Induction and protective role of antibodies in Legionella pneumophila infection
Abstract
Legionella pneumophila (Lpn) is a ubiquitous Gram-negative bacterium found in aquatic environments and is the causative agent of Legionnaires’ disease, a severe form of pneumonia. We have used Lpn-permissive A/J mice as a model to analyze the B cell response upon intravenous (i.v.) and intranasal (i.n.) infection with Lpn. A strong antibody (Ab) response was observed upon i.v. infection with wild-type (WT) Lpn and an icmT mutant strain, which is unable to replicate within permissive host cells. In contrast to i.v. infection, only WT but not icmT mutant Lpn was able to induce specific Ab responses upon i.n. infection. After primary i.n. infection with WT Lpn, a strict compartmentalization of Lpn-specific Ab isotypes was observed, as IgG was found exclusively systemically, while IgA was detectable only locally in the lung. Regardless of the infection route, isotype switching to IgG and to IgA was strictly dependent on CD4+ T cells, whereas IgM production was completely Th-independent. Finally, we analyzed the protective capacity of the Lpn-specific Ab response. Actively or passively immunized mice or mice that were infected with opsonized Lpn had 50–100-fold reduced bacterial titers compared to naive animals, clearly demonstrating the capacity of Ab to protect against infection with Lpn.
Abbreviations:
-
- Lpn:
-
Legionella pneumophila
-
- T4SS:
-
type IV secretion system
Introduction
The Gram-negative bacterium Legionella pneumophila (Lpn) is ubiquitously found in aquatic environments as an intracellular pathogen of amoebae as well as in biofilms 1–4. Lpn is the etiological agent of Legionnaires’ disease, a severe form of pneumonia 5. Humans are infected through inhalation of contaminated aerosols, which allow the bacteria to enter the lung and to replicate inside alveolar macrophages. Lpn replication leads to extensive leukocyte infiltration causing considerable, potentially fatal immunopathological tissue damage to the lung, in particular in elderly people and in individuals with reduced immunocompetence 4, 6.
The interactions of Lpn with macrophages is governed by the Icm/Dot type IV secretion system (T4SS), which promotes phagocytosis 7 and the formation of a replication-permissive, endoplasmic reticulum-derived “Legionella-containing vacuole” 8, 9. More than 40 Icm/Dot-secreted “effector” proteins have been identified, some of which modulate vesicle trafficking by subverting host cell GTPases or phosphoinositide metabolism 10–13.
In vitro studies using thioglycollate-elicited peritoneal or BM-derived macrophages have shown that most commonly used mouse strains are poorly or non-permissive for Lpn replication 14–16. Recent studies have shown that the NOD-LLR family protein Birc1e/Naip5 is involved in sensing Lpn flagellin. Engagement of Birc1e/Naip5 in macrophages leads to restriction of Lpn replication and initiation of proinflammatory cell death 17–19. A/J mice carry a natural mutation in the Birc1e/Naip5 locus rendering them more permissive for Lpn replication 20, 21. Therefore, this mouse strain is most commonly used as an in vivo model for Lpn infection 22.
Innate immunity, mainly through early and pronounced IFN-γ production by NK cells, was shown to be crucial for the initial control of Lpn 23, 24. However, early studies also documented cell-mediated and humoral immune responses upon Lpn infection, as indicated by seroconversion of patients who recovered from Legionnaires’ disease 25. Furthermore, a protective role of adaptive immune responses was shown in guinea pigs that had been vaccinated with (i) an avirulent strain of Lpn, (ii) the major secretory protease (Msp), (iii) the outer membrane protein OmpS, or (iv) the heat shock protein Hsp60. These vaccinations resulted in protection from lethal challenge with Lpn 26–29. Horwitz and co-workers speculated that T cells are involved in the protective mechanism. However, this was not formally shown, and all vaccination protocols mentioned above induced both Lpn-specific B as well as T cell responses. Additionally, results from vaccination studies using Lpn-loaded dendritic cells for immunization demonstrated that B cell-deficient mice were as susceptible to lethal Lpn challenge as CD4- or CD8-deficient mice 30. Furthermore, depletion of CD4+ or CD8+ T cells led to increased lethality when mice were challenged with very high doses of Lpn (LD50 or higher). These results suggest a role for cell-mediated immunity also in the control of primary infection 31.
In vitro studies with human monocytes suggested that antibodies (Ab) enhance uptake of Lpn into phagocytes but do not inhibit intracellular replication of the bacteria 32–34. Opsonization of Lpn with a mAb directed against LPS and subsequent infection of mice resulted in a decrease in intrapulmonary growth of the bacteria 35. However, the role of Ab in a physiological setting remains unclear.
