Monocyte-mediated T cell suppression by HIV-2 envelope proteins
Abstract
HIV-2 is associated with an attenuated form of HIV disease. We investigate here the immunosuppressive effects of the HIV-2 envelope protein, gp105. We found that gp105 suppresses activation of T cells through a monocyte-mediated mechanism. Suppression of T cell activation by gp105 depends on contact between monocytes and T cells, but not on CD4+CD25+ T cells. The TLR4 pathway is likely involved, since gp105 activates TLR4 signaling and induces TNF-α production by monocytes. Immunosuppression is viewed as the main pathophysiologic consequence of infection by HIV. However, the main immunologic defect caused by HIV, depletion of T cells, requires T cell activation. Our findings are consistent with a new concept that HIV-2 envelope proteins act on monocytes to suppress T cell activation and that this property may contribute to the benign course of HIV-2. We hypothesize that the HIV-2 envelope immunosuppressive properties limit bursts of T cell activation, thus reducing viremia and contributing to the slow rate of disease progression that characterizes HIV-2 disease.
Abbreviations:
-
- 1-MT:
-
1-methyl-D-tryptophan
-
- 3H-TdR:
-
[3H]-thymidine
-
- Env:
-
envelope
-
- L-NMMA:
-
NG-monomethyl-L-arginine
-
- PD-L1:
-
programmed death-L1
Introduction
Besides promoting viral attachment and entry, HIV envelope (Env) proteins modify immune responses 1. In parallel with its recognized immunogenicity, the HIV-1 Env modulates the function of T cells, B cells, Nk cells and APC 1–7. Several effects, such as suppression of proliferation, induction of anergy and apoptosis, and cytokine imbalances have been reported. Its relative importance varies according to the nature of Env used and the cell culture system studied. Although some of the effects of Env are thought to be mediated by CD4 binding, involvement of the chemokine receptors CCR5 and CXCR4 as well as C-type lectin receptors has been proposed 4–9.
The external domain of HIV-1 Env, gp120, is associated non-covalently with the transmembrane protein gp41, facilitating release of gp120 into the extracellular milieu, which can result in significant serum levels in infected patients 10. Therefore, gp120, whether as part of the intact virus or in a soluble form, can be taken up by neighboring uninfected cells, potentially modifying their function 1–9.
HIV-2 infection is considered a natural model of attenuated disease with limited impact on the survival of infected adults 11. Although HIV-2 infection exhibits the same clinical spectrum as HIV-1 infection, viremia is at a much lower level, and peripheral blood CD4 T cell counts decline at a slower rate in HIV-2-infected patients 12, 13. What attenuates HIV-2 relative to HIV-1 is unknown 14–20.
We explored whether and how HIV-2 Env might modify immune responses and in this way potentially account for the relatively benign course of infection. We compared HIV-1 gp120 with the external component of the HIV-2 Env, gp105. A more “open” structure of gp105 is thought to explain the broader range of co-receptor usage by HIV-2 than HIV-1 isolates 21 and the higher levels of neutralizing antibodies documented in HIV-2-infected patients 22. Moreover, gp105ROD was shown to bind to the CD8 molecule with high affinity, whereas HIV-1IIIB gp120 was unable to 23. We show here that HIV-2 Env markedly suppresses TCR-mediated responses through a mechanism dependent upon the contact between monocytes and T cells.
Results
Inhibitory properties of HIV-2 Env
To address the effects of HIV-2 Env on immune responsiveness, we measured proliferative responses of human T cells in the absence or presence of the whole external Env component from HIV-2ROD (gp105), a laboratory-adapted strain that uses CXCR4 as its co-receptor, the 165 aa peptide covering the C2-V3-C3 region of the Env of the ALI strain, an HIV-2 primary isolate that uses CCR5 as a co-receptor 24 as well as the Env protein (gp120) from HIV-1IIIB, HIV-1MN and SIVmac251. Freshly isolated PBMC from healthy donors were stimulated with immobilized anti-CD3 or the recall antigens Candida albicans or tetanus toxoid in the presence or absence of the HIV-2, HIV-1 or SIV Env proteins, and proliferative responses were tested. Both gp105 and the 165 aa peptide suppressed PBMC proliferation by more than 50%, without a statistically significant difference between them (Fig. 1). In contrast, HIV-1 or SIV Env only modestly suppressed the proliferation, and this suppression was significantly less than the one induced by HIV-2 Env (p<0.0001; unpaired t-test) as shown in Fig. 1B. The gp105-associated impaired proliferation was only found in response to TCR-mediated stimuli and was not observed with PMA plus ionomycin or PHA stimulation, as previously reported 25. This impairment in proliferation was not due to increased apoptosis as measured by annexin/propidium iodide 25. Both CD4+ and CD8+ T cells were suppressed as assessed by flow cytometry using BrdU incorporation 25. C2-V3-C3ALI was also found to inhibit only the TCR-mediated stimuli and was not associated with an increase in cell death (data not shown). Although the significant inhibition of T cell proliferation by anti-CD4 mAb precludes its use to block CD4 interaction 25, T cell suppression is likely to be independent of Env interaction with the CD4 receptor, since the C2-V3-C3ALI does not cover the CD4-binding region.
