Leukocyte-associated Ig-like receptor-1 has SH2 domain-containing phosphatase-independent function and recruits C-terminal Src kinase
Abstract
Most inhibitory receptors in the immune system contain one or several immunoreceptor tyrosine-based inhibitory motifs (ITIM) and recruit the SH2 domain-containing phosphatases SHP-1, SHP-2 and/or SHIP, which are generally believed to be essential for the inhibitory function. However, it has not been systematically investigated whether ITIM-bearing receptors exert their function through alternative interactions. Here we describe that leukocyte-associated Ig-like receptor (LAIR)-1 has inhibitory function in DT40 chicken B cells that lack both SHP-1 and SHP-2. In addition, we found that LAIR-1 did not recruit SHIP upon phosphorylation. Thus, LAIR-1 can function independently from SH2 domain-containing phosphatases and must recruit at least one other signaling molecule. Using a yeast-tri-hybrid system, we found that phosphorylated LAIR-1 bound the C-terminal Src kinase (Csk). The interaction required the SH2 domain of Csk and phosphorylation of the tyrosine in the N-terminal ITIM of LAIR-1. We propose that Csk is an additional player in the regulation of the immune system by ITIM-bearing receptors.
Abbreviations:
-
- Csk:
-
C-terminal Src kinase
-
- HRP:
-
horseradish peroxidase
-
- ITIM:
-
immunoreceptor tyrosine-based inhibitory motif
-
- LAIR-1:
-
leukocyte-associated Ig-like receptor-1
-
- RBL:
-
rat basophilic leukemia
-
- SH:
-
src homology
-
- SHP:
-
SH2 domain-containing tyrosine phosphatase
Introduction
An appropriate response of the immune system depends on a balance between activating and inhibitory signals. Inhibitory receptors play an important role in the regulation of immune cells. This is illustrated by the fact that mutations in inhibitory receptors or in the molecules through which they signal, are associated with autoimmune disease 1. Most inhibitory receptors in the immune system contain one or several immunoreceptor tyrosine-based inhibitory motifs (ITIM). This motif has the consensus sequence (I/V/L/S)-x-Y-x-x-(L/V/I), where x represents any amino acid 2. Upon engagement, ITIM-bearing receptors become phosphorylated and recruit the SH2 domain-containing inositol phosphatase SHIP and/or the SH2 domain-containing tyrosine phosphatases SHP-1 and SHP-2. The phosphatases subsequently dephosphorylate and thereby inactivate key molecules involved in cellular activation 2, 3. It is widely believed that the recruitment of SH2 domain-containing phosphatases is required for the inhibitory function of ITIM-bearing receptors. However, several studies, including our previous work on leukocyte-associated Ig-like receptor (LAIR)-1, suggest that the phosphatases are not the sole down-stream effectors of ITIM-bearing receptors.
LAIR-1 is an ITIM-bearing receptor that is broadly expressed in the immune system 4 and functions as an inhibitory receptor on NK cells, T cells and B cells 4–9. In addition, LAIR-1 inhibits the differentiation of peripheral blood precursors towards dendritic cells 10 and induces apoptosis in myeloid leukemia cells 11, 12. LAIR-1 is phosphorylated by Src family kinases and recruits SHP-1 and SHP-2 4, 13–15. Recently, we identified a mouse homologue of LAIR-1, but unlike human LAIR-1, it does not recruit SHP-1, although it has a similar inhibitory capacity 16. In addition, a human LAIR-1 mutant that lacks the N-terminal tyrosine does not detectably bind SHP-1 or SHP-2 but still has inhibitory function 15. Taken together, this suggests that LAIR-1 may also recruit other molecules.
Here we show that LAIR-1 has an inhibitory capacity in SHP-1- and SHP-2-deficient DT40 chicken B cells and does not recruit SHIP. This indicates that, in contrast to the established dogma, LAIR-1 has an inhibitory function independent of SH2 domain-containing phosphatases. Therefore, we searched for other LAIR-1-interacting proteins. Using a yeast-tri-hybrid screen, we have found that C-terminal Src kinase (Csk) interacts with phosphorylated LAIR-1. As Csk inactivates Src family kinases 17, 18, it may be a novel player in the regulation of immune responses by ITIM-bearing receptors.