Here, we characterized in detail the Lpn-specific B cell response after intravenous (i.v.) or intranasal (i.n.) Lpn infection and investigated the role of the induced polyclonal Ab pool in protection from re-infection with Lpn. In the present study, we have exclusively used the permissive A/J mice as a model. Our results demonstrate that i.v. as well as i.n. infection with Lpn leads to a strong Lpn-specific B cell response, which is accelerated and strongly increased after secondary infection. While T4SS-deficient Lpn induce Ab titers comparable to those induced by WT Lpn when administered i.v., the T4SS is indispensable for the induction of a B cell response via the i.n. infection route. In primary i.n. infections a strict compartmentalization of Ab isotypes was observed, as IgA was exclusively found in the bronchio-alveolar lavage fluid (BALF) while IgG was only detectable in the serum. We further demonstrate that the Lpn-specific IgM response is T cell independent, while isotype switching to IgG as well as to IgA is dependent on T cell help, regardless of the route of infection. Finally, we show that active immunization or passive immunization with polyclonal Ab leads to a strong reduction of the bacterial burden, indicating that polyclonal Ab confer protection from re-infection with Lpn.
Results
Antibody response upon intravenous infection with wild-type and T4SS mutant Lpn
The Icm/Dot T4SS plays an indispensable role in Lpn infections, as T4SS-deficient strains are incapable of establishing a replication-permissive vacuole and are degraded by the host cell via the phagolysosomal pathway. Upon infection with T4SS-deficient Lpn the innate immune response is virtually absent, as shown by the lack of neutrophil influx and inflammatory cytokines 23. In view of these profound differences of innate immune activation, we analyzed the induction of the Ab response generated upon infection with the WT Lpn strain JR32 or with the icmT mutant Lpn strain ΔT, lacking a functional Icm/Dot T4SS.
Surprisingly, Lpn-specific IgM and IgG Ab titers induced by WT or ΔT Lpn were comparable (Fig. 1A). In fact, the IgM response, peaking on day 7, was even slightly higher in Lpn ΔT-infected mice, while the generation of Lpn-specific IgG was slightly delayed. However, the serum IgG plateau levels reached by day 14 post infection were comparable in Lpn WT and ΔT-infected animals and were sustained for at least 30 days. In both settings, the Ab generated were highly specific for Lpn, as they were not cross-reactive with Escherichia coli, another Gram-negative bacterium (not shown).
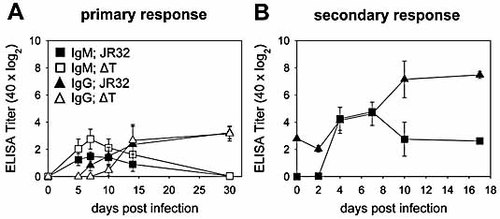
Antibody titers after intravenous Lpn infection. Lpn-specific IgM (squares) and IgG (triangles) titers in sera were determined by ELISA. (A) To measure the primary Ab response, mice were infected with Lpn JR32 (filled symbols) or Lpn ΔT (open symbols). (B) To measure the secondary Ab response, mice were primed i.v. with Lpn JR32 and subsequently re-infected with Lpn JR32 at day 32 after primary infection. Ab titers are presented as log2 of 40-fold prediluted sera and represent half-maximal OD. Results are representative of three (A) or two (B) independent experiments (four animals/group).
Secondary infection 32 days after priming with Lpn WT led to a strong increase of Lpn-specific IgG (10–20-fold) over the first 10 days after re-infection, which was maintained at a high level thereafter (Fig. 1B).
In order to analyze the effect of the early inflammatory response on the Ab isotypes generated, we measured the IgG subclasses induced after i.v. infection with Lpn WT and ΔT. While the levels of Lpn-specific IgG1 were similar in WT and ΔT-infected mice, IgG2b and IgG3 titers were found to be increased in ΔT-infected mice, while the amount of Lpn-specific IgG2a generated was lower than in WT Lpn-infected mice (Fig. 2). As IgG2a production is strongly enhanced by IFN-γ 36, the reduced levels of Lpn-specific IgG2a correlated with the lack of an early IFN-γ-dominated inflammatory response upon infection with T4SS-deficient Lpn 23.
