam | + | − | − | n.d. |
| Origin in chimeras | host | donor | donor | donor |
| Depletion in Langerin–DTR mice | yes | yes | no | yes |
| Duration of depletion in Langerin–DTR mice | weeks | days | n.a. | days |
| Depletion in Langerin–DTA mice | yes | no | no | no |
| Duration of depletion in Langerin–DTA mice | constitutive | n.a. | n.a. | n.a. |
- a) a)See text for references; n.d., not determined; n.a., not affected.
Initial studies with the Dutch and French Langerin–DTR–EGFP models demonstrated that DT treatment efficiently ablated LC in the epidermis, as well as LC and blood-derived CD8+ Langerinlo DC in the CLN. Contrary to expectation it was observed that the reconstitution of Langerin+ DC populations in CLN preceded the reconstitution of LC in the epidermis by a couple of weeks and reappeared with similar kinetics to the blood-derived CD8+ Langerinlo DC 30, 35. This suggested that either LC were capable of homing to CLN without trafficking through the epidermis or that another Langerin+ DC subset existed. Although Langerin+ MHC class II+ DC in the dermis had already been observed, they were always assumed to be transitory LC migrating from the epidermis to the draining LN 30, 35. Utilizing the three available mouse models (French, American and Dutch mice) and a variety of techniques, three groups were able to demonstrate the presence of a radio-sensitive Langerin-expressing dDC subset, which is independent of epidermal LC. This observation has important implications for the interpretation of previous functional studies on LC using the Langerin–DTR–EGFP ablation models.
Langerin+ dermal DC
Recently, three groups described the existence of a novel Langerin+ DC subset in the dermis that is independent of ‘classical’ LC of the epidermis 36, 41, 42. Bone marrow (BM) reconstitution studies with either congenic strains (CD45.1 BM into CD45.2 recipients or CD45.1 BM into Langerin–DTR–EGFP recipients) demonstrated that a Langerin+ skin-derived DC subset is capable of reconstituting both the dermis and CLN of host mice while the epidermal LC remain of recipient origin 36, 41, 42. This suggests that Langerin+ dDC and LC originate from two distinct developmental pathways. Furthermore, DT ablation studies in Langerin–DTR–EGFP versus CD45.1 parabiotics demonstrated that 18 days following DT administration, the epidermis of parabiotized Langerin–DTR–EGFP mice remained LC-free but both the dermis and CLN of Langerin–DTR–EGFP mice contained Langerin+ dDC 41. Bursch et al. 36 took advantage of the Langerin–DTA model, which displays constitutive ablation of LC in the epidermis and CLN from birth but exhibits normal numbers of other Langerin+ DC subsets. By crossing the Langerin–DTA with the Langerin–EGFP model they unequivocally demonstrate that Langerin+ CD8− DC can be found in both the skin dermis and CLN in a LC-free background. Together these studies demonstrate the true existence of a Langerin+ dDC subset.
During steady state, Langerin+ dDC originate from a radio-sensitive blood-derived precursor that is recruited to the dermis and can migrate to CLN independently of LC. Parallel analysis of skin and CLN of DT-treated BM chimeras (Langerin–DTR–EGFP BM into CD45.1 recipients) found that Langerin+ dDC reappear in the skin 4 days following DT treatment and with a slight lag in the CLN, as would be expected, demonstrating that this novel DC subset is capable of replenishing their tissue ‘niche’ significantly faster than LC of the epidermis 36, 42. Furthermore, Langerin+ dDC and Langerin− dDC exhibit significantly accelerated BrdU incorporation (50% have divided during the 7 days of BrdU administration) compared with significantly lower incorporation of BrdU in LC 42.
Although Langerin+ dDC are phenotypically very similar to LC in expression levels of maturation and co-stimulatory molecules, they can be distinguished from LC using CD103 (integrin-αIEL chain), CD11b (integrin-αM) and the adhesion molecule Ep-CAM (gp40) (Table 2) 36. Langerin+ dDC are CD11blo, CD103+, Ep-CAMlo, while LC are CD11bhi, CD103–, Ep-CAMhi. Both cell types maintain their phenotype in the skin and CLN. Interestingly, Langerin+ dDC are phenotypically very similar to Langerin+ DC populations found in the lung, and it is yet unclear whether these two populations are mutually exclusive or together constitute a Langerin+ interstitial DC subset 36, 40. Recent experiments demonstrate that clearance of influenza virus from the lung depends on this migratory Langerin+ DC subset 43.