Results
LAIR-1 has inhibitory function independent of SHP-1 and SHP-2
LAIR-1 recruits both SHP-1 and SHP-2 4, 13–15. To determine the contribution of each phosphatase in LAIR-1 signaling, we investigated the function of LAIR-1 in the chicken B cell line DT40. DT40 mutants have been generated that lack SHP-1, SHP-2 or both phosphatases (19 and Fig. 1A). We stably transfected the DT40 cells with an mFcγRIIB/hLAIR-1 chimera that contained the extracellular and transmembrane domains of mFcγRIIB and the intracellular domain of LAIR-1. The mFcγRIIB/hLAIR-1 chimera can be co-ligated to the BCR by cross-linking the BCR with mouse IgM anti-chicken IgM in combination with intact rabbit Ig anti-mouse IgM, which will also bind to the FcγRIIB moiety of the chimera through the Fc part. No co-ligation of the chimera occurs when rabbit F(ab’)2 anti-mouse IgM is used as a secondary antibody 20, 21. The expression of the mFcγRIIB/hLAIR-1 chimera was similar on all clones (Fig. 1B).
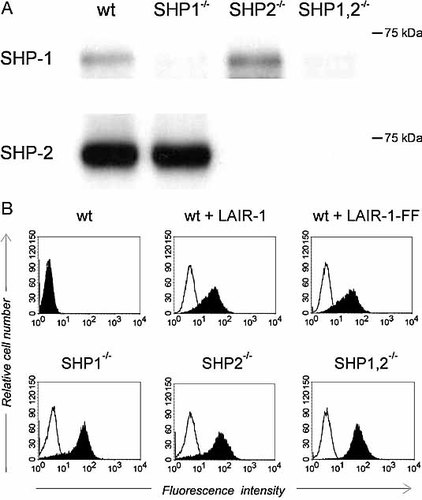
Generation of DT40 clones stably expressing the mFcγRIIB/hLAIR-1 chimera. (A) Expression of the phosphatases in the different DT40 cell lines was determined by immunoprecipitation of SHP-1 from pervanadate-treated cells and Western blot analysis using anti-phosphotyrosine antibodies (upper panel) and by Western blot analysis of whole lysates using anti-SHP-2 antibodies (lower panel). (B) Expression of the mFcγRIIB/hLAIR-1 chimera was analyzed by staining the DT40 clones with FITC-conjugated anti-mFcγRIIB antibodies (solid histograms) or isotype control (open histograms). Upper panels: untransfected DT40 (wt), wild-type DT40 transfected with the mFcγRIIB/hLAIR-1 chimera (wt + LAIR-1), wild-type DT40 transfected with the mFcγRIIB/hLAIR-1 chimera containing tyrosine-to-phenylalanine mutations (wt + LAIR-1-FF). Lower panels: SHP-1, SHP-2 or double-deficient cells transfected with the mFcγRIIB/hLAIR-1 chimera.
Using this system, we found that signaling by LAIR-1 inhibited BCR-induced calcium mobilization (Fig. 2A). The inhibitory function depended on the LAIR-1 ITIM, as mutation of the tyrosines within these ITIM abrogated the inhibition of calcium mobilization (Fig. 2B). We next investigated the contribution of each phosphatase to LAIR-1-mediated inhibition. In DT40 cells that were deficient for either SHP-1 or SHP-2, LAIR-1 inhibited the calcium mobilization to a similar extent as in wild-type cells (Fig. 2C, D), suggesting that SHP-1 and SHP-2 have redundant functions in LAIR-1 signaling. Surprisingly, LAIR-1 still had an inhibitory effect on BCR-induced calcium mobilization in cells lacking both phosphatases (Fig. 2E), indicating that LAIR-1 has a phosphatase-independent inhibitory function.
