Selective silencing of DNA-specific B lymphocytes delays lupus activity in MRL/lpr mice
Abstract
The pathological DNA-specific B lymphocytes in lupus are logical targets for a selected therapeutic intervention. We have hypothesized that it should be possible to suppress selectively the activity of these B cells in lupus mice by administering to them an artificial molecule that cross-links their surface immunoglobulins with the inhibitory FcγIIb surface receptors. A hybrid molecule was constructed by coupling the DNA-mimicking DWEYSVWLSN peptide to a monoclonal anti-mouse FcγRIIb antibody. This chimeric antibody was added to cultured spleen cells from sick MRL/lpr mice, immunized with diphtheria toxoid, resulting in reduction of the numbers of anti-DNA but not of anti-diphtheria IgG antibody-producing cells. Intravenous infusions with the DNA-peptide antibody chimera to 7-wk-old animals prevented the appearance of IgG anti-DNA antibodies and of albuminuria in the next 2 months. The administration of the DNA-peptide chimeric antibody to 18 wk-old mice with full-blown disease resulted in the maintenance of a flat level of IgG anti-DNA antibodies and in delay of the aggravation of the lupus glomerulonephritis. The use of chimeric antibodies targeting inhibitory B lymphocyte receptors represents a novel approach for the selective suppression of autoreactive disease-associated B cells in autoimmune diseases.
Abbreviation:
-
- SLE:
-
systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is the prototype systemic autoimmune disease, characterized by the generation of autoantibodies specific for native double-stranded (ds) DNA and to nucleic acid/protein complexes 1. Renal involvement is common and is a strong predictor of poor outcome. The presently used anti-inflammatory and immunosuppressive drugs delay the development of lupus glomerulonephritis, but do not cure the disease itself. These drugs have serious and sometimes even life-threatening side effects.
The pathological DNA-specific B cells in lupus patients are logical targets for therapeutic intervention. Their appearance is regarded as a major event in the development of the disease because of the specificity of the antibodies they produce. Other investigators have recently claimed, however, that their main role in the pathogenesis of lupus is that of antigen-presenting cells that help breaking T cell tolerance to self DNA/protein complexes 2, 3. Regardless of the precise role that anti-DNA B lymphocytes play in the induction of the disease, their selective elimination or suppression is a legitimate goal in the efforts to control SLE.
The continuous selective elimination of B-1 lymphocytes in (NZB×NZW)F1 mice since an early age has been shown to prevent the development of glomerulonephritis and to dramatically increase the life span of the animals 4. This approach is, however, not effective in MRL/lpr mice with spontaneous lupus, as B-1 cells are not involved in the disease. Recent clinical studies have shown that the depletion of most B cells by infusion of an anti-CD20 monoclonal antibody has a beneficial effect in SLE patients who do not respond to conventional treatment 5–7.
The development of a more specific therapy, targeting only selected disease-associated B lymphocytes remains, however, highly desirable. Single-signal anergy can be used to silence targeted disease-associated B cells. An example of such a B cell tolerogen is LJP 394, which has been shown to inactivate anti-dsDNA-specific B cells in vivo in murine immunized and spontaneous disease models, but the high initial expectations were not met in controlled clinical trials 8.
Immunoglobulin genes encoding dsDNA-specific IgG antibodies in lupus accumulate mutations in the same way as do VH and VL genes coding for antibodies against foreign antigens 9, 10. Both types of B lymphocytes are antigen driven, and we reason that if this is the case, autoreactive B cells should be susceptible to the same mechanisms that control the magnitude and duration of the IgG antibody response to foreign antigens 11. One of these mechanisms is the cross-linking of the surface immunoglobulin with the inhibitory FcγIIb receptors by IgG-containing immune complexes (for review see 12–15). FcγIIb is the only Fcγ receptor expressed by B lymphocytes. Mice deficient for it react with enhanced antibody production to T-dependent and -independent antigens 16 and develop fatal glomerulonephritis 17. Memory B cells from SLE patients have decreased expression of FcγIIb receptors 18.
Infusion of pre-formed DNA/anti-DNA immune complexes into MRL/lpr mice has resulted in suppression of the production of disease-associated autoantibodies and in reduction of the severity of lupus nephritis 19. Immune complexes could hardly be used for therapy, however, because of numerous standardization, stability and shelf life problems. We hypothesized that it should be possible to down-regulate selectively the activity of self-reactive B cells by using artificial molecules that cross-link immunoglobulin receptors with inhibitory receptors on targeted autoreactive B cells.
