Manipulation of NK cytotoxicity by the IAP family member Livin
Abstract
Natural killer (NK) cells are part of the innate immune system, capable of killing tumor and virally infected cells. NK cells induce apoptosis in the target cell by either granule- or receptor-mediated pathways. A set of inhibitory and activation ligands governs NK cell activation. As transformed cells often attempt to evade NK cell killing, up-regulation of a potential anti-apoptotic factor should provide a survival advantage. The inhibitor of apoptosis protein (IAP) family can inhibit apoptosis induced by a variety of stimuli. We have previously described a new IAP family member, termed Livin, which has two splice variants (α and β) with differential anti-apoptotic activities. In this study, we explore the ability of Livin to inhibit NK cell-induced killing. We demonstrate that Livin β moderately protects against NK cell killing whereas Livin α augments killing. We show that Livin β inhibition in Jurkat cells is apparent upon concomitant activation of an inhibitory signal, suggesting that Livin augments an extrinsic inhibitory signal rather than functioning as an independent inhibitory mechanism. Finally, we demonstrate that detection of both Livin isoforms in melanoma cells correlates with a low killing rate. To date, this is the first evidence that directly demonstrates the ability of IAP to protect against NK cell-induced apoptosis.
Abbreviations:
-
- BIR:
-
baculovirus IAP repeat
-
- IAP:
-
inhibitor of apoptosis protein
-
- KIR:
-
killer cell Ig-related receptor
-
- KIR2DL:
-
KIR two-domain long-tail receptor
-
- NCR:
-
natural cytotoxicity receptor
-
- PARP:
-
poly-ADP ribose polymerase
-
- RING:
-
really interesting new gene
Introduction
Natural killer (NK) cells play a major role in innate immunity responses, particularly against tumor and virally infected cells 1. The main NK cell functions include strong cytolysis of target cells combined with the release of cytokines and chemokines that have an inflammatory effect and ultimately modulate the adaptive response as well 2, 3. Although not antigen restricted, NK cell engagement is regulated by a set of surface inhibitory and activating receptors 4, allowing NK cells to correctly discriminate their targets from self cells 5. Killing activity is mainly mediated via the natural cytotoxicity receptors (NCR), which are expressed mainly by NK cells and include the NKp46, NKp44, NKp30 and NKG2D receptors 4, 6, 7. The tumor-derived NCR ligands are still elusive as the only NCR ligands identified so far are all of viral origin, including hemagglutinin for NKp46 8 and NKp44 8 and the CMV pp65 protein for NKp30 9. The NKG2D ligands are well characterized and include ULBP1, ULBP2, ULBP3 and ULBP4 10, and MICA and MICB 11. In contrast, inhibition of killing is attained mainly via recognition of MHC class I proteins by killer cell Ig-related receptors (KIR) 12–14. The inhibitory signal is delivered via the immunoreceptor tyrosine-based inhibitory motif (ITIM) found within the cytosolic tail of these receptors. Three families of MHC class I-binding inhibitory receptors are known to date 12. These include members of the Ig superfamily, namely KIR two-domain long-tail (KIR2DL) and KIR three-domain long-tail (KIR3DL) receptors 13 which recognize mainly HLA-C alleles and some HLA-B alleles carrying a Bw4 motif, respectively 15, the C-type lectin complex CD94/NKG2A which recognizes the non-classical HLA-E protein 16, and the leukocyte Ig-like receptor (LIR) family which has a broad recognition spectrum of both classical and non-classical HLA proteins 17. We have previously discovered a novel MHC class I-independent inhibitory pathway that is mediated through homophilic CEACAM1 interactions 18 or through heterophilic interactions of CEACAM1 with CEACAM5 19. Down-regulation of MHC class I proteins is a frequent event observed during the development of tumors 20. Selective MHC expression in tumors modulates adaptive and innate anti-tumor responses. By manipulating MHC class I protein expression, tumors may become vulnerable to NK cell-mediated killing 21 provided that NCR and NKG2D function 22.
NK cells kill mainly through the delivery of Granzyme B into the target cell, or by the binding of death receptors on its surface 23. Both pathways result in the induction of apoptosis 24. Upon its introduction into the target cell, Granzyme B can induce apoptosis by several mechanisms, such as activation of caspase 8 or via the mitochondrial pathway through Bid 25, 26. In addition, Granzyme B can directly cleave and activate caspase 3 27. As tumors seek to evade NK cell-mediated killing, an intracellular anti-apoptotic milieu provides a definitive advantage 28.