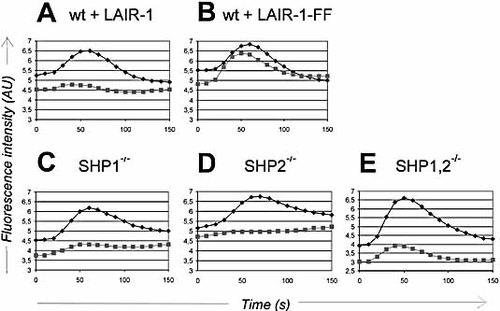
LAIR-1 inhibits BCR-induced calcium mobilization in phosphatase-deficient DT40 cells. DT40 transfectants were assayed for calcium mobilization upon BCR cross-linking with (gray squares) or without (black diamonds) co-ligation of the mFcγRIIB/hLAIR-1 chimera. (A, B) Wild-type DT40 cells transfected with the mFcγRIIB/hLAIR-1 chimera (wt + LAIR-1) or the mFcγRIIB/hLAIR-1 chimera containing tyrosine-to-phenylalanine mutations (wt + LAIR-1-FF). (C–E) SHP-1, SHP-2, or double-deficient DT40 cells transfected with the mFcγRIIB/hLAIR-1 chimera. The results shown are representative of three independent experiments. Similar results were obtained with another set of independent clones.
SHP-1 and SHP-2 exert their inhibitory function by dephosphorylation of key molecules involved in cellular activation 22. We therefore investigated the effect of LAIR-1-mediated signaling on BCR-induced tyrosine phosphorylation. Cross-linking of the chicken BCR resulted in the phosphorylation of a number of proteins, which was inhibited upon co-ligation of the mFcγRIIB/hLAIR-1 chimera (Fig. 3, left panel). Again, the inhibitory function depended on phosphorylation of the ITIM, as the LAIR-1 mutant lacking functional ITIM had no effect on tyrosine phosphorylation (Fig. 3, middle panel). However, in the absence of SHP-1 and SHP-2, co-ligation of the wild-type mFcγRIIB/hLAIR-1 chimera still reduced BCR-induced tyrosine phosphorylation. Thus, the ITIM of LAIR-1 are able to recruit at least one other molecule that mediates the inhibition of protein tyrosine phosphorylation and cellular activation.
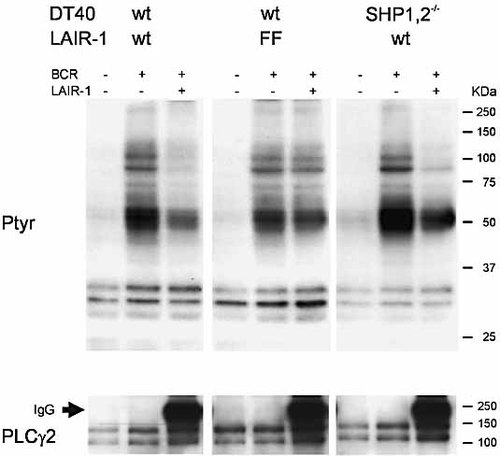
SHP-1 and SHP-2 are not required for LAIR-1-mediated inhibition of tyrosine phosphorylation. Wild-type (wt) and phosphatase-deficient DT40 cells transfected with the mFcγRIIB/hLAIR-1 chimera or the mFcγRIIB/hLAIR-1 chimera containing tyrosine-to-phenylalanine mutations (FF) were left untreated, stimulated through the BCR alone or stimulated through the BCR with co-ligation of the mFcγRIIB/hLAIR-1 chimera (LAIR-1) for 1 min. Cells were lysed immediately and Western blotting was performed using anti-phosphotyrosine antibodies. Staining for PLCγ2 was used as a loading control. The results shown are representative of three independent experiments.