A chimeric antibody was constructed by us by coupling the DWEYSVWLSN dsDNA-mimicking peptide 20–22 to a rat anti-mouse FcγRIIb-binding monoclonal antibody. In the present study, we have proven that by using this artificial antibody it is possible to suppress selectively the activity of disease-associated B lymphocytes and to change the natural course of a spontaneous autoimmune disease.
Results
Chemical coupling of peptides to the monoclonal antibodies
The comparison between the HPLC curves of the 2.4G2 antibody and of the antibody chimeras prepared using it showed that the latter was displaced as a result of the coupling of a certain number of peptides (Fig. 1A). Immunoglobulin aggregates were not formed during the conjugation procedure (data not shown). The final solutions of the chimeric antibody molecules contained 0.5 mg protein and only 0.24 EU/mL endotoxin.
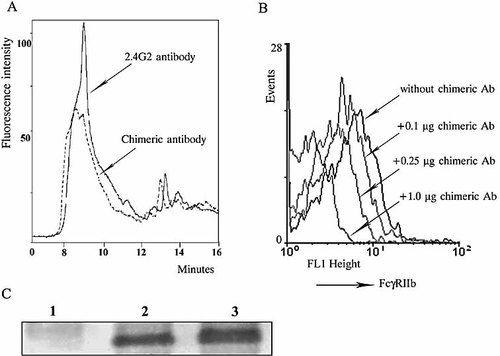
Characterization of the constructed chimeric antibody molecules. (A) The comparison between the HPLC curves of the 2.4G2 antibody and of the antibody chimeras prepared using it show that the chimera curves were displaced, indicating that a number of peptides were coupled to the antibody molecules. (B) The DNA-like chimera retains its reactivity to mouse FcγRII as it inhibits the binding of the FITC-conjugated 2.4G2 antibody. Spleen cells from an MRL/lpr mouse were incubated with increasing concentrations of the DNA-like chimera, followed by incubation with the FITC-conjugated 2.4G2 antibody. Each step was performed for 30 min at 4°C. The cells were washed and analyzed by flow cytometry. A dose-dependent inhibition of the signal from the cells treated with 2.4G2-FITC only was seen in the presence of 0.1, 0.25 or 1.0 µg of the DNA-like chimera per 106 cells. (C) After coupling of the DNA-mimicking peptide to the 2.4G2 antibody, the former retains its ability to be bound by anti-dsDNA antibodies. Samples (10 µg) from the irrelevant chimera 1 (lane 1), the irrelevant chimera 2 (lane 2) or the DNA-like chimera (lane 3) were subjected to SDS-PAGE (under non-reducing conditions using a 10% gel) and transferred to a nitrocellulose membrane. The latter was blocked, incubated with the dsDNA-specific 10F10 mouse monoclonal antibody, washed, incubated further with a goat anti-mouse IgG antibody conjugated to peroxidase, and developed.
There are many free carboxyl groups on the surface of the antibody molecules, and some of them are available for interaction with the reactive H2N group of the peptide spacer. The peptides could theoretically bind to groups within the antigen-binding sites, and as a result the interaction of the parent antibody to mouse FcγRIIb could be affected. This possibility was excluded as we showed that the native 2.4G2 antibody and the DNA-like chimera recognized equally well the targeted receptor on mouse splenocytes (not shown). In a separate experiment, the DNA-like chimera inhibited, in a dose-dependent manner, the binding of a commercial FITC-labeled 2.4G2 antibody to the same cells (Fig. 1B). In an independent study, the pure heavy and light chains from the native 2.4G2 antibody and from the DNA-like antibody chimera were separated by HPLC. Comparative mass spectral analyses of both showed that 14–16 peptides were bound per one DNA-like chimeric IgG molecule (N. Mihaylova et al., manuscript in preparation).
The recognition of the DNA-like peptide by the dsDNA-specific monoclonal antibody 10F10 in a Western blot (Fig. 1C) confirmed that the peptides coupled to the rat IgG molecules retained their DNA-mimicking epitope. The latter is available for interaction with dsDNA-specific antigen receptors on lupus B lymphocytes.
As the abilities of the antibody and of the peptide part of the constructed DNA-like chimera to bind to both the FcγIIb receptors and the surface dsDNA-specific immunoglobulin receptors are preserved, it is expected that this artificial molecule would cross-link these receptors on the surface of the targeted disease-associated B cells.