Function of Langerin+ dermal DC versus LC in CHS
Langerin+ dDC can capture, process and present antigen to effector cells in the CLN. OVA peptide-pulsed Langerin+ dDC are capable of presenting antigen peptide and stimulating T-cell proliferation 36. Ginhoux et al. 41 took advantage of the YAe monoclonal antibody, which recognizes an MHC class II I-E-derived peptide presented in the context of MHC class II I-Ab. They generated chimeras using F1 (C57BL/6 I-Ab–/– × BALB/c I-Ed+) mice reconstituted with C57BL/6 I-Ab+/+ BM; thus only donor cells and not LC would be capable of presenting I-Ed-derived peptide. LC in the skin did not stain for YAe, whereas both Langerin+ dDC and Langerin– dDC, and only donor-derived Langerin+ DC in the CLN stained positive for YAe.
As already mentioned, Langerin+ dDC- and CD8+ DC-expressing Langerin are deleted after DT administration into Langerin–DTR–EGFP mice. However, unlike epidermal LC, which do not repopulate the epidermis until at least several weeks after DT administration, these other Langerin+ DC begin to return within a few days and reach approximately 50% of their steady-state levels by 2 wk. This provided a time window in which to assess their relative roles in CHS. Using a standard dose of hapten, CHS responses are greatly diminished in Langerin–DTR–EGFP mice when DT is administered on day −1 before sensitization with DNFB. However, when mice are sensitized 7 or 13 days after DT treatment, at a time when only the epidermal LC remain absent, CHS responses have returned to normal 36. CHS experiments using a standard dose of FITC as a hapten give a similar result (Conrad Hauser, unpublished observations). From these studies, it appears that Langerin+ dDC but not LC are critical for the development of efficient CHS responses (Table 2).
These observations are apparently inconsistent with a study showing that the Dutch Langerin–DTR–EGFP mice sensitized with a low dose of oxazalone 4 wk after DT, when most LC are still absent but all other Langerin+ DC populations have largely recovered from the toxin treatment, exhibit reduced CHS responses 35. The most likely explanation to reconcile these deviating observations with the two inducible Langerin–DTR models is that the dose of hapten is important. At standard doses of hapten sensitization, antigen is delivered to both the LC in the epidermis as well as DC in the dermis. When both DC populations acquire the antigen, presentation by LC is redundant for the development of a response. However, when antigen is limiting and preferentially acquired by LC, then their absence leads to diminished CHS.
The identification of a novel population of dDC that are eliminated in Langerin–DTR–EGFP mice but not in Langerin–DTA mice most likely explains why Langerin–DTA mice do not develop decreased CHS responses and partially reconciles the discordant findings between the LC-deficient models. However, Langerin–DTR–EGFP mice treated with DT 7 or 13 days (or 4 wk) before sensitization when they still lack all or most epidermal LC do not display the enhanced CHS observed in Langerin–DTA mice. If LC are regulatory, then their selective absence should lead to enhanced CHS similar to that observed in the Langerin–DTA mice. One interpretation is that the still reduced levels of dDC and/or CD8+ Langerin+ DC prevented what otherwise would have been an enhanced response in the absence of LC. Alternatively, the steady-state absence of epidermal LC in Langerin–DTA mice could inhibit the development of regulatory mechanisms such as the development of regulatory T cells and result in enhanced CHS.
Recently, it was shown in a minor-mismatch skin transplantation model using Langerin–DTA mice that the presence of LC in the donor skin is required for long-term graft acceptance 44. This supports the notion that LC are regulatory cells and is consistent with the observed enhancement of CHS responses in these mice. It is interesting to note that in this model, the regulatory capacity of LC can be transferred with the skin and is not dependent on cells in lymphoid tissue where regulatory T cells would be expected to reside.
Conclusions
The generation of three independently derived mouse strains that target LC has enabled the first functional examination of a discrete DC subset in vivo and led to a wealth of new insights. The availability of multiple LC-deficient models has enabled us to examine the role of LC from slightly different angles (Fig. 2, Table 1). From work with the two Langerin–DTR–EGFP mouse strains in the setting of an acute depletion of LC, it appears that when antigen is targeted preferentially to LC such as with low-dose hapten, then LC appear to be stimulatory and their absence leads to diminished CHS responses. However, when antigen is simultaneously targeted to LC and dDC such as in the setting of normal hapten doses, then LC appear largely redundant and CHS responses are mediated by dDC. This is consistent with a model in which all skin DC have similar function and the number that migrate to the CLN carrying antigen determines the ultimate outcome.