LAIR-1 does not recruit SHIP
An alternative molecule involved in the signaling of several ITIM-bearing receptors is the 5′-inositol phosphatase SHIP. To investigate whether SHIP binds to phosphorylated LAIR-1, we transfected 293T cells with FLAG-tagged LAIR-1 (Fig. 4A). FLAG-tagged human FcγRIIB was used as a positive control 23, 24. The cells were treated with pervanadate to induce extensive phosphorylation of the receptors 15, and the FLAG-tagged proteins were immunoprecipitated. While a significant amount of SHIP co-immunoprecipitated with FcγRIIB, no SHIP was found in association with LAIR-1 (Fig. 4B). In agreement with this observation, the mFcγRIIB/hLAIR-1 chimera inhibited BCR-induced calcium mobilization in SHIP-deficient DT40 cells (Fig. 4C, D), while co-ligation of wild-type FcγRIIB has no inhibitory effect in these cells 20. Thus, we conclude that SHIP does not play a role in LAIR-1-mediated signaling.
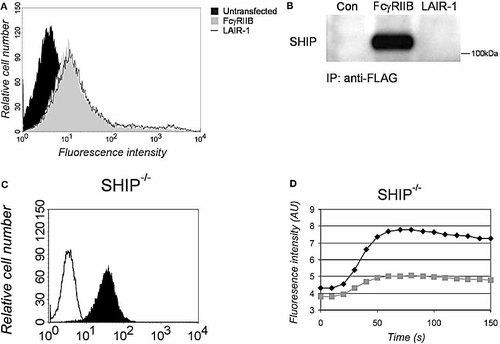
SHIP does not bind to LAIR-1. (A) 293T cells (black histogram) and 293T cells transfected with FLAG-tagged FcγRIIB (gray histogram) or FLAG-tagged LAIR-1 (open histogram), were stained with anti-FLAG antibodies followed by PE-conjugated goat anti-mouse antibodies and analyzed by flow cytometry. (B) The cells described in (A) were treated with pervanadate and the phosphorylated receptors were immunoprecipitated using anti-FLAG coated beads. Western blotting was performed with anti-SHIP antibodies. (C) SHIP-deficient DT40 cells transfected with the mFcγRIIB/hLAIR-1 chimera were stained with FITC-conjugated anti-mFcγRIIB antibodies (solid histogram) or isotype control (open histogram). (D) The cells were assayed for calcium mobilization upon BCR cross-linking with (gray squares) or without (black diamonds) co-ligation of the mFcγRIIB/hLAIR-1 chimera. The results shown are representative of three independent experiments. Similar results were obtained using another clone.
Human and mouse LAIR-1 bind Csk
To identify proteins that selectively bind to phosphorylated LAIR-1, we performed a yeast-tri-hybrid screen with a human fetal brain library using the intracellular tail of human LAIR-1 as bait. We used the Ylck4.1 strain, in which expression of Lck can be induced, enabling the identification of phosphotyrosine-dependent interactions. Protein interactions were detected by the expression of the β-galactosidase reporter gene. We identified two clones that were positive in the reporter assay. Both clones contained a segment of Csk carrying the SH2 domain and the kinase domain (amino acids 84–450). This Csk fragment bound to LAIR-1 in a phosphorylation-dependent manner, as the yeast cells did not show β-galactosidase activity when grown on media containing methionine, which suppresses Lck expression. The intracellular tail of mouse LAIR-1 (mLAIR-1) also bound Csk in yeast (Fig. 5A).
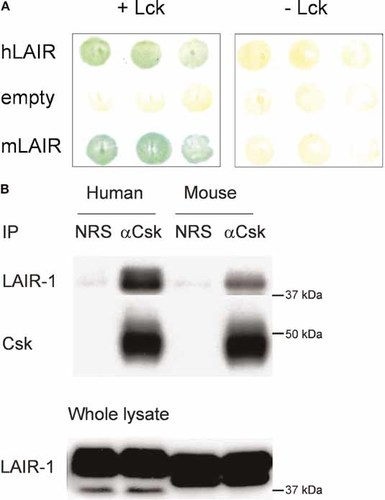
Csk binds to phosphorylated LAIR-1. (A) Ylck stably transfected with either Gal4BD-human LAIR-1 (human) or Gal4BD-mouse LAIR-1 (mouse) was transfected with Gal4 AD-CskΔSH3 and grown on selective medium in the absence (+Lck) or presence (-Lck) of methionine for 48 h. The yeast was transferred to a nylon membrane and assayed for Gal4-promotor activity using a standard β-galactosidase assay. Yeast stably transfected with Gal4BD alone (empty) was used as a negative control. (B) RBL cells stably transfected with human LAIR-1 or a human LAIR-1/mouse LAIR-1 chimera (mLAIR) were lysed and immunoprecipitation was performed with normal rabbit serum (NRS) or rabbit anti-Csk antibodies. Immune complexes were separated by SDS-PAGE and Western blotting was performed with anti-LAIR-1 and anti-Csk antibodies. Equal amounts of whole cell lysates were loaded to confirm LAIR-1 expression. The results shown are representative of three independent experiments.