The DNA-like chimera targets dsDNA-specific B lymphocytes only
MRL/lpr mice with lupus, immunized twice with diphtheria toxoid, have large numbers of both dsDNA- and toxoid-specific B and plasma cells. Their splenocytes were cultured for 5 days, and the numbers of anti-diphtheria toxoid and anti-dsDNA IgG antibody-producing cells were counted by the ELISpot technique (Fig. 2A). The addition of bacterial LPS did not increase further the numbers of antibody producers, showing that most B cells with the studied specificities were already activated. None of the constructed chimeras added to the medium during the period of cultivation influenced the numbers of diphtheria toxoid-specific IgG antibody-producing cells (Fig. 2A, lower panel). The DNA-like chimera caused a dose-dependent decrease of the numbers of in vitro differentiated anti-dsDNA antibody-producing cells (Fig. 2A, upper panel).
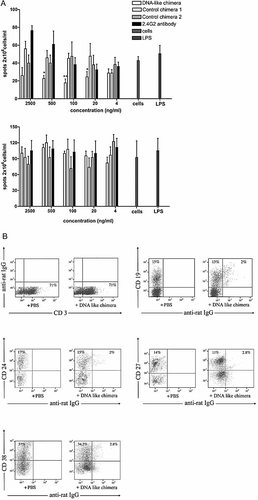
(A) The effect of the DNA-like chimera is selective as it affects the differentiation of the targeted dsDNA-specific B cells only. Spleen cells from diphtheria toxoid-immunized MRL/lpr mice with lupus were cultured in medium alone, in the presence of LPS, the DNA-like chimera, the pure 2.4G2 antibody, or of the irrelevant chimeras for 5 days. The numbers of plasma cells producing anti-dsDNA (upper figure) or anti-diphtheria toxoid IgG antibodies (lower figure) were determined by the ELISpot technique (see Materials and methods section). * p <0.05; ** p <0.01; Student's t-test. (B) The DNA-like chimera binds to cells of the B lymphocyte lineage only. Spleen cells from an MRL/lpr mouse were incubated with the DNA-like chimera (at 1 µg/106 cells) followed by incubation with a goat anti-rat IgG FITC-conjugated antibody, a PE-Cy5 conjugate of the anti-CD19 antibody, and one of the following PE-conjugated antibodies: anti-mouse CD3, anti-mouse CD24, anti-mouse CD27 or anti-mouse CD38. Each step was performed for 30 min at 4°C. The cells were washed and analyzed by flow cytometry.
The DNA-like chimera was shown to bind only to B lineage cells at different stages of differentiation, but not to T cells (Fig. 2B). Culturing of splenocytes from a lupus mouse in the presence of the same chimera increased the percentage of apoptotic (Annexin V-positive) B lymphocytes (Fig. 3), but not T lymphocytes (not shown).
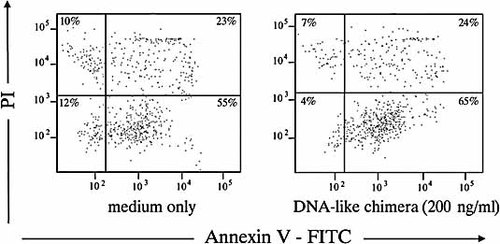
The constructed DNA-like chimera induces B cell apoptosis. Spleen cells from a sick 12-wk-old female MRL/lpr mouse were cultured for 4 days in complete RPMI medium only or in the same medium containing 200 ng/mL of the DNA-like chimera. At the end of the cultivation, gated CD19+ cells were analyzed for staining with propidium iodide and Annexin V-FITC.
The DNA-like chimera decreases anti-dsDNA IgG antibody levels and disease activity
The in vivo effects of the DNA-like antibody chimera were studied in two groups of female lupus-prone MRL/lpr mice: in 7-wk- and in 18-wk-old ones. The younger animals were still disease free at that age but the appearance of the first signs of lupus were imminent. The older group had already high levels of IgG anti-dsDNA antibodies and of albuminuria at the start of the experiment. The period of treatment was limited to 6 wk, because of the appearance of anti-rat immunoglobulin antibodies after that point (data not shown).
The administration of the chimeric antibody to 7-wk-old mice resulted in prevention of the sharp increase of the IgG anti-DNA antibody levels during the next 6 wk (p <0.05, Student's t-test, compared to the PBS-treated group). Differences in proteinuria levels were also present (Fig. 4A). The DNA-like chimera-treated mice had low protein levels in the urine for the first 10 wk, which increased gradually in the next 10 wk. A rapid increase of the urine protein concentration was observed in the PBS-injected controls during the same period.