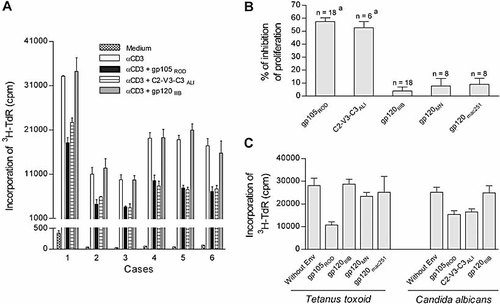
Effects of the HIV-1 and HIV-2 Env proteins on TCR-mediated lymphocyte proliferation. PBMC were cultured with different stimuli in the absence or presence of one of the following HIV Env proteins: HIV-2ROD gp105, the large peptide covering the C2-V3-C3 region of HIV-2ALI, HIV-1IIIB gp120, HIV-1MN gp120 or SIVmac251 gp120. Lymphocyte proliferation was measured by 3H-TdR incorporation. (A) Results of 3-day proliferation in response to immobilized anti-CD3 mAb in six different healthy subjects. Bars represent the individual mean cpm ± SEM of triplicates. (B) Percentages of inhibition induced by each protein on anti-CD3-mediated proliferation, calculated as the fold reduction in cpm levels of cells cultured in the presence of Env protein versus cells cultured in its absence. Bars represent the mean ± SEM of all the subjects studied under the different conditions (ap<0.0001 as compared to the percentage of inhibition of proliferation in the presence of gp120IIIB, gp120MN or gp120mac251, unpaired t-test). (C) Lymphocyte proliferation after 6-day culture in response to Candida albicans or tetanus toxoid in the presence of different Env proteins in one individual. Bars represent the mean cpm ± SEM of quadruplicates.
HIV-2 Env does not act directly on T cells
To determine whether HIV-2 Env acts directly on T cells, we purified T cells from PBMC and tested whether proliferation was suppressed by HIV-2 Env. As Fig. 2 shows, neither gp105ROD nor C2-V3-C3ALI suppressed proliferation of purified T cells stimulated with anti-CD3. Moreover, there were also no suppressive effects when CD28 co-stimulation was added to the anti-CD3 stimulus. Thus, the suppression of T cell proliferation does not result from a direct effect of HIV-2 Env on T cells.
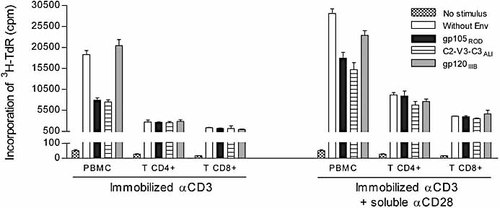
HIV-2 Env proteins do not act on purified T cells. PBMC and purified CD4+ and CD8+ T cell subsets were stimulated with immobilized anti-CD3 with or without anti-CD28 mAb in the absence or presence of HIV-2ROD gp105, HIV-2ALI C2-V3-C3 or HIV-1IIIB gp120. Proliferation was measured by 3H-TdR incorporation. Bars represent individual mean cpm ± SEM of triplicates in a representative case out of four.