To confirm the interaction of Csk with LAIR-1 in eukaryotic cells, we performed co-immunoprecipitation studies in rat basophilic leukemia (RBL) clones stably expressing human LAIR-1 or a human LAIR-1/mouse LAIR-1 chimera. Both molecules were selectively co-immunoprecipitated using anti-Csk antibodies (Fig. 5B). As the interaction between LAIR-1 and Csk is phosphorylation dependent in yeast, our results suggest that there must be constitutive LAIR-1 phosphorylation in RBL cells. Indeed, we have found that in unstimulated RBL cells, although barely detectable, there is phosphorylation of LAIR-1. This is sufficient for a low level of SHP-1 recruitment, which requires the phosphorylation of the ITIM (15 and data not shown). As shown below, Csk did not interact with the LAIR-1 mutant in which both tyrosines were mutated, indicating that the interaction with Csk also requires phosphorylation of LAIR-1 in RBL cells.
The interaction of Csk with LAIR-1 requires the SH2 domain of Csk and the tyrosine residue in the N-terminal ITIM of LAIR-1
Csk consists of an SH3 domain, an SH2 domain and a kinase domain. The Csk fragment that was obtained in the yeast-tri-hybrid screen contained the SH2 domain and the kinase domain. As the interaction of Csk with LAIR-1 is phosphotyrosine dependent, we investigated whether Csk binds to LAIR-1 through its SH2 domain. We therefore transformed yeast cells, stably expressing the Gal4 binding domain-LAIR-1 fusion protein, with Gal4 activation domain fusion proteins of either full-length Csk, Csk lacking the 83 N-terminal amino acids containing the SH3 domain (CskΔSH3) or Csk lacking the SH2 domain (CskΔSH2). As shown in Fig. 6A, full-length Csk and CskΔSH3 bound to LAIR-1, while CskΔSH2 did not. This indicates that indeed the SH2 domain of Csk binds to phosphorylated LAIR-1.
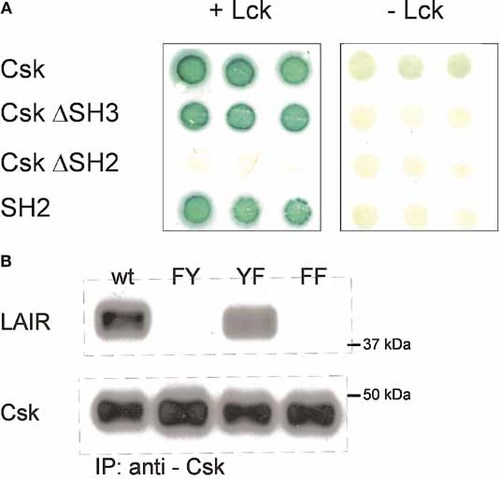
The interaction of Csk with LAIR-1 requires the SH2 domain of Csk and the N-terminal tyrosine of LAIR-1. (A) Ylck stably transfected with Gal4BD-human LAIR-1 was transfected with Gal4 AD-Csk constructs containing either full-length Csk, CskΔSH3, CskΔSH2 or the SH2 domain alone and grown on selective medium in the absence of methionine (+Lck) or in the presence of methionine (-Lck) for 48 h. The colonies were assayed for Gal4-promotor activity using a standard β-galactosidase assay. (B) RBL cells stably expressing either wild-type (wt) LAIR-1 or LAIR-1 mutants containing tyrosine-to-phenylalanine mutations in the N-terminal ITIM (FY), C-terminal ITIM (YF) or both ITIM (FF) were lysed and immunoprecipitation was performed with anti-Csk antibodies. Immune complexes were separated by SDS-PAGE and Western blotting was performed with anti-LAIR-1 and anti-Csk antibodies.