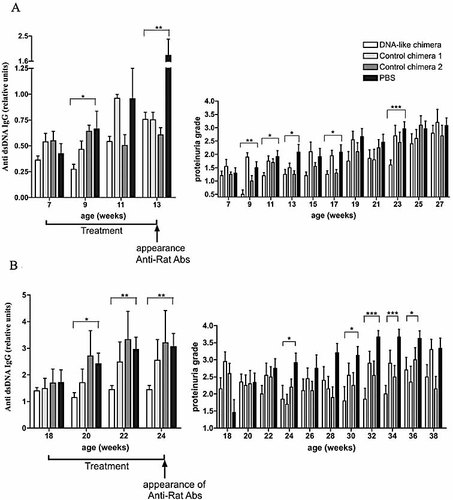
Intravenous infusions with the DNA-like chimera delay the appearance of IgG anti-dsDNA antibodies (left figures) and of proteinuria (right figures) in 7-wk-old (A) or 18-wk-old (B) female MRL/lpr mice. Groups of mice (ten animals per group) were treated intravenously twice weekly (20 µg/dose) with the DNA-like chimera, with irrelevant chimera 1, with irrelevant chimera 2, or with PBS alone. * p< 0.05; ** p <0.01; Student's t-test.
Treatment that started at the age of 18 wk resulted in the maintenance of a flat level of IgG anti-DNA antibodies in the next 6 wk. This result correlated with a flat level of proteinuria during the same period (Fig. 4B).
IgG antibodies against the SSA, SSB and Sm antigens were detected in the sera of all mice treated with the DNA-like chimera, with the control chimeras or with PBS. No significant differences were seen between the groups (not shown).
By 5 months of age, all mice in the control groups had severe lymphadenopathy, while the DNA-like chimera-treated animals had smaller peripheral lymph nodes and spleens (Fig. 5A, B). We are presently investigating the mechanisms of this effect.
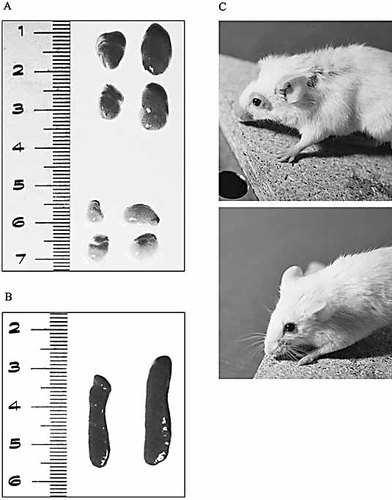
Comparison between the lymphoid organ sizes of the DNA-like chimera and of the PBS-treated MRL/lpr mice. Inguinal and axillary lymph nodes (A) and spleens (B) in control (A – upper group, B – right spleen) and DNA-like chimera-treated animals (A – lower group, B – left spleen). At 5 months of age, control MRL/lpr mice had severe skin lesions (above), while the DNA-like chimera-treated ones were lesion free (below) (C).
MRL/lpr mice develop a spontaneous progressive skin disease (Fig. 5C, upper panel), and by 5 months of age all have large plaque-like cutaneous lesions on their posterior neck. No lesions were detected in any of the DNA-like chimera-injected animals (Fig. 5C, lower panel).
The same treatment affected the deposition of IgG-containing immune complexes in the glomeruli. While massive mesangial deposits were observed in the animals treated with the control chimeras, the depositions detected in the chimera-treated mice were of the segmentary mesangial type (Fig. 6, upper panel). The 6-wk-long administration of the DNA-like chimera resulted also in a significant improvement in the kidney histology. The microscopical structure of the organs of chimera-treated mice was preserved although an interstitial infiltrate was present. In contrast, the kidney sections of irrelevant peptide chimera-treated animals were characterized by a mononuclear infiltrate and a massive mesangial proliferation, and those of the irrelevant antibody chimera-treated ones by a massive mononuclear infiltrate surrounding the small blood vessels. Finally, PBS-treated MRL/lpr controls presented mononuclear interstitial infiltrate and glomerulopathy with a global mesangial proliferation and epithelial crescents (Fig. 6, lower panel).