HIV-2 Env immunosuppression does not require CD25+ T cells, IL-10 or TGF-β1,2,3
We next asked whether T cell proliferation might be suppressed by regulatory T cells. CD4+CD25+ T cells, a subset that expresses the highest level of the lineage regulatory marker FoxP3, have been proposed to play a significant role in models of experimental chronic infections by contributing to the suppression of T cell responses 26. Depletion of CD25+ cells using magnetic anti-CD25 mAb-coated microbeads did not abrogate the suppressive effects of the gp105ROD (Fig. 3A), nor did the blocking of IL-10 and TGF-β, cytokines that are known to suppress T cell responses (Fig. 3B), with antibodies at a concentration (10 μg/mL) known to exhibit neutralizing activity 27. No statistically significant differences were found between the inhibition of proliferative responses observed in bulk PBMC versus CD25-depleted PBMC or between the inhibition observed in the presence versus in the absence of the neutralizing antibodies. These findings should not be taken to mean that regulatory T cells play no part in the response to HIV-2 but rather that whatever part they may play is probably not conditioned by gp105ROD.
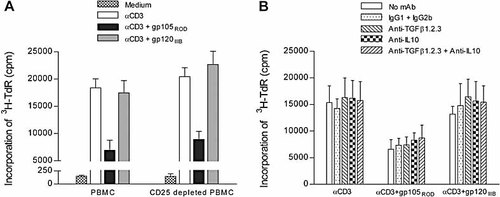
The anti-proliferative effect of HIV-2 Env proteins is independent of CD25+ cells or the immunosuppressive cytokines TGF-β or IL-10. (A) Bulk PBMC and PBMC depleted of CD25+ cells were stimulated in triplicate with immobilized anti-CD3 in the absence or presence of HIV-2ROD gp105 and HIV-1IIIB gp120, and proliferation was measured by 3H-TdR incorporation. Bars represent mean cpm ± SEM of three different healthy subjects. (B) PBMC were cultured in triplicate with immobilized anti-CD3 mAb for 3 days in the absence or presence of HIV-2ROD gp105 or HIV-1IIIB gp120, with or without neutralizing mAb against IL-10 and/or against TGF-β1,2,3 or the respective isotype controls IgG2b and IgG1.. Bars represent mean cpm ± SEM of three different healthy subjects.
Immunosuppression by HIV-2 Env depends on monocytes
We next tested whether gp105 suppresses T cell responses by acting on monocytes. The suppression of T cell proliferation was completely abrogated by the depletion of CD14+ cells (Fig. 4A) and was fully recovered when these cells were added back to purified T cells (Fig. 4B). Thus, the suppression of T cell proliferation mediated by HIV-2 Env requires the presence of monocytes.
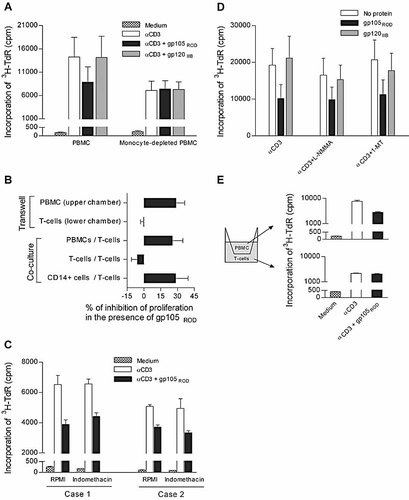
The anti-proliferative effect of HIV-2 Env proteins depends on the contact between monocytes and T cells. Cells were stimulated in triplicate with immobilized anti-CD3 for 3 days in the absence or presence of HIV-2ROD gp105 or HIV-1IIIB gp120, and proliferation was measured by 3H-TdR incorporation under the following conditions: (A) bulk PBMC and PBMC depleted of CD14+ cells; (B) purified T cells cultured alone and co-cultured with PBMC or CD14+ cells, as well as PBMC and purified T cells cultured in the upper and lower chamber, respectively, of a transwell system, as described in E; (C) PBMC in the presence of indomethacin; (D) PBMC in the presence of 1-MT or L-NMMA; (E) cells cultured in a transwell system with PBMC in the upper chamber and purified T cells in the lower chamber, with both chambers coated with anti-CD3 mAb. Bars represent: (A and D) mean cpm ± SEM of three different healthy subjects; (B) mean percentage inhibition of proliferation ± SEM of three different donors, calculated as the fold reduction in cpm levels of cells cultured in the presence of gp105 versus in its absence; (C) individual mean cpm ± SEM of triplicate of two different donors; (E) individual mean cpm ± SEM of a representative case out of three.