We have previously shown that the ITIM of LAIR-1 contribute differentially to the recruitment of SHP-1 and SHP-2 15. To investigate which tyrosine is required for the interaction with Csk, we performed co-immunoprecipitations in RBL cells stably expressing wild-type LAIR-1 or mutants of LAIR-1 with tyrosine-to-phenylalanine mutations in the N-terminal ITIM (FY), the C-terminal ITIM (YF) or both (FF). The expression level of each LAIR-1 mutant was similar 15. The mutant in which the C-terminal ITIM was mutated still bound Csk, while mutation of the N-terminal ITIM abrogated Csk binding (Fig. 6B). Thus, the SH2 domain of Csk binds to the phosphorylated tyrosine residue in the N-terminal ITIM of LAIR-1.
Discussion
Many ITIM-bearing receptors recruit SHP-1 and SHP-2 upon phosphorylation, and it is generally thought that these phosphatases are necessary for their inhibitory function. Here we investigated the role of these phosphatases in LAIR-1-mediated signaling. Surprisingly, we found that LAIR-1 inhibited BCR-induced calcium mobilization and tyrosine phosphorylation in DT40 cells lacking both SHP-1 and SHP-2. This is in contrast to previous studies with other ITIM-bearing receptors that recruit both SHP-1 and SHP-2: killer cell Ig-like receptor (KIR), platelet endothelial cell adhesion molecule (PECAM)-1 and CD66a are no longer effective in SHP-1- and SHP-2-deficient DT40 cells, while the inhibitory function of paired Ig-like receptor (PIR)-B is strongly reduced 19–21, 25. The inhibitory function of LAIR-1 in the absence of SHP-1 and SHP-2 is not due to recruitment of SHIP, since we did not find an interaction between SHIP and LAIR-1 under the conditions in which SHIP does bind to FcγRIIB. This is in accordance with the fact that neither ITIM of LAIR-1 contains a leucine at the Y+2 position, a residue that is essential for the recruitment of SHIP to FcγRIIB 26. In addition, LAIR-1 function was not affected in SHIP-deficient DT40 cells. Thus, we conclude that LAIR-1 has an inhibitory function independent of SH2 domain-containing phosphatases and that there must be at least one other protein that can associate with LAIR-1 and inhibit cellular activation.
Using a yeast-tri-hybrid system, we found that phosphorylated LAIR-1 bound to Csk. This interaction was mediated by the SH2 domain of Csk and required phosphorylation of the N-terminal ITIM of LAIR-1. Mutants of LAIR-1 that lack the N-terminal ITIM did not bind Csk, which may be explained by the limited phosphorylation of these mutants 15, or by the specificity of the SH2 domain of Csk for the N-terminal tyrosine.
Although in the last few years several Csk interacting molecules have been identified (listed in 27), there is no specific consensus sequence that predicts binding of Csk. Most Csk-interacting proteins do not contain ITIM. The ITIM-containing SHP-2 interacting transmembrane adaptor protein (SIT) binds Csk, but via a tyrosine residue outside the ITIM 28. However, previously, it was reported that signal regulatory protein (SIRP)α and Ig-like transcript (ILT)-2 bind Csk 29, 30. In contrast, KIR3DL1 was reported not to recruit Csk 31. In accordance with these reports, we found in the yeast-tri-hybrid system that Csk also bound to FcγRIIB and SIRPα, but not to KIR3DL1 and KIR2DL2 (data not shown). Thus, Csk appears to interact with several, but not all ITIM-bearing receptors. It remains to be investigated what determines whether a particular ITIM-bearing receptor recruits Csk. Since the sequence of the N-terminal ITIM of LAIR-1, VTYAQL, is also present in KIR3DL1, which does not bind Csk, it is likely that not the ITIM consensus sequence, but another motif that includes the same tyrosine, is involved in Csk binding.