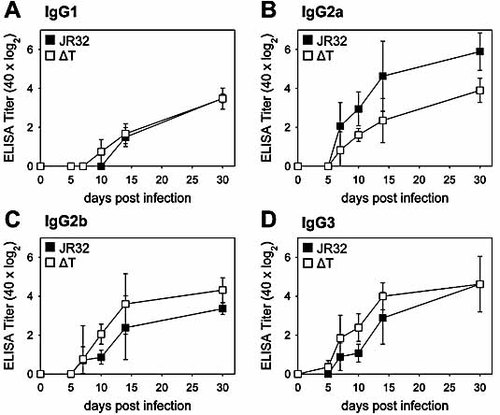
Infection with WT or T4SS mutant Lpn generates distinct levels of IgG subclasses. Mice were infected with Lpn JR32 (filled symbols) or Lpn ΔT (open symbols). Ab titers are presented as log2 of 40-fold prediluted sera and represent half-maximal OD. Titers for Lpn-specific IgG1 (A), IgG2a (B), IgG2b (C), and IgG3 (D) are shown. ELISA plates were coated with Lpn WT bacteria. Results are representative of two independent experiments (four animals/group).
Antibody responses are only induced after intranasal infection with wild-type Lpn
As the natural route of Lpn infection in humans is via inhalation, we characterized the Ab response upon i.n. infection and compared it with the results of the i.v. infection. While i.n. infection with WT Lpn resulted in a robust Lpn-specific Ab response, i.n. infection with Lpn ΔT did not induce detectable levels of Lpn-specific IgM or IgG, neither in the serum nor in the BALF (Fig. 3A). Upon WT Lpn infection IgM titers in serum reached similar levels as during i.v. infection and peaked on day 7. Similar IgM kinetics were observed in the BALF. Interestingly, upon infection via the i.n. route, a strict compartmentalization of isotype-switched Lpn-specific Ab isotypes was observed. IgG was only found systemically, while IgA was exclusively found locally in the lung. IgA was readily detectable in the BALF as early as day 7 post infection and reached maximal levels by day 14. In contrast, IgG in the serum was detectable only very late (day 30) and at low levels.
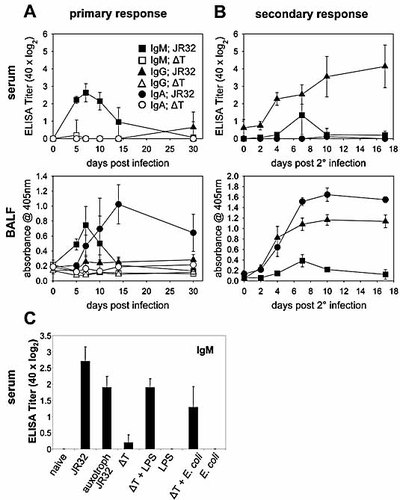
Antibody titers after intranasal Lpn infection. Lpn-specific Ab titers in serum and BALF were analyzed by ELISA. Serum titers were determined as in Fig. 1. (A and B) IgM (squares), IgG (triangles), and IgA (circles) levels are depicted. (A) To measure the primary Ab response, mice were infected i.n. with Lpn JR32 (filled symbols) or Lpn ΔT (open symbols). (B) To measure the secondary Ab response, mice were primed i.n. with Lpn JR32 and re-infected with Lpn JR32 at day 32. (C) Mice were infected i.n. with Lpn JR32, thymidine-auxotroph Lpn JR32, Lpn ΔT, or Lpn ΔT together with E. coli LPS or E. coli bacteria. Lpn-specific IgM titers were determined in serum on day 7 post infection. Results are representative of two independent experiments (four (A and B) or three (C) animals/group).
Secondary infection 32 days after primary infection via the i.n. route resulted in highly elevated serum IgG levels, which increased throughout the time period analyzed. Similarly, IgA levels in the BALF showed characteristics of a memory response, increasing approximately sixfold within 7 days after boosting and then remained at constant levels (Fig. 3B). Interestingly, substantial levels of IgG could now be detected in the BALF as well, while IgA was produced exclusively in the lung, as observed in primary infections.
Next we addressed the question whether the lack of Lpn-specific Ab in mice infected intranasally with Lpn ΔT was due to a limiting amount of antigen produced by the non-replicating strain or due to the lack of inflammation in this setting. We tested this by infecting mice i.n. with thymidine-auxotroph Lpn, which are able to induce an inflammatory response 23 but depend on an exogenous thymidine source for replication. Additionally, we infected mice with Lpn ΔT and deliberately induced inflammation by co-administration of E. coli LPS or E. coli bacteria. On day 7 post infection, Lpn-specific IgM was readily detectable in the serum of mice infected with thymidine-auxotroph WT Lpn or with Lpn ΔT if administered together with an inflammatory stimulus (Fig. 3C). These data indicate that intracellular replication and thus an increased bacterial load is not required for the induction of antibody responses as replication-defective thymidine-auxotroph Lpn readily induced IgM responses. However, the level of inflammation during primary infection seems to be relevant for the efficient induction of antibody responses, as co-administration of inflammatory stimuli with Lpn ΔT led to induction of specific IgM responses.