Absence of an impact of HIV-2 Env on factors released from monocytes
Next, we asked whether the suppression of T cell proliferation by HIV-2 gp105 was associated with substances secreted by monocytes. To test whether prostaglandins produced by monocytes inhibit lymphocyte proliferation, we added indomethacin, an inhibitor of prostaglandin synthase, to the cultures and then asked whether T cell proliferation was modified. As can be seen in Fig. 4C, gp105 suppressed T cell proliferation in the presence of indomethacin, suggesting that PGE2 or other prostaglandins were not involved. Nor was NO involved in the suppression of T cell proliferation by HIV-2 Env, as addition of NG-monomethyl-L-arginine (L-NMMA), an inhibitor of inducible NO synthase, to the cultures did not reverse immune suppression (Fig. 4D). Moreover, suppression did not depend on IDO, the rate-limiting enzyme in the catabolism of tryptophan, as an IDO inhibitor (1-methyl-D-tryptophan, 1-MT) did not reverse gp105-mediated suppression (Fig. 4D). No statistically significant differences were found between the inhibition of proliferative responses observed in the presence versus in the absence of indomethacin, L-NMMA or 1-MT.
T cell-monocyte interaction is required for suppression of T cell responses
We next asked whether contact between monocytes and T cells is required for the suppressive properties of gp105. Toward that end, we assessed the suppressive properties of gp105ROD in a 0.4 μm-transwell system that physically separated purified T cells from monocytes or bulk PBMC. As shown in Fig. 4E, gp105ROD inhibited proliferation of PBMC in the upper chamber but not T cell proliferation in the lower chamber of the transwell system, suggesting that suppression depended on contact between T cells and APC. Furthermore, gp105ROD-mediated suppression of T cell responses was recovered when PBMC or isolated monocytes were added back to purified T cells (Fig. 4B). These results suggest that inhibition of T cell activation by HIV-2 Env requires contact between monocytes and T cells and further support the exclusion of the soluble mediators tested.
HIV-2 Env activates TLR4 signaling
HIV-2 Env from strains that use both CCR5 (ALI) and CXCR4 (ROD) induced marked TNF-α production by monocytes, while HIV-1 Env (IIIB) did not (Fig. 5A), raising the possibility that gp105 activates TLR4 signaling in those cells. The same findings were observed after stimulation of purified monocytes instead of PBMC (data not shown). To determine whether gp105 activates TLR4, HEK 293 cells stably transfected with TLR4, MD2 and CD14 were transiently transfected with an NF-κB-luciferase reporter vector to reveal NF-κB activation after TLR4 signaling 28. As illustrated in Fig. 5B, gp105 stimulated TLR4 signaling in a dose-dependent manner, while gp120 had no effect on TLR4 activation. HIV-2 gp105 acted specifically on TLR4, because HEK 293 cells that lack TLR4 did not respond at any concentration of gp105 (Fig. 5C). Moreover, the addition of Rhodobacter sphaeroides diphosphoryl lipid A (RsDPLA), an LPS-like TLR4 antagonist, abrogated gp105-stimulated NF-κB-luciferase activity (Fig. 5C). A 6-h exposure of HEK 293 cells that express TLR4 to HIV-2 gp105 did not suppress subsequent stimulation with LPS or heparan sulfate, another TLR4 agonist 29 (data not shown). These findings indicate that gp105 is not contaminated by LPS and that signaling through TLR4 is by a mechanism distinguishable from signaling by LPS. Thus, HIV-2 Env is able to activate TLR4 signaling, suggesting a possible involvement of TLR4 on the immunosuppressive effects of HIV-2 Env.