Csk is a kinase that phosphorylates Src family kinases at the C-terminal inhibitory tyrosine residue 17, 18. Disruption of the Csk gene in mice results in embryonic death, indicating that Csk plays an essential role in development 32. By overexpression of Csk, it has been shown that Csk negatively regulates signaling by the TCR and FcϵRI 33, 34, while down-regulation of Csk by RNA interference lowers the threshold for TCR signaling 35. In addition, mice in which the Csk gene is deleted in granulocytes develop multifocal inflammation and are hyperresponsive to LPS, indicating an essential role for Csk in setting an activation threshold in granulocytes 36. Taken together, these studies show that Csk has an important control function in immune cells.
A major step in the understanding of the function of Csk was the identification of Csk binding protein/phosphoprotein associated with glycosphingolipid-enriched microdomains (Cbp/PAG), a membrane-associated protein that is localized to lipid rafts 37, 38. In resting T cells, Cbp serves as an anchor for Csk at the cell membrane, where it can act on Src family kinases and sets a threshold for T cell activation 37, 38. Our data suggest that Csk may also regulate cell function by binding to an ITIM-bearing receptor. So far, we have been unable to identify an unambiguous effect of Csk on LAIR-1 function, using either RNA interference or overexpression of Csk mutants to modify Csk function. This may be due to the fact that LAIR-1 itself is phosphorylated by Src family kinases 15; therefore, by inactivating Src family kinases, Csk may also indirectly affect the function of LAIR-1.
In conclusion, we have found that LAIR-1-mediated inhibition does not solely depend on SHP-1 and SHP-2. In addition, we found that Csk interacts with phosphorylated LAIR-1. Further research should elucidate the role of Csk in the function of LAIR-1 and possibly other ITIM-bearing receptors.
Materials and methods
cDNA constructs
Human LAIR-1 and LAIR-1 containing tyrosine-to-phenylalanine mutations in the ITIM have been described before 4, 15. The mFcγRIIB/hLAIR-1 chimera containing the extracellular and transmembrane domain of mFcγRIIB and the intracellular domain of LAIR-1 (amino acids 187–289) was generated by PCR as previously described for the mFcγRIIB/PECAM-1 chimera 21 and cloned into the pMx expression vector. Human FcγRIIB cDNA has been described before 39. N-terminal FLAG-tagged FcγRIIB and LAIR-1 were generated by cloning the cDNA to the 3′ site of the FLAG sequence in the pMx vector (generated at the DNAX Research Institute, Palo Alto, CA). cDNA encoding human Csk was generated by Neet et al. 40. cDNA encoding Csk lacking the SH2 domain (amino acids 80–163, CskΔSH2) or the SH2 domain of Csk alone (amino acids 77–172) were generated by PCR.
Cell lines and culture
The DT40 chicken B cell line and SHP1–/–, SHP2–/–, SHP1,2–/– and SHIP–/– DT40 cells have been described before 19, 20 and were purchased from RIKEN Cell Bank (Tsukuba Science City, Japan). The DT40 cells were grown on RPMI 1640 (Gibco, Paisley, UK) supplemented with 10% fetal calf serum (Integro, Dieren, The Netherlands), 1% chicken serum (Sigma), 4 mM glutamine (Gibco), 50 μM β-mercaptoethanol and antibiotics. To generate DT40 cells expressing the mFcγRIIB/hLAIR-1 chimera, the cells were transfected by electroporation. The cells were grown on selective media and cloned by the limiting dilution method, 24 h after transfection. To confirm expression of the chimera, the cells were stained with FITC-conjugated anti-mFcγRIIB 2.4G2 antibodies or FITC-conjugated isotype control (Pharmingen BD) and analyzed by flow cytometry.