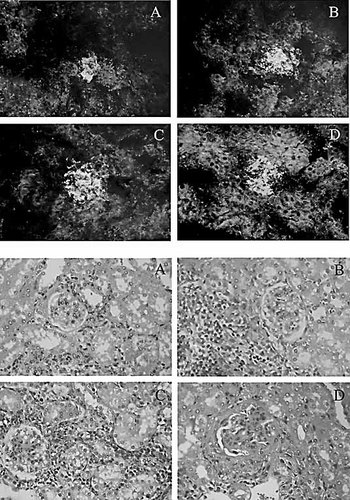
Upper panel: Immunofluorescence analysis of IgG deposition in the glomeruli of DNA-like chimera-treated (A), PBS-treated (D) and control chimera-treated (B, C) mice. The kidney sections were stained with FITC-labeled goat-anti-mouse IgG. Representative pictures are shown. Bottom panel: Kidney sections stained with hematoxylin/eosin from DNA-like chimera-treated (A), PBS-treated (D) and control chimera-treated (B, C) mice. Representative pictures are shown.
The administration of the DNA-chimera to 7-wk-old MRL/lpr mice did not prolong their lives (data not shown). However, the same treatment of 18-wk-old sick animals significantly prolonged their survival (Fig. 7; p <0.02, Log Rank test), proving that an approach mimicking the natural mechanism for avoiding unwanted immune responses could be used for the treatment of autoimmune disorders.
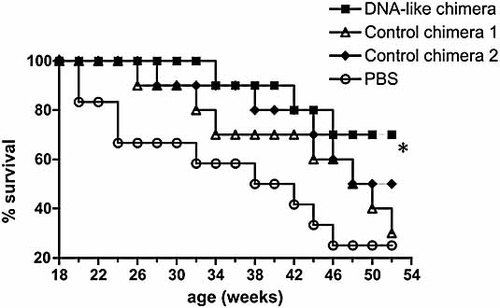
DNA-like chimera treatment significantly prolongs the survival of MRL/lpr mice (at least ten animals per group). * p = 0.02; Log Rank test.
Discussion
We have shown in the present study that it is possible to suppress selectively the differentiation of autoreactive B lymphocytes by using an antibody chimera that binds to the inhibitory FcγRIIb, as well as to the immunoglobulin receptors on targeted B cells. We have also shown that the intravenous administration of the same chimera prevents the development of the symptoms of lupus in young MRL/lpr mice and prevents the progression of the disease when the treatment was started after its onset.
The DNA-like chimera used in the study was generated using the rat 2.4G2 monoclonal antibody that binds to mouse FcγRIIb and has some reactivity to mouse FcγRIII. It is inevitable that a portion of the administered molecules interact with untargeted cells. The overall avidity of binding of the chimera to the targeted DNA-specific B lymphocytes is, however, theoretically equal to the product of the binding affinities of the antibody to the FcγRIIb and of the DNA-mimicking peptide to the anti-DNA immunoglobulin receptor. This theoretical value could not be expected to be seen in vivo, but there should be some degree of selectivity of interaction of the chimera with the surface of the targeted disease-associated B cells in lupus mice.
The beneficial effects seen lasted until the treated mice started producing antibodies to the rat immunoglobulin that was part of the chimera. If antibody chimeras targeting the inhibitory FcγIIb receptor on selected autoreactive B cells in autoimmune patients are to be used in the future, they should be constructed using humanized antibodies or Fv antibody fragments binding only to the human FcγIIb receptor isoform 18, 23, 24. While the intravenous injections of the DNA-like chimera prevented the appearance of IgG anti-dsDNA antibodies in young MRL/lpr mice, the effect of the same treatment starting after the onset of the disease was limited to ensuring a flat level of antibodies with this specificity. This difference could be explained by the type of B lineage cells affected by the administration of the DNA-like chimeric molecules. Animals at 7 wk of age are expected to have dsDNA-specific B lymphocytes, but few plasmacytes producing antibodies with the same specificity. Animals that have already developed lupus have high numbers of IgG anti-dsDNA antibody-producing plasma cells in their lymphoid organs. The chimeric antibody is not expected to easily reach their surface against a gradient of secreted anti-dsDNA antibodies. There is a fraction of long-lived anti-dsDNA IgG antibody-producing plasma cells in mice with SLE that survive for very long periods in the newly described plasma cells niches (the bone marrow, the inflamed kidneys, etc.) 25. The selective elimination of these plasmacytes remains a formidable challenge. The long-term administration of a DNA-like chimera is expected to block the replenishment of the pool of disease-associated plasma cells. In our experiments, the treatment with a DNA-like chimera delayed the progression of the disease in both young and older sick MRL/lpr mice, but the survival was prolonged only in the older group. The period between the end of the therapeutic infusions in the younger group (wk 13) and of the 50% survival time (wk 38) of the PBS-injected control mice was obviously long enough for the disease to re-establish itself.