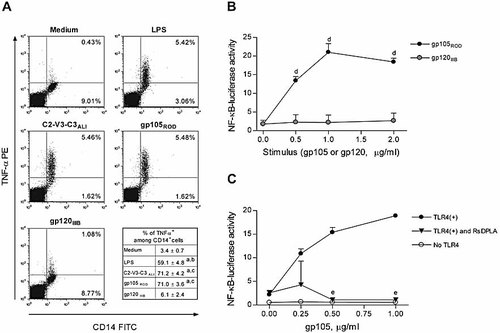
HIV-2 Env proteins activate TLR4 signaling. (A) PBMC were cultured for 6 h in the absence or presence of HIV-2ROD gp105, HIV-2ALI C2-V3-C3, HIV-1IIIB gp120 or LPS (positive control) with brefeldin A (last 5 h). After surface staining for CD14, cells were intracellularly stained for TNF-α. Dot plots show a representative example (out of three) of the analysis done in a large gate including monocytes and lymphocytes. Values in the lower right table represent the proportion of monocytes induced to produce TNF-α in the presence of the Env proteins or LPS (mean ± SEM of three different donors). There are no statistically significant differences between the TNF-α production induced by the two HIV-2 Env (ap<0.006 as compared to TNF-α production in medium alone, bp<0.005 as compared to TNF-α production in the presence of gp120 IIIB, cp<0.001 as compared to TNF-α production in the presence of gp120 IIIB; paired t-test). (B, C) HEK 293 cell lines stably expressing the TLR4 signaling complex (filled/shaded symbols) or control cells lacking TLR4 expression (open symbols) were transfected with an NF-κB-firefly luciferase reporter vector and the control pTK-Renilla luciferase vector. The cells were then incubated with various concentrations of HIV-2 gp105 or HIV-1 gp120 Env proteins (B) or with gp105 in the presence of 0.1 μg/mL Rhodobacter sphaeroides diphosphoryl lipid A (RsDPLA) (C) for 6 h. Activation of TLR4 is reported as a ratio of the NF-κB-stimulated firefly luciferase activity to the constitutively expressed Renilla luciferase internal control. gp105 activated TLR4 signaling in a dose-dependent manner (dp<0.002 as compared to the absence of protein), and TLR4 stimulation by gp105 was blocked by RsDPLA (ep<0.002 as compared to the absence of RsDPLA). Shown in the graphs are the mean ± SEM of triplicate wells, which are representative of three independent experiments.
Discussion
Here we report that HIV-2 Env, acting on monocytes, suppresses TCR-mediated responses and that HIV-2 Env may do so by stimulating TLR4. One might expect that immunosuppression generated by Env proteins would make HIV-2 immunodeficiency worse, as it would add to the impact of CD4+ T cell depletion. Contrary to this concept, however, the Env of HIV-2 is more suppressive than Env of HIV-1, and subjects with HIV-2 infection do not suffer striking immunodeficiency, at least compared to those infected with HIV-1 11–20. Since it has limited impact on the survival of the majority of infected adults, HIV-2 infection is considered a natural model of attenuated HIV disease 11, 20. Why the CD4 depletion and viremia of HIV-2 infection are less severe than in HIV-1 infection remains unknown 12–20. We postulate that the HIV-2 Env may limit the bursts of T cell activation and therefore contribute significantly to the slower rate of disease progression that characterizes HIV-2 disease.
Chronic immune activation represents a main driving force for the progressive decline in CD4 counts and other manifestations of AIDS 16, 17, 30–34. The plasma HIV RNA load predicts no more than 10% of the subsequent CD4 cell loss in untreated HIV-1-infected patients, suggesting that other factors, such as the persistent immune activation, determine this outcome 35. Although specific responses against HIV may contribute, much and perhaps most T cell activation is thought to be driven by bystander processes. Among the bystander processes may be bacterial translocation at the altered gut mucosa and the subsequent increased levels of circulating bacterial products such as LPS 36. In this context, it is plausible that monocytes/APC are important modulators of the state of activation. Interactions between HIV-1 components and those cells have been extensively reported 37.
Suppressor monocytes/macrophages have been found in several conditions associated with chronic inflammation, including leishmaniasis, tuberculosis and hepatitis C 38–41. Our findings suggest that HIV-2, like other pathogens, may direct monocytes or DC to hamper T cell stimulation 38–42. Suppression of T cell responses by these pathogens is thought to enable evasion of clearance and promote persistence of the microorganism. It is possible that suppression of T cell responses by HIV-2 Env also promotes persistence of the virus; however, the dominant impact of suppression of cell-mediated immunity may be a reduction in the immunopathology associated with the immune response, thereby contributing to the more benign outcome of HIV-2 infection.