In this regard, a novel family of intracellular anti-apoptotic proteins that have become prominent in the field of cancer is the inhibitor of apoptosis protein (IAP) family. IAP molecules possess the potential to suppress apoptosis induced by a diverse range of stimuli 29, mainly by binding and inhibiting specific intracellular proteases, primarily caspases 3, 7 and 9 29. These proteins contain one or more repeats of the highly conserved baculovirus IAP repeat (BIR) domain, located at the N terminus. With the exception of NIAP and Survivin, human IAP contain a zinc binding motif known as really interesting new gene (RING) that is located at the C terminus 30. The BIR domain was shown to play a role in the anti-apoptotic function of IAP 31, 32; however, its exact function is still a matter of intense study 33. The BIR domain also has a regulatory function as the binding site of several IAP antagonists such as SMAC/Diablo and HtrA2 34. The IAP Livin contains a single BIR domain at the N-terminus as well as a C-terminus RING domain 35–38. We have demonstrated that Livin encodes two splice variants, termed Livin α and β 35. The two proteins are highly similar, except for 18 amino acids located between the BIR and the RING domains, which are present in the α but not the β isoform. Despite the high similarity, we have shown different anti-apoptotic properties of the two isoforms 35. In a previous study, we have shown that Livin undergoes caspase-mediated cleavage to produce a truncated protein (tLivin). tLivin not only loses its anti-apoptotic ability but also gains a paradoxically marked pro-apoptotic effect. Thus, Livin is in fact a regulator of apoptosis rather than a mere anti-apoptotic protein.
Livin expression was detected in nasopharyngeal carcinoma 39, neuroblastoma 40, lung cancer 41, 42, hematological malignancies 43 and superficial bladder cancer 44. Nevertheless, Livin was most closely linked to melanoma, with high levels of expression detected in numerous melanoma cell lines 35, 38. Malignant melanoma is increasing in incidence with a high mortality rate, due to the chemoresistant phenotype of most tumors. We have previously shown that Livin has a differential expression pattern in primary cultures derived from melanoma patients, while ubiquitous expression of Survivin and XIAP was observed. We found a correlation between Livin expression levels and the resistance of the cells to chemotherapy, both in vitro and in melanoma patients receiving chemotherapy. High levels of expression were also correlated with a lower survival rate 45.
To date, the ability of IAP to inhibit NK cell-mediated apoptosis has not been studied. We therefore sought to explore potential roles for Livin in the regulation of NK cell-mediated killing. In this study, we demonstrate for the first time that Livin β provides protection from NK cell-mediated killing. Interestingly, in Jurkat cells, this protection is only apparent upon concomitant inhibitory KIR recognition of MHC class I. Livin α, on the other hand, induces a paradoxical pro-apoptotic effect, resulting in increased lysis. We further explore possible direct interactions between Granzyme B and Livin. Finally, we shed light on the ability of Livin α to promote apoptosis.
Results
Expression of Livin β provides protection from NK cell-mediated killing
In order to study the ability of Livin to inhibit NK cell-mediated apoptosis, Jurkat cells and the EBV-transformed B cell line 721.221 were stably transfected with Livin α, Livin β, empty vector (designated Mock) or Bcl-2. NK cells isolated from the peripheral blood of various healthy donors were assayed for specific lysis of target cells. Bulk NK cultures were tested against Jurkat cells (Fig. 1A) or 721.221 cells (Fig. 1B) stably expressing the various constructs at different effector-to-target ratios. A marked inhibition of NK cell-mediated apoptosis was noted in Livin β-expressing cells as compared to Livin α-expressing cells or cells transfected with an empty vector. Notably, no inhibition was observed in Bcl-2-expressing target cells.
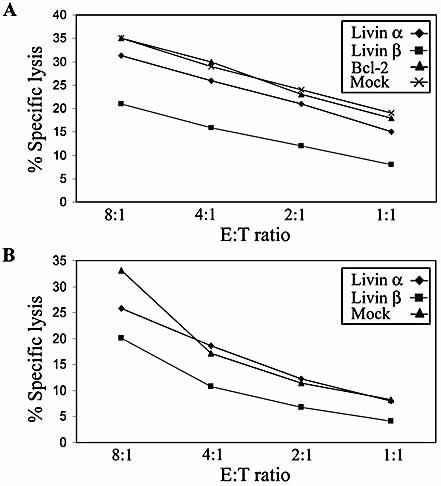
Livin β conveys moderate protection against NK cell-mediated cytotoxicity. Specific lysis assays were carried out with bulk NK clones and either Jurkat cells (A) or (B) 721.221 EBV-transformed B cells stably expressing Livin α, Livin β, empty vector (designated Mock) or Bcl-2. A representative experiment of three similar repeats is demonstrated on each panel. E:T signifies effector (NK cells)-to-target (Jurkat or 721.221 cells) ratio.