Wild-type Lpn infection generates higher numbers of Lpn-specific memory B cells
Both i.v. and i.n. infection with WT Lpn lead to an accelerated and increased Ab response in secondary infections, characteristic for a memory response. We therefore wanted to quantify the Lpn-specific memory B cells generated after primary Lpn infection by flow cytometry 37. Positive gating on B cells using the B220 marker (or the CD19 marker, not shown) and exclusion of IgM- and IgD-expressing naive B cells allows for the identification of isotype-switched B cells, representing the memory B cell pool (Fig. 4A). In addition, cells were stained for their Lpn-binding capacity in order to quantify the Lpn-specific memory B cells and for IgG to identify the isotypes represented in this population.
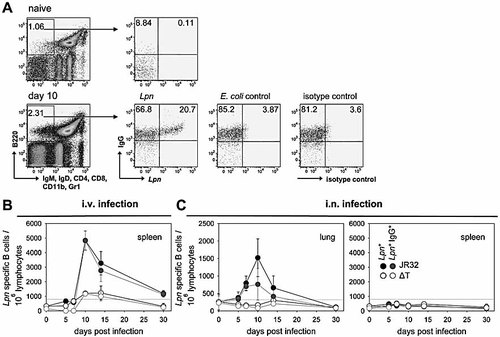
Induction of memory B cells. (A) Memory B cells were quantified using flow cytometry by gating on B220+ live lymphocytes that were IgM– and IgD– (for the exclusion of naïve B cells) and Gr-1–, CD11b–, CD4–, CD8– (for the exclusion of non-B cells). Additionally, cells were stained for IgG and binding to whole Lpn. Representative stainings of splenocytes from naive and immunized mice, as well as control stainings are shown (A). The numbers of Lpn-specific memory B cells were determined after i.v. (B) and i.n. (C) infection with Lpn JR32 or ΔT (filled and open symbols, respectively). Black symbols represent total numbers of Lpn-specific memory B cells and grey symbols depict IgG+ cells within this population. The background as determined by the isotype control (dotted line in B and C) is indicated. Results are representative of two independent experiments (four animals/group).
A sharp increase in the number of memory B cells was observed between day 7 and day 10 post infection. At this time point, up to 5000 Lpn-specific memory B cells per 106 lymphocytes were detectable in the spleen following i.v. infection (Fig. 4B). Thereafter, the numbers of memory B cells steadily decreased to barely above background level by day 30 after infection. As expected, virtually all Lpn-specific memory B cells stained positive for IgG, correlating with the Ab isotype found in serum. Surprisingly, Lpn ΔT infection induced only low numbers of memory B cells (Fig. 4B), despite Ab levels after primary infection with Lpn ΔT being comparable to those detected after Lpn WT infection (Fig. 1).
Analysis of the Lpn-specific memory B cell numbers in the lung after i.n. infection also showed a peak on day 10 post infection and a decrease to background levels by day 30 (Fig. 4C). As no Lpn-specific Ab could be measured after i.n. infection with Lpn ΔT, the lack of detectable memory B cells in this setting was expected. Although only Lpn-specific IgA but no IgG was detected in the BALF after primary infection (Fig. 3A), approximately half of the memory B cells in the lung expressed the IgG isotype. This could account for the high levels of IgG found in the BALF after secondary infection (Fig. 3B). While low levels of IgG were detected in serum after i.n. infection with Lpn (Fig. 3A), memory B cells in the spleen did not rise above background level throughout the experiment (Fig. 4C).
Isotype switching upon Lpn infection is dependent on T cell help
While isotype switching to IgG generally depends on helper T cells, IgA can be generated in the intestinal mucosa in the absence of T cell help 38, 39. Moreover, specific IgA has been reported in T cell-deficient mice following rotavirus infection 40 and in response to (neo-)antigens expressed by commensal bacteria 41. To test whether the IgA generated upon Lpn infection is produced via a T-independent mechanism, we analyzed the generation of Lpn-specific IgM, IgG, and IgA responses in the presence or absence of helper T cells. Mice were depleted of CD4+ T cells by i.p. injection of a depleting anti-CD4 Ab prior to infection, followed by weekly administrations throughout the duration of the experiment. Flow cytometric analysis of cell types present in blood at regular intervals confirmed the absence of CD4+ T cells during the time of analysis (not shown).
As expected, the IgM response upon i.v. or i.n. Lpn infection in CD4-depleted mice was comparable to that of untreated animals, indicating that this response was entirely Th cell-independent (Fig. 5, upper panels). However, Th cells were indispensable for isotype switching to IgG in serum (i.v. and i.n. infection), as well as to IgA in the BALF (i.n. infection; Fig. 5, lower panels).