Several mechanisms have been proposed for monocyte-mediated suppressive effects 6, 38–41, 43, 44. Prostaglandins, NO and induction of IDO have been classically implicated, but our studies using inhibitors of these pathways suggest that they contribute little to suppression of T cell proliferation in the presence of HIV-2 Env. Moreover, IL-10 and TGF-β, two cytokines frequently implicated in T cell suppressive responses, did not contribute appreciably to suppression of T cell responses. Recently, monocytes have been shown to express programmed death-L1 (PD-L1) and, through the PD-L1/PD-1 pathway, suppress TCR-mediated responses 45. Our preliminary results clearly show that some recombinant HIV-2 and HIV-1 Env proteins up-regulate PD-L1 expression in monocytes in vitro as assessed by flow cytometry. However, this pathway is not implicated, since neutralization using anti-PD-L1 mAb did not reverse suppression of lymphocyte proliferation (data not shown). Another possibility that we tested was that the suppressive properties of HIV-2 Env are mediated by CD4+CD25+ regulatory T cells, which are known to be able to exert inhibitory effects on the activation and function of DC 46 and monocytes/macrophages 43, but depletion of CD25+ cells did not impact the suppressive effects of HIV-2 Env.
We found that the contact between monocytes and T cells is essential for the suppression of TCR-mediated proliferation by HIV-2 Env. A contact-dependent mechanism was also shown to be involved on the suppression of T cell mitogenesis by immunosuppressive macrophages in other persistent infections such as mycobacterial infection 40. Continuous interplay between several receptors on the surface of monocytes and DC mediates a delicate balance of positive and negative signals that ultimately determines the fate of the T cell responses. Although TLR, which recognize pathogen-associated molecular patterns, trigger the functional maturation of APC leading to the initiation of antigen-specific adaptive immune responses, they have also been shown to induce immune suppression 47. A switch from a pro-inflammatory to a suppressive cytokine profile in the response of DC to TLR agonists was clearly documented during the establishment of the chronic murine infection by Plasmodium yoelii 48. Recently, the HCV Core protein was found to hamper antiviral immunity through TLR2-mediated monocyte activation 41. The mouse mammary tumor virus was also shown to interact with TLR4 on DC and macrophages, leading to suppression of cytotoxic immune responses 47.
Considering the previous reports of the interaction of mouse mammary tumor virus and murine leukemia retrovirus Env proteins with TLR4 47, 49, we also investigated the possibility of HIV-2 Env signaling through TLR4. The HIV-2 Env induces TNF-α production by monocytes at levels similar to those found with TLR4 agonists. Indeed, we found that HIV-2 Env induces dose-dependent activation of NF-κB in HEK 293 cells that express stable levels of TLR4. The HIV-1 Env recombinant protein produced using the same expression system did not induce NF-κB. Corroborating the controls used for the exclusion of LPS contamination, we found that the same HIV-2 Env recombinant proteins were unable to induce maturation of monocyte-derived DC (data not shown). Others have shown that TLR4 can form a complex with CXCR4 inside lipid rafts 50 and that CXCR4 and its ligand SDF-1 may interfere with TLR4 signaling 51. Thus, gp105 may activate TLR4, at least in part, by disrupting the interaction between CXCR4 and TLR4. Of note, anti-CXCR4 mAb have also been shown to activate TLR4 signaling 51, precluding its use to investigate this possibility. If gp105 activates TLR4 indirectly in this manner, it may explain how TLR4 on cells stimulated by gp105 remain responsive to LPS, since the receptor would be available for activation by ligand.
The differences between the biological properties of HIV-1 Env and HIV-2 Env may reflect differences in protein structure, as HIV-2 and HIV-1 Env proteins share only 40% homology 52. It is plausible that the more “open” structural conformation of HIV-2 Env may allow the protein to act on the surface of monocytes and, through this interaction, modulate the observed suppression. Although the number of available HIV-2 Env proteins to study is small and assumptions regarding the in vivo relevance of their suppressive effects should be made cautiously, it is noteworthy that these effects were also observed using an Env protein from a primary viral isolate. Our studies were performed using a recombinant polypeptide to optimize the homogeneity of responses. Clearly, the response of any given individual to a naturally expressed polypeptide might depart, in one direction or another, from what we report here. Nevertheless, if our contention about the impact of gp105 on the biology of HIV-2 is upheld, then the overall direction and dimension of responses to recombinant gp105 will faithfully represent what happens in vivo.