The DNA-like chimera described here obviously targets dsDNA-specific B cells at an earlier stage of their differentiation. If the arguments developed above are correct, this property could be used in the future for the prevention of selected autoimmune diseases. A large-scale study of frozen sera obtained from individuals years before they develop lupus has shown that in 55% of all cases, the appearance of IgG anti-dsDNA antibodies preceded by years the clinical onset of the disease. At least one of the lupus autoantibodies tested was present long before the diagnosis was made (up to 9.4 years earlier; mean period 3.3 years) 26. So there is a sufficiently long period in which disease-associated B cells could be specifically targeted – silenced or eliminated. This would delay or even prevent the development of the autoimmune disease.
Materials and methods
Antibodies
The rat 2.4G2 hybridoma producing a monoclonal IgG2b antibody specific to mouse FcγRII (CD32) (ATCC HB-197) was adapted to grow in the serum-free CHO medium (Gibco, Gaithersburg, MD). The antibodies from the supernatant were isolated by 50% ammonium sulfate precipitation and subsequent dialysis. Their purity was determined by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under non-reducing conditions using a silver staining kit (PlusOne, Pharmacia Biotech AB, Uppsala, Sweden) and by blotting using an anti-rat κ chain alkaline phosphatase conjugate (Pharmingen BD, San Diego). Care was taken to diminish all possible sources of endotoxin during the purification and subsequent conjugation steps.
The rat hybridoma I/9 producing a monoclonal anti-idiotype [anti-B10-anti-(T,G-A-L)] IgG2b antibody 27 (kindly provided by Dr. Gloria Laszlo, Immunology Research Group, Hungarian Academy of Sciences, Budapest, Hungary) was cultured and the antibody purified as described above. The following antibodies from Pharmingen BD (San Diego, CA) were used in the flow cytometry experiments: 2.4G2-FITC, anti-mouse CD3-FITC, PE conjugates of anti-mouse CD24, CD27 and CD38, as well as a PE-Cy5 conjugate of anti-mouse CD19.
Mice
Groups of female MRL/lpr mice (7-wk–old ones with initial disease and 18-wk-old ones with severe disease) were obtained from the Taiwan National University Animal Service and from Harlan Farm, Blackthorn, UK). The mice were kept under specific pathogen-free conditions, and the manipulations were approved by the Animal Care Commission at the Institute of Microbiology in accordance with International Regulations.
The animals (five and ten per group in the first and second experiment, respectively) were injected i.v. twice weekly for 6 wk with 20 µg of the DNA-like chimera, with the same amount of the control chimeras or with PBS alone. Every 14 days, they were bled and the sera kept frozen at –20°C. Albuminuria was measured using Multistix strips (Beyer Diagnostics Manufacturing Ltd., Bridgend, UK) and graded semi-quantitatively (0, none; 1, 30–100 mg/dL; 2, 100–300 mg/dL; 3, 300–500 mg/dL; 4, >500 mg/dL).
Construction of chimeric antibody molecules
Two synthetic peptides – the DNA mimotope peptide (DWEYSVWLSN) and an irrelevant peptide containing the same amino acids but in a shuffled order (WSLDYWNEVS) – were used in the study. The synthesis of Ac-DWEYSVWLSN-NH-(CH2)6-NH2 and Ac-WSLDYWNEVS-NH-(CH2)6-NH2 was carried out using Fmoc-based manual solid-phase peptide synthesis protocols on 2-Cl-Trt resin. The peptides were purified (⩾95% purity) by reversed-phase sample displacement chromatography 28 on a series of 3 × 50 mm Nucleodur 100-5 C18 columns (Macherey-Nagel, Germany). The DNA-mimicking DWEYSVWLSN peptide was coupled to the 2.4G2 antibody (DNA-like chimera) and to the irrelevant antibody I/9 (irrelevant chimera 2). The irrelevant peptide WSLDYWNEVS was coupled to the 2.4G2 antibody (irrelevant chimera 1).