NK cell cytotoxicity is tightly regulated by various inhibitory and excitatory signals 4. To confirm that the reduced lysis of Livin β-expressing Jurkat cells was not due to a difference in other inhibitory signals, we tested the expression of MHC class I proteins on the various Jurkat transfectants using the W6/32 mAb. Importantly, all of the Jurkat cells expressed MHC class I with similar intensity (Fig. 2A). We further tested more specifically the ability of each transfectant to bind either KIR2DL1 or KIR2DL2 inhibitory receptors. This was performed by staining the Jurkat transfectants with chimeric KIR2DL1-Ig or KIR2DL2-Ig fusion proteins. In agreement with the similar expression levels of MHC class I, all Jurkat transfectants showed a similar staining profile using either KIR2DL1-Ig or KIR2DL2-Ig (Fig. 2B), with a significantly higher staining intensity of KIR2DL2-Ig, implying that the latter is the major inhibitory receptor on Jurkat cells. Since reduced killing could also reflect differences in engagement of NCR, we analyzed the recognition pattern of various NCR of the Jurkat transfectants. As the cellular ligands of the NCR are still to be determined, we utilized NCR-Ig fusion proteins: NKp46-Ig, NKp30-Ig and CD16-Ig. The binding intensity of each NCR-Ig fusion protein was divided by the background fluorescence, to provide fold binding values. Importantly, as with the inhibitory receptors, no significant difference was noted in NCR binding to the different targets (Fig. 2C). These results demonstrate that the main activating and inhibitory signals are similarly expressed by the various Jurkat transfectants, thus confirming the ability of Livin β to inhibit NK cell-mediated cytotoxicity.
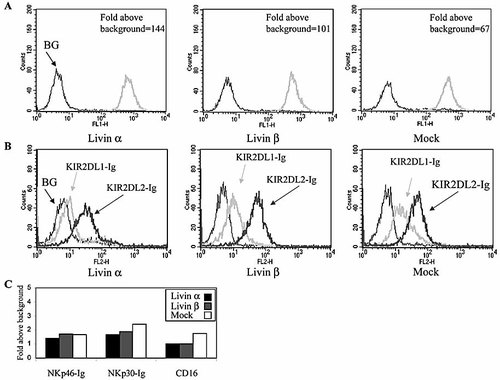
Target cells show similar expression of inhibitory and activation ligands. Target Jurkat cells were tested for their pattern of inhibitory ligands and activation signals. (A) Staining with w6/32, an anti-MHC1 antibody. (B) Staining with KIR2DL1-Ig and KIR2DL2-Ig fusion proteins. (C) Staining for NCR binding by NKp46-Ig, NKp30-Ig and CD16-Ig fusion proteins.
Concomitant engagement of inhibitory receptors is needed for Livin β inhibitory effect
To further explore the ability of Livin β to protect from NK cell-mediated killing, various NK clones were tested against the Jurkat transfectants. Notably, NK clones could be differentiated according to specific inhibition by Livin β. Indeed, out of more than 100 NK clones tested, most clones (roughly 75%) were moderately, yet specifically inhibited by Livin β as compared to mock-transfected and Livin α-transfected Jurkat cells (Fig. 3A; see representative NK clones 67 and 94). Interestingly, NK clones that were not inhibited by Livin β displayed increased lysis of the Livin α-transfected cells as compared to mock-transfected cells (Fig. 3B; see representative NK clones 85 and 89). We next explored the KIR expression profile of the various clones. NK clones were analyzed for their expression of KIR2DL1 and KIR2DL2, using the specific mAb HP3E4 and GL183, respectively. Remarkably, strong expression of KIR2DL2 was observed only by the clones that were inhibited by Livin β (Fig. 3C; representative clones 67 and 94) whereas NK clones 85 and 89 showed no expression of KIR2DL2 (Fig. 3D). No significant difference could be detected in the expression of KIR2DL1 between the different NK clones, except for NK clone 67 which expressed low levels of KIR2DL1 (Fig. 3C). The inhibitory effect of Livin β in KIR2DL2-positive NK clones was consistent in eight other clones tested, regardless of their KIR2DL1 expression (data not shown). These results are in agreement with the pattern of inhibitory NK receptor recognition of Jurkat cells (Fig. 2B), and suggest that the ability of Livin β to protect Jurkat cells from NK cell killing requires an inhibitory signal. However, the inhibitory activity did not depend on the absolute killing rate. Moreover, at different effector-to-target ratios, as the killing rate decreased, the relative protection by Livin β was unchanged. These results taken together suggest that the inhibitory effect exerted by Livin β is evident only when another inhibitory signal is transmitted, and in fact augments its effect rather then acting as a single determinant. To test the specificity of the KIR2DL2 interaction in Jurkat cells, we used the GL183 antibody, directed at KIR2DL2. NK clones that were inhibited by Cw3-expressing 721.221 cells (data not shown) were tested against Jurkat cells transfected with either empty vector, as a control, or Livin β. Clones against which protection was noted were pretreated either with or without GL183 antibody and then incubated with Jurkat cells. Indeed, the moderate protection provided by Livin β was abolished after blocking with GL183 (Fig. 3E).