HIV-2 arose by cross-species transmission of SIVsm found naturally in sooty mangabey monkeys 53. Acute SIVsm infection appears to induce suppressive cytokines in the natural hosts that limit the pro-inflammatory characteristics of SIV-associated immunodeficiency 54 and prevent subsequent CD4 depletion and progression to AIDS 33. HIV-2 disease is characterized by a much slower rate of progressive immune activation and CD4 T cell decline than HIV-1 infection 12, 13, 17, 19. Because HIV-2 strains are no less cytopathic than HIV-1 strains, host factors probably limit HIV-2 disease progression 55.
The DC/T cell microenvironment has been shown to be an ideal site for HIV-1 propagation through the transfer of the virus from DC to activated antigen-specific T cells 56. Thus, the action of HIV-2 Env on monocytes/DC during antigen presentation may prevent the spread of the virus among T cells. Even a modest reduction in transmission might have a drastic effect on the virus produced in local infection bursts and contribute to reduced viremia 57. Moreover, it might delay the progressive disruption of secondary lymphoid environments by immune activation, thus reducing the rate of CD4 decline 16.
These data point to a new line of research into the benign course of HIV-2 centered on monocytes and raise the possibility of using HIV-2 Env as an immunomodulatory tool.
Materials and methods
Cell isolation
PBMC were isolated by Ficoll-Hypaque density gradient (Amersham Pharmacia Biotech, Little Chalfont, UK) from healthy donor venous blood or from leukocyte-enriched buffy coats (Portuguese Institute of Blood, Lisbon) and resuspended at 1 × 106 cells/mL in RPMI 1640 (Gibco-Invitrogen, Paisley, UK) supplemented with 10% heat-inactivated human AB serum (Sigma-Aldrich, St Louis, MO), 100 U/mL penicillin/100 μg/mL streptomycin (Gibco-Invitrogen) and 2 mM L-glutamine (Gibco-Invitrogen). T cells were isolated from PBMC by negative selection using a cocktail of biotin-conjugated mAb against CD14, CD16, CD19, CD36, CD56, CD123 and Glycophorin A, followed by anti-biotin microbeads. The T cell purity was >99%, as assessed by flow cytometry as previously described 25. T cells were further fractionated using CD4 microbeads into CD4+ T cells (>97% pure) and a CD4neg fraction, corresponding to >94% CD8+ T cells. PBMC were depleted of monocytes using CD14+ microbeads and of CD25+ cells using CD25 microbeads (purity >95%). All the separations were performed in a VarioMACS (Miltenyi Biotec, Bergish Gladbach, Germany) using Miltenyi Biotec microbeads. Purified monocytes were shown to have levels of CCR5 and CXCR4 expression similar to those in bulk PBMC. This study was approved by the Ethical Board of the Faculty of Medicine of Lisbon.
Proliferation assays
Cells (1 × 105) were cultured in round-bottom 96-well plates (Costar, Corning Incorporation, NY) with immobilized anti-CD3 (1 μg/mL, clone HIT3a; BD Biosciences, San Jose, CA) or the recall antigens Candida albicans (40 μg/mL; Greer, Lenoir, NC) or tetanus toxoid (dilution 1:800; Connaught, Swiftwater, PA) in the presence or absence of the following Env proteins (1 μg/mL): gp105 from HIV-2ROD and gp120 from HIV-1IIIB, HIV-1MN or SIVmac251 produced using a baculovirus expression system (EU program EVA, MRC, UK); or a 165-aa peptide covering the C2-V3-C3 Env region of the primary isolate HIV-2ALI, provided by Nuno Taveira, produced in an E. coli expression system and negative for endotoxin by the Limulus amebocyte assay (Pyrotell, East Falmouth, MA; detection limit 0.125 EU/mL). In some experiments, anti-TGF-β1.2.3 or anti-IL-10 as well as their respective IgG1 or IgG2b isotype controls, alone or in combination (10 µg/mL; R&D Systems, Minneapolis, MN), soluble anti-CD28 mAb (1 μg/mL; BD Biosciences), indomethacin (1 μg/mL; Sigma-Aldrich), 1-MT (200 μM; Sigma-Aldrich) or L-NMMA (1 mM; Sigma-Aldrich) were added to immobilized anti-CD3. All cultures were performed in triplicate for 3 days (anti-CD3 stimulation) or in quadruplicate for 6 days (antigen stimulation) at 37°C in a humidified atmosphere of 5% CO2. Lymphocyte proliferation was measured by [3H]-thymidine (3H-TdR; Amersham Pharmacia Biotech) incorporation after a pulse of 1 μCi during the last 18 h ofculture, counted in a gaseous scintillation β-counter (Packard, Meriden, CT). Results are expressed as mean cpm ± SEM. Percentages of inhibition were calculated as the fold reduction in cpm levels of cells cultured in the presence of Env protein versus cells treated in its absence.