The coupling of the antibodies to the peptides was carried out using the classical 1-ethyl-3-(3′-dimethylaminopropyl) carbodiimide.HCl (EDC; Fluka AG, Buchs, Switzerland) cross-linking technique 29, 30. During the synthesis of the peptides, a spacer [H2N(CH2)6H2N] was added to their C terminus with the aim to allow the peptide to form its own structure. To avoid cross-linking of the immunoglobulin molecules during the conjugation step, their concentration was kept low. The antibody (in concentration 0.1 mg/mL in sterile 0.1 M sodium phosphate buffer, pH 6.0) was mixed with a 20-fold molar excess of the peptide [dissolved in 10% v/v N,N-dimethylformamide (Sigma-Aldrich, Taufkirchen, Germany) in the same buffer to 0.02 mg/mL final concentration]. The reaction was started by the addition of carbodiimide at 60-fold molar excess over the antibody. The reaction mixture was stirred overnight at 4°C, dialyzed against PBS and concentrated by ultrafiltration. HPLC analyses using a model 1046A fluorescence detector (Hewlett-Packard), operating at excitation and emission wavelengths of 280 and 340 nm, respectively, were performed. The level of endotoxin in the final chimera solutions was determined by a Limulus amebocyte lysate assay (E-Toxate, Sigma).
Flow cytometry
The binding of the DNA-like chimera to mouse spleen cells was compared to that of the unconjugated 2.4G2 antibody itself. Cells were washed with PBS (containing 2.5% FCS and 0.05% sodium azide) and incubated with the 2.4G2 antibody or with the DNA-like chimera (1 µg/106 cells) for 30 min at 4°C, followed by two washes. Next, the cells were incubated with goat anti-rat κ light chain biotin-conjugated antibody (Pharmingen BD), followed by incubation with FITC-conjugated avidin (Calbiochem, Darmstadt, Germany). Flow cytometric analysis was carried out on a FACSCalibur flow cytometer; 10 000 cells were collected for each sample.
The retained ability of the DNA-like chimera to interact with FcγRII was confirmed as it competed successfully with a FITC-conjugated antibody with the same specificity for binding to FcγRII on mouse spleen cells. The splenocytes were incubated with increasing concentrations (1.0, 0.5, 0.25 and 0.1 µg/106 cells) of the DNA-like chimera (as described above), washed, and incubated with the same amount of a FITC-conjugated 2.4G2 antibody (Pharmingen BD).
The binding of the DNA-like chimera to mouse T cells or subsets of B cells was studied. Spleen cells were washed with PBS (containing 2.5% FCS and 0.05% sodium azide) and incubated with the DNA-like chimera (at 1 µg/106 cells) for 30 min at 4°C, followed by two washes. Next, the cells were incubated with a goat anti-rat IgG FITC-conjugated antibody, a PE-Cy5 conjugate of an anti-mouse CD19 antibody, and with one of the following anti-mouse antibody-PE conjugates: anti-CD3, anti-CD24, anti-CD27 or anti-CD38. The analysis was performed on a FACSCalibur flow cytometer; 10 000 cells were collected for each sample.
Detection of apoptosis
Spleen cells from a sick 12-wk-old female MRL/lpr mouse were cultured for 4 days at 2 × 106 cells/mL in complete RPMI 1640 medium or in the same medium containing 200 ng/mL of the DNA-like chimera. The apoptosis of gated B (CD19+) and T (CD3+) cells was measured using the Annexin V-FITC apoptosis detection Kit I (Pharmingen BD).
PAGE and Western blotting
All constructed chimeras were subjected to SDS-PAGE and Western blot analysis. SDS-PAGE was performed using 10% gels and a MiniProtean II system (Bio-Rad, Richmond, CA) in the presence of 0.1% SDS. After the electrophoresis, the proteins were transferred to a nitrocellulose membrane (0.45 µm; Sartorius, Germany) using a MiniTrans Blot device (Bio-Rad) in a buffer containing 48 mM Tris and 110 mM glycine in the presence of 20% v/v methanol. The membranes were blocked overnight in a TBS buffer containing 0.4% Tween-20, and were incubated for 1 h with the dsDNA-specific mouse IgG2a monoclonal antibody 10F10 (kindly provided by Dr. Bor-Luen Chiang, College of Medicine, National Taiwan University, Taipei, Taiwan). After washing, the membranes were incubated in an optimal dilution of a goat anti-mouse IgG antibody, conjugated to peroxidase (from Sigma). The reaction was developed using sodium nitroprusside and o-dianisidin-dihydrochloride-(3,3′-dimethoxybenzidine) (Sigma).