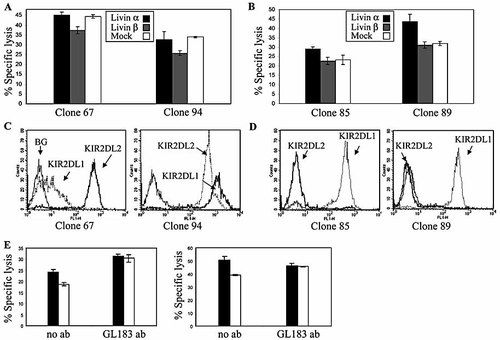
Different Livin isoforms manifest different effects on NK cell cytotoxicity. NK cell-mediated specific lysis assays performed with NK clones against Jurkat target cells. Representative clones are shown against which Livin β (A) showed a protective effect or (B) did not show a protective effect. (C, D) Expression of KIR2DL1 and KIR2DL2 by the indicated NK clones. Staining was performed by specific antibodies. (E) Empty vector (EV)-transfected (black) and Livin β-transfected (white) Jurkat cells were incubated with NK clones at an E:T ratio of 4 : 1. KIR2DL2 interaction was blocked using the GL183 mAb. Two representative clones are presented (t-test p <0.05).
NK cell-mediated apoptosis induces Livin cleavage
Granzyme B can induce apoptosis in target cells by direct cleavage and activation of caspases such as caspase 8 and caspase 3, but also can mimic caspase action and cleave some of their substrates, such as Bid and ICAD (Fig. 4A). We have previously demonstrated that, following apoptotic stimuli, full-length Livin is cleaved by effector caspases to produce a truncated form (tLivin) with paradoxical pro-apoptotic activity. To explore the relevance of Livin cleavage in NK cell cytotoxicity, we performed similar killing assays. At the indicated time points, Jurkat cells were lysed and Western blot analysis was performed. As shown in Fig. 4B, full-length Livin α and Livin β were detected as approximately 39- and 37-kDa proteins, respectively. Cleavage of both Livin α and β was noted as early as 1 h post exposure to NK cells, with a reverse ratio of full-length Livin to tLivin over time. No cleavage was detected after 4 h in the absence of NK cells (designated “4-”; Fig. 4B). NK cells were tested separately for Livin expression and demonstrated no detectable Livin levels. The cleavage rates of Livin α and β were similar. Indeed, no significant inhibition by Livin β was noted against the NK clone used in this experiment (data not shown), explaining the similar cleavage rates of Livin α and β.
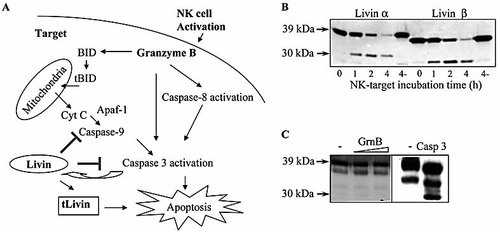
NK cell-mediated cytotoxicity induces cleavage of Livin. (A) Diagram demonstrating Granzyme B-mediated apoptosis pathways. (B) Following NK target incubation, Jurkat cells were lysed and Western blot analysis was performed using an anti-Livin antibody. “4-” represents control at 4 h of incubation without NK cells. (C) Recombinant Livin α was incubated with active Granzyme B; Western blot analysis was performed with anti-Livin antibody, to determine whether Granzyme B can specifically cleave Livin. The triangle represents escalating activity of Granzyme B as determined by a specific colorimetric assay. Active caspase 3 was used as a positive control, indeed generating the tLivin fragment of 30 kDa in size. A nonspecific lower band is noted, most probably due to the purification process of the recombinant protein.