Transwell experiments
Flat-bottom 24-well plates with a 0.4 μm transwell (BD Falcon, BD Biosciences) were used. Both the upper and lower chambers were coated with anti-CD3 mAb as described above. T cells (0.9 × 106) were added to the lower compartment, and PBMC or CD14+ cells (0.9 × 106) were cultured in the upper chamber in the presence of medium alone, HIV-2 gp105ROD or HIV-1 gp120IIIB. To control for diffusion of the Env protein in the transwell system, experiments were performed in which PBMC were cultured in both chambers with the HIV-2 Env protein added to the upper chamber, and the proliferation levels were found to be similar in both chambers (data not shown). In parallel, 0.9 × 106 T cells were stimulated in a co-culture assay with the same quantity of PBMC, CD14+ or T cells in the absence or presence of the Env protein.
Single-cell analysis of intracellular TNF-α
PBMC were cultured for 6 h at 5 × 105 cells/tube in the absence or presence of the HIV Env protein or LPS from E. coli 0111:B4 (2 μg/mL). Brefeldin A (10 μg/mL; Sigma-Aldrich) was present during the last 5 h of culture to block cytokine secretion. Intracellular cytokine staining was performed as previously described 25 using PE-conjugated anti-TNF-α mAb (BD Biosciences) after surface staining with FITC-conjugated anti-CD14 mAb (Sanquin, Amsterdam, The Netherlands). Briefly, cells were fixed with 2% formaldehyde (Sigma-Aldrich) and permeabilized with PBS containing 1% BSA, 0.1% sodium azide and 0.5% saponin (all from Sigma-Aldrich). Data were acquired using a FACSCalibur flow cytometer and CellQuest software (BD Biosciences), and analysis was conducted within a large manual setting gate including monocytes and lymphocytes defined on forward/side scatter, with thresholds set according to the isotype-matched negative controls. Results are expressed as the percentage of TNF-α+ cells within CD14+-gated cells.
TLR4-stimulated NF-κB activation
Activation of TLR4 was measured in HEK 293 cells stably expressing TLR4, MD2 and CD14 as previously described 28. Briefly, HEK 293 cells expressing the TLR4 signaling complex or control cells were transfected with 0.1 μg pTK-Renilla luciferase (Promega) and 0.1 μg NF-κB-firefly luciferase 58 using Superfect Transfection Reagent (Qiagen, Valencia, CA). Following transfection, the cells were cultured for 24 h at 37°C in medium with low (0.5%) FBS. After various treatments, the culture medium was aspirated, and expression of Renilla and firefly luciferase was assayed simultaneously using the Dual-Luciferase Reporter Assay System (Promega) and a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA). Activation of NF-κB is reported as a ratio of the firefly luciferase activity to the constitutively expressed Renilla luciferase internal control and is the mean of triplicate wells.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 4 (GraphPad Software Inc., San Diego, CA). Data were compared using the paired t-test or Wilcoxon test and unpaired t-test. p values <0.05 were considered significant.
Acknowledgements
We thank Nuno Taveira, José Marcelino and Helena Barroso from the Faculty of Pharmacy, Lisbon for producing the peptide C2-V3-C3 HIV-2ALI and for helpful scientific discussion of this work; the NIBSC Centralized Facility for AIDS Reagents supported by EU Programme EVA (contract QLK2-CT-1999–00609) and the UK Medical Research Council for providing recombinant HIV-1 gp120IIIB and HIV-2 gp105ROD; and the Portuguese Institute of Blood for providing the buffy coats. This work was supported by grants from “Fundação para a Ciência e a Tecnologia” (FCT) and “Comissão Nacional de Luta Contra a SIDA” (PSIDA/ESP/49655/2003 to A. E. S.). R. C. received a scholarship from FCT.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
Appendix
Conflict of interest: The authors declare no financial or commercial conflict of interest.