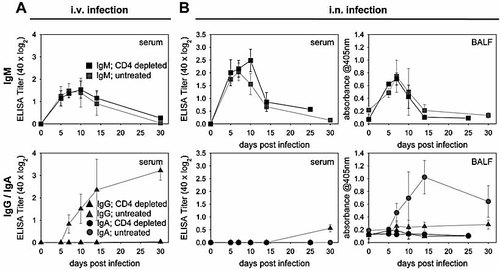
Isotype switching upon Lpn infection is dependent on CD4+ T cells. Mice were injected i.p. with a CD4-depleting Ab at day -3 and day -1 and weekly thereafter to ensure absence of CD4+ T cells throughout the experiment. At day 0, the mice were infected i.v. (A) or i.n. (B) with Lpn JR32. Lpn-specific IgM (squares), IgG (triangles), and IgA (circles) titers in serum and BALF were determined by ELISA. Results are representative of two (A) or one (B) independent experiments (four animals/group).
Antibodies protect mice from re-infection with Lpn
As Ab have been shown to be important for protection against infection with several pathogenic intracellular bacteria such as Mycobacterium tuberculosis and Listeria monocytogenes 42, 43, we investigated their role in pathogen control upon Lpn challenge. Mice were vaccinated twice (at day -64 and day -32) i.v. or i.n. with WT Lpn before they were challenged via the same route. The determination of bacterial titers 2 days after the challenge revealed a 10–100-fold reduction in CFU in vaccinated mice compared to the naïve controls (Fig. 6A and C). In order to determine whether this effect was mediated by Ab, or if other components of the immune system were involved, mice were passively immunized by i.v. transfer of 200 µL hyperimmune serum 1 and 2 days before i.v. challenge. When bacterial titers were determined in spleens 2 days after infection, mice that had received Ab showed a marked reduction in CFU, reaching a similar protection level to that observed in vaccinated mice (Fig. 6A). To corroborate this finding in the i.n. setting, Lpn opsonized with Ab from the BALF of immune mice were used for the challenge. As Ab-opsonized bacteria might activate the complement cascade, their viability might be reduced. However, Lpn have been reported to be resistant to complement mediated lysis 34, 44 and should therefore not be affected by this treatment. To confirm this, PBS-treated and opsonized Lpn were plated on agar plates, and indeed, no differences in viability could be observed (Fig. 6B). When mice were challenged with opsonized Lpn, the bacterial load in the lung and in the BALF 2 days after challenge was reduced 200- or 50-fold, respectively, relative to the controls and was comparable to that found in vaccinated mice (Fig. 6C). Of note, if mice were vaccinated i.n. with Lpn ΔT, only marginal protection against WT Lpn was observed, as would be expected due to the poor induction of antibody responses in the lung after Lpn ΔT priming (not shown).
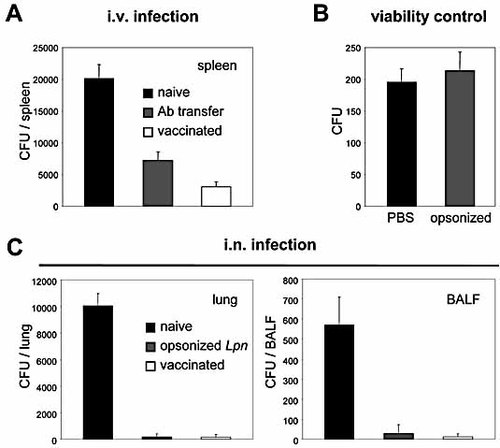
Antibodies protect mice from re-infection with Lpn. (A) Mice received 200 µL hyperimmune serum at day -2 and day -1 (Ab transfer, grey bar), were vaccinated twice with Lpn JR32 (day -64 and day -32, white bar), or were left naive (black bar). On day 0, the mice were challenged i.v. with Lpn JR32, and after 2 days CFU were determined in the spleen. (B) Lpn were opsonized with Ab from memory mice (opsonized, grey bar) or with PBS as a control (PBS, black bar), and 200 CFU were plated on agar plates. (C) Mice were vaccinated twice with Lpn JR32 (day -64 and day -32, white bars), or were left naive (white or grey bars). On day zero mice were challenged i.n. with opsonized Lpn JR32 (grey bars) or untreated Lpn (black or white bars) and CFU were determined in the lung and BALF 2 days later. Results are representative of one (A) or two (B and C) independent experiments (three animals/group).