ELISA for anti-DNA antibodies
Methylated bovine serum albumin (Calbiochem; 10 µg/mL in PBS) was coated on 96-well Maxisorp immunoplates (Nunc, Roskilde, Denmark). The plates were washed and incubated with S1 nuclease-treated protein-free calf thymus DNA (Sigma) at a concentration of 2.5 µg/mL in PBS overnight at 4°C. The plates were then washed and blocked with 0.1% gelatin for 1 h at room temperature, followed by two washes. Next, the plates were incubated with sera dilutions for 1 h at 37°C. After five washes, the plates were incubated with peroxidase-conjugated anti-mouse IgG (γ chain-specific) antibody (Pharmingen BD) for 1 h at room temperature. The plates were washed and developed using a 2,2′-azido-bis (3-ethyl benz-thiazoline-6-sulfonic acid) solution (ABTS; Sigma) and read at 405 nm. All serum dilutions were tested in triplicates. Significance between IgG anti-dsDNA antibody levels in the DNA-like chimera and in the PBS-treated groups was determined by Student's t-test 31.
ELISA for other lupus antibodies
IgG anti-SSB/La, anti-SSA/Ro and anti-Sm IgG antibodies in sera of immunized animals were detected using commercial ELISA kits (from Alpha Diagnostic Int., San Antonio, USA) according to the manufacturer's instructions.
ELISpot assays
Nitrocellulose membranes (0.45 µm; Sartorius, Germany) with a size covering the working area of a blotting manifold apparatus (Bio-Dot, Bio-Rad) were coated with either 50 µg/mL calf thymus dsDNA or with 30 µg/mL diphteria toxoid (from BulBio, Sofia, Bulgaria) for 30 min at room temperature. Spleen cells were obtained from sick MRL/lpr mice 10 days after the second subcutaneous immunization with 2.5 µg alum-adsorbed diphtheria toxoid. These cells were cultured for 5 days at 2 × 106 cells/mL in RPMI 1640 (Gibco) containing 10% FCS, 4 mM L-glutamine, 50 µM 2-mercaptoethanol and antibiotics in the presence of the DNA-like chimera, or of the control chimeras, or of 10 µg/mL lipopolysaccharide (LPS) from E. coli (Sigma, L-2630 – used as a positive control), or of medium alone. The same number of cells from each well was then transferred to a well of the manifold apparatus on the pre-coated membranes. Next, after washing, the cells were incubated for five additional hours in a humidified 5% CO2 atmosphere at 37°C. After washing, the membranes were incubated with Fc-specific anti-mouse IgG conjugated with alkaline phosphatase (Sigma) for 2 h at room temperature and developed using the nitroblue tetrazolium/bromo-chloro-indolyl-phosphate chromogenic substrate (Sigma). The spots were counted blindly under a microscope by an independent observer.
Separately, samples from the same spleen cell suspension were added without additional in vitro culturing to the manifold apparatus containing DNA- and diphtheria toxoid-pre-coated membranes, and the IgG anti-dsDNA and anti-diphtheria toxoid antibody-producing cells were determined as described above. This number (showing the in vivo differentiated plasma cells) was subtracted from the number obtained after the 5-day-long in vitro cultivation.
Assays for glomerular IgG deposition
Kidneys from treated mice were frozen; cryostat sections were stained with a FITC-conjugated anti-mouse IgG antibody (Vector Laboratories Inc., Burlingame, CA) and viewed under a fluorescence microscope (Carl Zeiss, Jena, Germany). The second kidney of the animals was fixed, imbedded in a paraffin block and stained with hematoxylin/eosin using standard methods.
Statistical analysis
Animal experiments were repeated twice with five mice per group in the first and with ten mice per group in the second experiment. Differences in survival were compared using the Log Rank test. All in vitro experiments were repeated at least three times. Values in the figures were expressed as mean ± SD. Student's t-test was used to compare means between groups. A value of p <0.05 was considered to be statistically significant.
Acknowledgements
This study was supported by the Bulgarian National Science Fund (grant L1304/03), by a National Science Council of Taiwan travel grant (both to A.T.) and by the Howard Hughes Medical Institute (grant #55000340) and a Swiss National Science Foundation grant (to T.V.).
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
Appendix
Conflict of interest: The authors declare no financial or commercial conflicts of interest.