To investigate whether Granzyme B is able to directly cleave Livin, or whether Livin cleavage results from caspase activation, purified recombinant Livin α and β were incubated with recombinant active Granzyme B. No Granzyme B-mediated cleavage of Livin was detected (Fig. 4C). The activity of Granzyme B was confirmed by a colorimetric assay. Control active caspase 3 readily cleaved Livin (Fig. 4C).
The pro-apoptotic effect of Livin α is cleavage dependent
As shown above, Livin β inhibited the killing activity of certain NK clones that expressed the KIR2DL2 receptor (Fig. 3). Interestingly, in the KIR2DL2-negative clones, where Livin β showed no inhibition, Livin α induced a pro-apoptotic effect (Fig. 3). We and others reported this contradictory behavior where ectopically expressed Livin α produced a pro-apoptotic effect in response to certain apoptotic stimuli such as etoposide or menadione 35, 36. We have previously demonstrated that caspase-mediated cleavage of Livin generates a truncated protein with paradoxical pro-apoptotic function 45. We therefore generated Livin α with a substitution mutation at the cleavage site and tested whether the pro-apoptotic effect is cleavage dependent. The mutation replaced aspartic acid at residue 52 by alanine (Livin α(D52A)). EBV-transformed 721.221 B cells were stably transfected with either Livin α, Livin α(D52A) or the empty vector as a control. In agreement with previous reports, Livin α produced a pro-apoptotic effect in response to etoposide as compared to empty vector control (Fig. 5A). This effect was not seen with Livin α(D52A), for which the apoptosis rate resembled that of the control (Fig. 5A). When cells were treated with anti-Fas/CD95 antibodies at low concentrations (0.2 µg/mL), Livin α was able to protect the cells from apoptosis, as compared to control cells (Fig. 5B). The cells transfected with Livin α(D52A) showed a significantly lower rate of apoptosis as compared with the cells expressing Livin α. At higher concentrations of anti-Fas/CD95 antibodies (0.4–1 µg/mL), Livin α induced a slightly pro-apoptotic effect, while Livin α(D52A) retained its ability to protect cells from apoptosis (Fig. 5B). The levels of expression of Livin α and Livin α(D52A) upon transfection were highly similar, as tested by flow cytometry analysis of IRES-expressed EGFP (data not shown), as well as by Western blot analysis (Fig. 5C). These findings indicate that cleavage of Livin α directly affects its ability to protect cells from apoptosis. In order to test whether the ratio of the full-length to its truncated form correlates with the apoptotic rate, cells were lysed, normalized for total protein and analyzed by Western blotting. As expected, no cleavage of Livin α(D52A) was detected (Fig. 5C), while cleavage of Livin α is readily seen. Concomitantly with the appearance of the cleavage fragment, depletion of full-length Livin α is observed. The apoptosis rate was further confirmed by poly-ADP ribose polymerase (PARP) cleavage. PARP is a caspase 3 substrate whose cleavage correlates with the apoptosis rate (Fig. 5C).
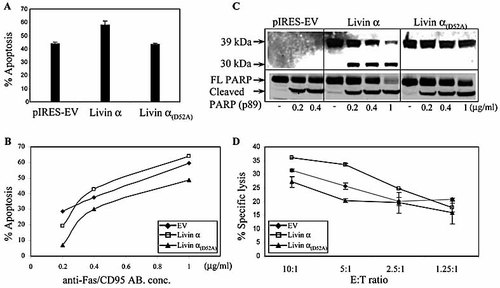
The pro-apoptotic effect of Livin α is cleavage dependent. 721.221 cells stably expressing the indicated plasmids were treated with (A) etoposide (1 µg/mL) for 18 h or (B) anti-CD95/Fas antibody for 18 h at the indicated concentrations. The apoptosis rate was determined using Annexin V/propidium iodide staining with flow cytometric analysis. (C) Concomitantly, cells treated with anti-CD95/Fas antibody were lysed and Western blot analysis was performed using a monoclonal anti-Livin antibody or anti-PARP antibody. The ratio between full-length PARP and the cleavage fragment (p89) serves as a marker of apoptosis. (D) Specific lysis assays were carried out with an NK line and Jurkat cells expressing the above constructs (EV, empty vector).
Similar results were obtained testing for NK cytotoxicity. Jurkat cells expressing Livin α showed a markedly higher killing rate as compared to control cells, designated empty vector (Fig. 5D). Notably, cells expressing Livin α(D52A) showed a decreased killing rate compared to control (Fig. 5D). Remarkably, at a low effector-to-target ratio, a reversed trend is observed where Livin α is able to moderately inhibit NK cell killing (Fig. 5D). This effect is similar to that observed in cells treated with a low anti-Fas/CD95 antibody concentration (Fig. 5C).