Discussion
In this study, we have characterized the B cell response after i.v. and i.n. Lpn infection and show that Ab protect against re-infection with Lpn. Despite the lack of an early inflammatory response after infection with T4SS mutant Lpn 23, i.v. infection led to IgM and total IgG titers that were comparable to those induced by WT bacteria (Fig. 1). However, when IgG subclasses were analyzed, infection with Lpn ΔT resulted in reduced IgG2a titers (Fig. 2B). As IgG2a production is strongly enhanced by IFN-γ 36, the reduced levels are likely to be due to the lack of IFN-γ early on in the course of infection with T4SS-deficient Lpn 23. In addition, the number of Lpn-specific memory B cells in Lpn ΔT-infected mice was reduced compared to Lpn WT-infected mice (Fig. 4B).
In contrast to i.v. infection, the T4SS proved to be crucial for the induction of Lpn-specific Ab after i.n. infection (Fig. 3A). As T4SS-deficient Lpn are unable to replicate within the host cells, infection with T4SS-deficient Lpn is associated with a reduced antigen load compared to that found after infections with replication-competent WT Lpn. In addition and most importantly, infection with Lpn ΔT does not induce an inflammatory response, as measured by neutrophil influx and production of inflammatory cytokines 23. To test whether the differences in antigen load or the absence of an early inflammatory response were responsible for the absence of Lpn-specific Ab, we infected mice with thymidine-auxotroph Lpn or with Lpn ΔT together with an additional inflammatory stimulus. In both situations, Lpn-specific IgM was readily detectable on day 7 post i.n. infection (Fig. 3C). These results show that intracellular replication is not a prerequisite for the induction of antibody responses. They also show that the antigen dose used for infection with Lpn ΔT is not limiting, but rather that the level of inflammation reached during primary infection plays a crucial role in the induction of antibody responses in the lung.
Blander et al. 26 reported a strong humoral immune response after infection with mutant Lpn that fail to replicate within host cells when they immunized guinea pigs. However, the number of mutant bacteria used for these immunizations was 1000-fold higher than that of WT Lpn. As we cannot rule out that such high doses of bacteria induce inflammation despite the lack of a T4SS, these results could still support the necessity of inflammation for B cell priming.
One of the striking findings of our study was the strict compartmentalization of the Ab isotypes generated after i.n. Lpn infection. While i.n. infections with a number of different pathogens induce production of IgA as well as IgG in the BALF 45–47, primary infection with Lpn exclusively induces IgA (Fig. 3A). Amongst the Lpn-specific memory B cells in the lung, approximately half were IgG negative and are likely to express IgA (Fig. 4C). Due to the lack of a suitable Ab, we were unable to directly verify whether these cells indeed express the IgA isotype. Surprisingly, although Lpn-specific IgG Ab were absent in the BALF, approximately 50% of the Lpn-specific B cells in the lung tissue stained positive for IgG. Since B cells can switch to IgA sequentially via IgG2b, the Lpn-specific B cells could represent an intermediate stage in class switching to IgA. Alternatively, these cells could have switched to IgG+ memory cells without differentiation into Ab secreting cells upon primary Lpn infection and thus may account for the rapid appearance to relatively high IgG titers in the BALF after secondary infection with Lpn.
In addition, we show that CD4 T cell help is essential for isotype switching in i.v as well as in pulmonary infection (Fig. 5). While switching to IgG generally depends on helper T cells, previous studies showed that antigen-specific IgA in the intestinal mucosa can be generated in the absence of T cell help 40, 41. This T cell-independent IgA is mainly derived from the B1 B cell subset 48. In view of the similarities in isotype prevalence and organization of the mucosa-associated lymphoid tissues in the intestinal and the respiratory mucosa, it is possible that similar mechanisms exist in the lung. However, we show that in Lpn infection isotype switching to IgA is strictly T cell dependent.
In serum transfer and opsonization experiments we have shown that Ab protect against re-infection with Lpn, as indicated by the strong reduction in CFU (Fig. 6). Under our experimental conditions, bacteria are cleared from the lung or spleen of naïve mice within approximately 4 days after infection, while in immune mice they are only detectable until day 2 after infection. This short timeframe suggests that pre-formed Ab rather than memory B or T cell responses are responsible for the accelerated bacterial clearance in immune mice. This would also argue against a protective effect of T cells claimed as by Horwitz and Blander. In their studies, using a guinea pig model, they speculated that cell-mediated immunity to Lpn is crucial for protection 27, 28, 49, 50. However, they also reported the induction of a humoral response to Lpn and have not formally shown whether T cells or Ab conferred protection. Additionally, their animals received a lethal bacterial challenge dose, which was much higher than the one we used in this study. It is possible that at such high doses, additional components of the immune system other than preformed Ab become more important. Another study, using a mouse model, also claimed a role for T cells in protection from lethal Lpn challenge 31. However, in that intra-tracheal infection model Lpn is not cleared until day 10–11 post infection, while in our i.n. model mice had virtually cleared the bacteria by day 4. These differences could account for the discrepancies with our findings. Furthermore, Kikuchi and colleagues 30 showed that while vaccinated CD4- or CD8-deficient mice succumb to lethal challenge with Lpn, the same was observed for vaccinated B cell-deficient mice. Additionally, opsonization of Lpn with a mAb was shown to decrease the intrapulmonary growth of the bacteria in the first 3 days post infection 35, supporting the protective role of Ab. While we have not formally ruled out a role for T cells in the protection observed in immune mice, we nevertheless clearly show that Ab are sufficient to reduce the bacterial burden to a level that is comparable to that found in immune mice.