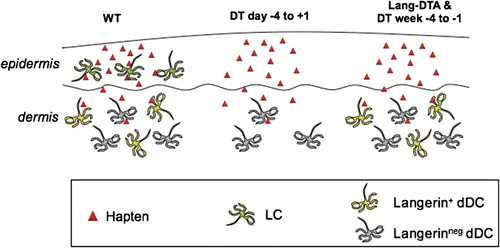
Absence of specific skin-resident DC subsets explains CHS responses in LC ablation models. (Left) In WT mice, all three populations of skin-resident DC (LC, Langerin+ dDC and Langerinneg dDC) are present and CHS responses to standard hapten doses are normal (see also Table 1). (Middle) When Langerin–DTR mice are treated with DT, LC, Langerin+ dDC and LN-resident Langerin+ DC (not depicted) are rapidly and efficiently eliminated, resulting in diminished CHS responses compared with WT mice irrespective of the hapten dose. Langerin+ dDC begin to repopulate the dermis 4 days after DT treatment and reach 50% of their steady-state level after 2 wk. (Right) Thus, when mice are sensitized 1–4 wk after DT administration, only LC will still be mostly absent, while Langerin+ dDC will have largely recovered. These mice develop normal CHS responses to standard hapten doses, indicating that LC are not required for CHS responses. Langerin+ dDC (and possibly Langerin– dDC as well) are required and presumably transport haptenated antigen to skin-draining LN where naive hapten-specific T cells can be activated. Unlike responses to standard hapten doses, CHS after sensitization with low hapten doses (not shown in this figure) are reduced at both early and late time points after DT administration. Thus, in situations of limited antigen, LC are required for efficient T-cell priming, probably due to reduced dissemination of antigen into the dermis. On the other hand, Langerin–DTA mice lack LC and have normal numbers of Langerin+ dDC analogous to Langerin–DTR mice 1–4 wk after DT administration. However, Langerin–DTA mice develop enhanced CHS responses. This discordance may result from the still somewhat reduced number of Langerin+ dDC in Langerin–DTR mice or from the constitutive absence of LC in Langerin–DTA mice.
In contrast, Langerin–DTA mice have a constitutive absence of only epidermal LC and not other Langerin+ DC. CHS responses and skin graft rejection are exaggerated in these mice. In this setting, dDC are presumably sufficient to mediate CHS/graft rejection but the absence of LC leads to a loss of regulation and an exaggerated response. These data are more consistent with a model in which LC have a unique function distinct from other skin DC. A central unresolved issue is whether it is the constitutive absence of LC and/or the selective absence of LC in the Langerin–DTA mice that leads to enhanced immune responses.
In an effort to sort out these functions, we are actively examining how LC might mediate their effects. We are in the process of systematically comparing CHS using high- and low-dose hapten in both Langerin–DTR and Langerin–DTA mice within the same laboratory to ensure consistent experimental technique. Generation of Langerin–DTA×Langerin–DTR–EGFP F1 animals will allow us to compare CHS responses after acute LC depletion with steady-state LC depletion within the same experiment. In addition, we have already generated BAC-transgenic mice with LC-specific expression of Cre recombinase 45. We are breeding them to a number of mice with ‘floxed’ genes of interest and plan to use them to elucidate which genes participate in LC-mediated immune regulation. To complement this model, we are also in the process of generating mice in which Cre is ‘knocked-in’ to the murine langerin locus, which we expect to result in Cre expression in all Langerin+ DC subsets. We anticipate that these new efforts will provide further insight into the functional role of LC in cutaneous immunity.
Acknowledgements
We would like to thank our laboratory members for their continuous support and hard work and wish to apologize to any colleagues whose important contributions we were unable to cite due to space limitations. D. H. K. is supported by the Al Zelickson professorship in dermatology and by NIH grant K08 AR651092. B. E. C. is a fellow of the Landsteiner Foundation for Blood Transfusion Research (LSBR, grant 0414F) and a VIDI fellow of The Netherlands Organization for Scientific Research (NWO, grant 917-76-365). A. K. is supported by a Lectureship at QUB and the CCRCB, and the discussed work was funded by INSERM, CNRS, Fondation pour la Recherche Medicale (Defis de la Recherche en Allergie), ARC, and the European Communities (MUGEN Network of Excellence).
Conflict of interest: The authors declare no financial or commercial conflict of interest.