Livin expression correlates with low killing rate in melanoma cells
Most tumors positive for Livin express both Livin α and Livin β. In order to demonstrate the physiological relevance for Livin expression, we utilized two metastatic melanoma cell lines derived from the same patient, designated cell lines LB33 A1 and B1 46, 47. The primary Mel A1 expressed the HLA class I molecules A24, A28, B13, B44, Cw6 and Cw7. The patient was vaccinated repeatedly with autologous melanoma cells and achieved remission. Four years later she relapsed, and another cell line was generated, designated Mel B1. These cells showed no expression of the original HLA class I molecules except for A24. We have previously found that the expression of Livin (both isoforms) was only detected in Mel B1 and not in Mel A1, while both cell lines similarly expressed other IAP family members: XIAP and Survivin (Fig. 6A). Specific lysis assays of Mel A1 and Mel B1 against an NK cell line demonstrated that Mel B1 cells are highly resistant to NK cell killing in comparison to Mel A1 cells (30% versus 60%, respectively) (Fig. 6B). This result is even more significant as the loss of MHC class I molecules on the Mel B1 cell line should render it less resistant to NK cell killing.
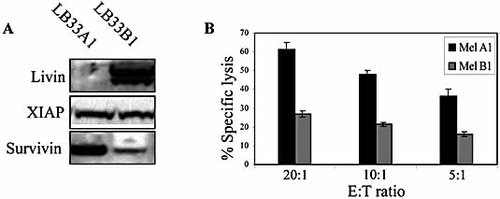
Livin expression correlates with low killing rate in melanoma cells. (A) Melanoma cell lines, designated Mel A1 and Mel B1, were lysed and Western blot analysis was performed using anti-Livin, anti-XIAP and anti-Survivin antibodies. (B) NK cell-mediated specific lysis assays were performed with an NK cell line against Mel A1 and Mel B1 cells.
Discussion
Tumors develop due to rapid proliferation, decreased death, or a combination of both factors. The natural mechanisms that evolved to ensure that randomly mutated cells will not develop into a malignant tumor include: contact inhibition, nutritional deprivation and immune surveillance. One of the first encounters of a transformed cell with the immune system involves NK cells. NK cells, as part of the innate immune system, are designed to recognize and eliminate tumor or virally infected cells 3, 22. Target recognition is by no means indiscriminate, and is governed by a set of various activating and inhibitory receptors which enable NK cells to spare normal cells 22. NK cytotoxicty is mediated by two main mechanisms: receptor mediated, such as FAS and TNF, and granule mediated, both of which induce apoptosis in the target cell 23. One of the mechanisms through which tumor cells are believed to acquire resistance to apoptosis is by overexpression of IAP. Indeed, overexpression of several IAP has been detected in various cancers 48 and IAP could block apoptosis induced by a variety of exogenous stimuli, governing chemotherapy resistance and tumor progression.
The possible role of IAP in allowing tumor cells to escape immune surveillance, and in particular NK cell cytotoxicity, has been poorly addressed. Recently, it has been shown that by expression of active SMAC, which leads to IAP inactivation, cells become more susceptible to a mouse LAK cell line, which is derived mainly from NK cells 49. Here, we utilized NK cells derived from healthy donors to study the ability of the IAP family member Livin to protect from NK cell cytotoxicity. Livin encodes two splice variants termed Livin α and β 35. The two proteins are highly similar but exhibit different anti-apoptotic properties. Here, we show that Livin β, but not Livin α, was able to inhibit NK cell-induced killing and this inhibition was observed when another inhibitory NK receptor was activated as well. This effect was evident in Jurkat cells where the inhibitory effect was only observed upon activation of KIR2DL2. Livin β inhibition was only moderate, reaching up to 30% inhibition (Fig. 1A). Cells expressing Bcl-2 did not show any resistance to NK cell-mediated killing, suggesting that the major pathway of apoptosis induction by NK cells in our experiments is mitochondria independent. Furthermore, we did not find a correlation between basal killing activity and inhibition by Livin β, suggesting that in Jurkat cells Livin β augments the effect of an ongoing inhibitory interaction rather than providing protection as a single determinant. Blocking the KIR2DL2 interaction eliminated the ability of Livin β to protect Jurkat cells from NK cytotoxicity (Fig. 3E). In other cells, such as the MHC-I negative 721.221 cells and the melanoma cell lines, we assume that Livin β tips the balance of other activating and inhibitory receptors toward inhibition.