It is generally believed that Ab are important in infections with extracellular pathogens by limiting pathogen spread, while in intracellular infections the cell-mediated immunity plays a major role. However, an increasing number of studies have demonstrated a role for Ab in the protection from intracellular pathogens. In the case of Listeria monocytogenes, Ab taken up by host cells together with the bacteria neutralize the listeriolysin O protein and thus block the escape of L. monocytogenes from phagosomes into the cytoplasm 42. Other studies have shown a protective role of Ab in infections with M. tuberculosis, Shigella flexneri, Ehrlichia chaffensis, and other intracellular pathogens 43, 47, 51–53. However, the mechanism of protection is different for each pathogen. We hypothesize that in Lpn infection Ab can confer protection via two potential mechanisms. In the presence of specific Ab Lpn might be taken up preferentially by cells other than macrophages, wherein they fail to replicate efficiently. Alternatively, the presence of specific Ab could lead to uptake of Lpn via Fc receptors, which might interfere with the formation of the replication-permissive vacuole and cause Lpn to enter the phagolysosomal pathway leading to degradation by the host cell. Further studies will focus on elucidating the mechanism by which Ab confer protection against Lpn challenge and on identifying the major B cell antigens of Lpn.
In summary, we show that i.v. as well as i.n. infection with Lpn induces a specific Ab response, which is accelerated and strongly increased in secondary infections. In primary i.n. infection, a strict compartmentalization of Ab isotypes was observed, where IgA could only be detected in BALF, while IgG was only detectable in serum. In addition, we show that Lpn-specific IgM responses are T cell-independent, while isotype switching to IgG as well as to IgA is dependent on T cell help, regardless of the route of infection. Furthermore, we demonstrate that active immunization or passive immunization with Ab confers protection against re-infection with Lpn.
Materials and methods
Mice, bacteria, and immunizations
A/J mice were bred at RCC (Füllinsdorf, Switzerland) or Biosupport (Zurich-Schlieren, Switzerland) and were used at 7–20 weeks of age (sex- and age-matched within the experiments). All animal experiments were performed in accordance with institutional policies and have been reviewed by the cantonal veterinary office.
The Lpn strains used in this study were JR32 (WT Philadelphia-1) 54, GS3011 (icmT deletion mutant lacking a functional Icm/Dot T4SS; ΔT) 8, and thymidine-auxotroph derivatives of Lpn JR32 23. Prior to infections, Lpn was grown for 3 days at 37°C on charcoal yeast extract agar plates. For stainings of Lpn-specific B cells, the bacteria were incubated for 1 h at room temperature with 100 μg/mL gentamicin and then washed twice with PBS.
For i.v. infections Lpn were suspended in pre-warmed PBS, and mice were injected i.v. with 5 × 106 CFU Lpn JR32 or ΔT in a volume of 200 µL. In protection experiments, mice were immunized on days -64 and –32 and challenged on day zero with the same dose of Lpn JR32. For i.n. infections mice were anesthetized with isofluran, and 5 × 106 CFU Lpn JR32, ΔT, or thymidine-auxotroph Lpn JR32 in 20 µL pre-warmed PBS were directly applied to one nostril using a Gilson-type pipette. Where indicated, 107 CFU E. coli strain DH5α or 1 µg LPS were included in these 20 µL. For i.n. protection experiments, Lpn was opsonized by incubation for 1 h at room temperature with neat BALF from secondarily infected mice containing Lpn-specific IgG and IgA or with PBS as control. Mice were challenged i.n. with 5000 CFU Lpn JR32.
CD4+ T cells were depleted 1 and 3 days before infection by i.p. injection of 0.2 mg purified YTS 191.1 mAb and weekly administration thereafter 55. Depletion was analyzed by flow cytometry and was >99%.