Tumors such as melanoma that show high levels of Livin usually express both isoforms. To further demonstrate the physiological relevance of Livin in escaping NK cell surveillance, we used two melanoma cell lines derived from the same patient. Mel A1 was derived at diagnosis, following which the patient underwent autologous vaccination. The relapsed melanoma cells Mel B1 had different HLA class I molecules, rendering it resistant to the anti-tumor cytolytic T lymphocyte (CTL) response, yet possibly more sensitive to NK cell killing. Remarkably, the Mel B1 cells showed high levels of both Livin isoforms, and were highly resistant to NK cell killing, as compared to Mel A1 cells with no detectable Livin expression. These results provide new insights regarding the role of Livin, and possibly other IAP, in tumor development, allowing tumor cells to evade the anti-tumor immune response.
We recently demonstrated that Livin interaction with caspase is bidirectional as Livin undergoes caspase-mediated cleavage 45, and that cleavage of Livin produces a truncated form with a marked paradoxical pro-apoptotic activity. Similarly, we demonstrated here that, following NK cell-induced apoptosis, Livin also underwent cleavage. Cleavage of Livin is mainly caspase mediated, as Granzyme B was unable to directly cleave Livin.
Livin possesses both the ability to protect from cell death and to promote it once it is cleaved. How then is the balance achieved? We and others reported that cells transfected with Livin α were protected from certain apoptotic stimuli, such as anti-Fas/CD95 antibodies, while showing a higher rate of apoptosis, compared to control, when treated with other apoptotic stimuli such as etoposide 35, 36. A similar effect was observed with NK cell-mediated cytotoxicty, in which marked enhanced lysis was noted in cells transfected with Livin α. In contrast, when an inhibitory signal is transmitted such as via KIR2DL2, enhanced killing of Livin α-expressing cells was not observed (Fig. 3A, B).
As mentioned, Livin undergoes caspase-mediated cleavage, which generates a pro-apoptotic truncated form (tLivin). We therefore hypothesize that the enhanced apoptosis observed in cells transfected with Livin α resulted from the generation of the pro-apoptotic tLivin. Indeed, in cells expressing mutated uncleavable Livin α, no pro-apoptotic effect was noticed in response to all apoptotic stimuli tested, including NK cytotoxicity (Fig. 5). Moreover, cells expressing Livin α(D52A) were resistant to both anti-Fas/CD95 antibody treatment and NK cell-mediated cytotoxicity, as compared to control. Native Livin α showed an enhanced apoptotic rate at high anti-Fas/CD95 antibody concentrations and at high effector-to-target ratios (specific lysis assays). Remarkably, however, it showed a moderate ability to inhibit apoptosis at low anti-FAS/CD95 antibody concentration, and low effector-to-target ratio. We can conclude that Livin α is a weak anti-apoptotic agent against the stimuli tested. Under a strong apoptotic stimulus, caspase activation generates pro-apoptotic tLivin, and paradoxically enhances apoptosis. Yet a weak apoptotic signal allows Livin α to confer inhibition. Livin β is unable to confer similar pro-apoptotic effects, most probably due to a higher potency of the anti-apoptotic full-length protein.
Interestingly, the Mel B1 cells, as most tumors, express both Livin isoforms (Livin α and β) and were highly resistant to NK cell killing. Presumably, the strong anti-apoptotic activity of Livin β precludes any significant caspase activation, thus allowing Livin α to exert its anti-apoptotic effect as well. This synergic effect might explain the only moderate ability of Livin β to inhibit NK killing when expressed alone, as compared to the marked resistance of the Mel B1 cells. These results further signify the physiological role of Livin in tumors, which usually express both isoforms.
The dual pro- and anti-apoptotic abilities of Livin suggest fine-tuning of apoptosis activation. At the initiation of the apoptotic cascade, Livin displays its anti-apoptotic activity, similar to the mutated uncleavable Livin. This might allow the cell to stop transient apoptotic stimuli early on, before effector caspases are activated resulting in potential cell damage. However, upon exposure to continuous apoptotic stimuli and effector caspase activation, cleavage of Livin results in a pro-apoptotic product that ensures cell death, and its commitment to apoptosis.
Materials and methods
Cells and apoptosis induction
The Jurkat human T cell leukemia/lymphoma cell line and the 721.221 EBV-transformed B cell line were grown in RPMI 1640. Media were supplemented with 10% fetal calf serum, 100 U/mL penicillin, 100 µg/mL streptomycin, and 1 mM L-glutamine. Primary NK cells (NK clones) were isolated from PBL using the human NK cell isolation kit and the autoMACS instrument (Miltenyi Biotec, Auburn, CA) and grown as described 50. The melanoma cell lines LB33 Mel A1 and B1 (a generous gift from P. G. Coulie) 51 were grown in DMEM.
Plasmid constructs and cell transfection
Transfections with the pIRES2-EGFP plasmids (QIAGEN, Germany) encoding the various constructs of Livin described were carried out by electroporation of 721.221 cells 50. The retroviral vectors pLXSN (Clontech, CA) containing the cDNA of either the Livin α or β splice variant were prepared as described 45. Jurkat cells were infected with the packaged particles, and were placed under selection using G418 (Sigma).
Cytotoxicity assays and apoptosis assays
The cytotoxic activity of NK cells against the various targets was assessed in 5-h 35S-release assays, as described 50. In order to block the KIR2DL2 interaction, NK clones were pre-incubated with the mAb GL183 (0.5 µg/well, 200 µL) for 1 h prior to incubation with target cells. In all assays, spontaneous release did not exceed 20%. To induce apoptosis, cells were treated with the anti-CD95 mAb clone DX2 (R&D Systems), staurosporine, a protein kinase C inhibitor, and the Topoisomerase II inhibitor etoposide (Sigma). Flow cytometric analysis of the apoptotic cells was performed by Annexin-V-Cy5 and propidium iodide staining according to the manufacturer's instructions (MBL).
Ig fusion proteins
The Ig fusion proteins used in these studies were the KIR2DL1-Ig, KIR2DL2-Ig, NKp30-Ig, NKp46-Ig and CD16-Ig. Briefly, the sequences encoding the extracellular portions of the receptors were amplified by PCR from cDNA isolated from human NK clones. These PCR-generated fragments were cloned in frame into a mammalian expression vector containing the Fc portion of human IgG1. Constructs were transiently transfected into COS-7 cells, and the protein produced was purified from supernatants using a protein G column 18.
Flow cytometry and mAb
mAb used in this work were the pan anti-MHC class I W6/32 mAb, the HP3E4 mAb directed against KIR2DL1, KIR2DS1 and KIR2DS4, and the GL183 mAb directed against KIR2DL2. Cells were stained with mAb or Ig fusion proteins. Second reagents were FITC-conjugated F(ab’)2 goat anti-mouse or anti-human IgG (Jackson ImmunoResearch Laboratories), directed against mAb or Ig fusion proteins, respectively. The mAb were used at a final concentration of 3 µg/mL, and the Ig fusion proteins at 100 µg/mL. The staining procedure was as follows: 30 000 cells were washed once in FACS medium (1 × PBS, 0.5% BSA, 0.05% NaN3) and then incubated in 100 µL FACS medium containing either mAb or Ig fusion proteins for 1 or 2 h on ice, respectively. Cells were then washed twice in FACS medium and incubated on ice for 1 h with the appropriate secondary antibody. Following the incubation, cells were washed twice, resuspended in 200 µL FACS medium and analyzed on a FACSCalibur (BD Biosciences).
Cell lysis, Western blot analysis and antibodies
Whole cell lysates were prepared using lysis buffer containing 20 mM Tris-HCl, 2 mM EDTA, 6 mM β-mercaptoethanol, 1% Nonidet P40 (NP40), 0.1% SDS and protease inhibitors [1 mM PMSF, protease inhibitor cocktail (Sigma) diluted 1 : 10, and complete inhibitor cocktail (Roche, Germany) diluted 1 : 25]. Cells (0.25 × 106 to 1×106) were lysed in 100 µL lysis buffer at 4°C for 20 min with vigorous vortexing.
The membrane was exposed to the antibodies in blocking solution (PBS, 1% casein, 0.05% Tween-20) for 1 h, followed by three 5-min washes with PBS. An mAb against Livin (clone 88C570; Imgenex) was diluted 1 : 3000. For these antibodies, Envision-HRP (DAKO, Denmark) was used as a secondary antibody for the enhanced chemiluminescence (ECL) reaction. A polyclonal antibody against PARP (Cell Signaling) was diluted according to the manufacturer's recommendation and anti-rabbit IgG HRP-linked antibody (Cell signaling) was used as a secondary antibody. The ECL reaction was performed as described previously [45].
Recombinant proteins and Granzyme B assay
Recombinant Livin was generated in bacteria and purified on Nickel columns as described 45. Livin concentration was determined by Coomassie gel analysis. Recombinant Granzyme B (Biomol) and caspase 3 (R&D Systems) activities were tested by a colorimetric assay system (Biomol).
Appendix
Conflict of interest: The authors declare no financial or commercial conflicts of interest.