ELISA
ELISA plates (PS Microplate 96-well, Greiner-Bio One, Frickenhausen, Germany) were coated with 4 × 106 CFU Lpn JR32 or ΔT per well in coating buffer (0.1 M NaHCO3, pH 9.6), and ELISA experiments were performed according to standard protocols using HRP-conjugated secondary Ab against mouse IgM, IgG, and IgA (Sigma-Aldrich Chemie, Buchs, Switzerland) or goat anti-IgG subclass Ab (Southern Biotech, Birmingham, AL, USA) followed by HRP-conjugated donkey anti-goat IgG (Abcam, Cambridge, UK). Plates were developed with 100 µL/well of a solution containing 0.2 mg/mL 2,2′-azino-di-(3 ethylbenzthiazoline sulfonic acid), 0.1 M NaH2PO4, 0.04% H2O2, pH 4; plates were read at 405 nm in a Victor3 reader (Wallac 1420, PerkinElmer, Waltham, MA, USA). All serum Ab titers are presented as log2 of 40-fold pre-diluted sera. Titers represent half-maximal OD.
To determine Ab titers in the lung, 1 mL PBS was used for the lavage, and 100 µL of the neat BALF was used in ELISA experiments. BALF samples from secondary infections were pre-diluted fourfold.
Cell preparations and flow cytometry
Single-cell suspensions of splenocytes were prepared by passing whole organs through a metal mesh using syringe plungers.
For the preparation of cells from the lung, mice were anaesthetized with 260 µL ketamine/xylacine/acepromacine mixture (2 mg ketamine; 0.4 mg xylacine; 60 µg acepromacine in PBS) by i.p. injection and the lungs were perfused via the right heart ventricle with approximately 5 mL PBS to remove circulating blood. Cells present in the lung lumen were removed by repeated flushing of the lumen with 1 mL PBS. The lungs were cut into small pieces and digested 2 × 20 min at 37°C with complete RPMI containing 2.4 mg/mL collagenase type I (Gibco, Invitrogen, Basel, Switzerland) and 0.2 mg/mL DNase I (Roche Diagnostics, Rotkreuz, Switzerland), and mononuclear cells were purified by gradient centrifugation over 30% Percoll. Complete RPMI consisted of RPMI (Invitrogen) with 10% FCS (Omnilab, Mettmenstetten, Switzerland), 2 mM L-Glutamine (Gibco), and 1% penicillin–streptomycin (Gibco).
Flow cytometric analysis of Lpn-specific B cells was based on techniques previously described 37. Single-cell suspensions of splenocytes or lung cells were incubated with gentamicin-treated Lpn JR32 or E. coli (strain DH5α) as controls, and Fc receptors were blocked with anti-mouse CD16/32 (2.4G2). The cells were then incubated with polyclonal rabbit anti-Lpn serum (m-TECH/Monoclonal Technologies, Alpharetta, USA) or an isotype control (rabbit polyclonal HA-probe, Santa Cruz Biotechnology, Santa Cruz, CA) and biotinylated goat anti-mouse IgG (Jackson ImmunoResearch Europe, Suffolk, UK), followed by Cy5-conjugated donkey anti-rabbit IgG (Jackson), PE-Cy7-conjugated anti-CD45R/B220 (BD Bioscience, Basel-Allschwil, Switzerland), a mixture of FITC-conjugated Ab (anti-IgD, anti-CD11b, anti-Gr-1, BD Bioscience; anti-CD4 (YTS191) and anti-CD8 (YTS156), self-made; anti-IgM, Jackson), and APC-Cy7-conjugated streptavidin (BD Bioscience). After staining, the cells were resuspended in 0.5 µg/mL propidium iodide to label and allow the exclusion of dead cells. All staining steps were performed for 20 min at 4°C in FACS buffer (PBS, 2% heat-inactivated FCS, 5 mM EDTA, and 0.02% sodium azide). The data were acquired with a LSRII flow cytometer (BD Bioscience) and analyzed using the FlowJo software (Treestar, San Carlos, CA).
Determination of bacterial titers
Organs were excised, homogenized using a TissueLyser (Qiagen, Hombrechtikon, Switzerland), and serial dilutions were plated on charcoal yeast extract agar plates. Bacteria in the BALF were extracted with 1 mL pre-warmed PBS and plated directly. Colonies were enumerated after 3 days of incubation at 37°C.
Acknowledgements
We thank Cytos Biotechnology AG (Zurich-Schlieren, Switzerland) for the kind support with animal breeding and Petra Wolint for technical assistance. We are grateful to Nicola Harris, Martin Bachman, Dominique Gatto, members of the Institute for Experimental Immunology at the University Hospital in Zürich, and members of the Hilbi and Oxenius Group for helpful discussions. This work was supported by the Roche Research Fund for Biology, the Bonizzi-Theler Stiftung, the GEBERT-RÜF-STIFTUNG, the Swiss National Science Foundation, the Vontobel Foundation, and by UBS AG on behalf of a client.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
Appendix
Conflict of interest: The authors declare no financial or commercial conflicts of interest.