RBL-2H3 is a rat basophilic leukemia cell line 41. RBL transfectants stably expressing either wild-type LAIR-1, LAIR-1 mutants or a hLAIR-1/mLAIR-1 chimera have been described previously 15, 16. Human embryonic kidney 293T cells were obtained from American Type Culture Collection (Manassas, VA). 293T cells, and RBL cells were grown in RPMI 1640 (Gibco) supplemented with 10% fetal calf serum (Integro) and antibiotics.
Antibodies
Polyclonal anti-chicken SHP-1 antibodies were a generous gift of Dr. T. Kurosaki (Kansai Medical University, Moriguchi, Japan). Polyclonal anti-SHP-2 and PLCγ2 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The PY20 anti-phosphotyrosine antibody was purchased from BD Transduction Laboratories (Franklin Lakes, NJ). Mouse anti-chicken IgM mAb M4 was purchased from Southern Biotechnology Associates (Birmingham, AL). Intact and F(ab’)2 rabbit anti-mouse IgM were obtained from Zymed (San Francisco, CA). Polyclonal anti-SHIP antibodies were kindly provided by Dr. J. Cambier (University of Colorado Health Sciences Center, Denver, CO). Monoclonal mouse IgG1 antibody directed against human LAIR-1, 8A8, has been described before 16. For immunoprecipitation polyclonal rabbit anti-Csk antibodies were used (purchased from Santa Cruz Biotechnology). For Western blot monoclonal anti-Csk antibody (BD Transduction Laboratories) was used. Horseradish peroxidase (HRP)-conjugated rabbit anti-mouse antibody (DAKO) or HRP-conjugated goat anti-rabbit antibody (Pierce) were used as secondary antibodies in Western blotting.
Calcium mobilization assay
DT40 cells (107/mL) were loaded with 3 μM Fluo 4-AM (Molecular Probes, Eugene, OR) in PBS for 20 min at 37°C. The cells were washed and resuspended in PBS supplemented with 1 mM MgCl2 and 1 mM CaCl2 . Triggering of the B cell receptor (BCR) with or without co-ligation of the mFcγRIIB/hLAIR-1 chimera was performed as described previously 20, 21. Co-ligation of the mFcγRIIB/hLAIR-1 chimera is achieved by cross-linking the FcγRIIB moiety of the chimera by the Fc part of the intact antibody used to cross-link the BCR, while F(ab’)2 fragments do not cross-link the chimera. Briefly, the cells were incubated with intact or F(ab’)2 rabbit anti-mouse IgM (final concentrations 9.5 μg/mL and 6.3 μg/mL respectively) for 5 min at room temperature. Mouse IgM anti-chicken IgM was added (clone M4, final concentration 25 μg/mL) and the fluorescence intensity was measured with 10-s intervals in the Fluoroskan Ascent (Thermo Labsystems, Franklin, MA) with excitation by 485 nm and emission at 527 nm.
Yeast-tri-hybrid assay
The yeast strain Ylck4.1 42 and the pYTH9B vector were generously provided by Dr K. Fuller (GlaxoSmithKline, UK). cDNA encoding the intracellular parts of human and mouse LAIR-1 (amino acids 187–287 and 165–263, respectively) were cloned in frame to the DNA binding domain of Gal4 in the pYTH9B vector. Stable transformants were generated by integration of the constructs into the Trp locus.
For screening, yeast cells stably expressing the BD-hLAIR-1 fusion protein were transformed with 50 μg human fetal brain library in the pACT2 vector (BD Biosciences) using a lithium acetate/Tris EDTA/polyethylene glycol protocol 42. Transformants were grown on selective media containing 50 mM 3-amino-1,2,4-triazole (Sigma) for 10–14 days. Large colonies were re-streaked on selective plates, grown for 48 h and filter-lifted on Hybond-N membranes (Amersham). β-Galactosidase activity was determined by a freeze-thaw fracture assay. Yeast clones that turned blue in this assay were considered as possible positives. Library DNA was recovered by mechanically disrupting the yeast cells with acid-washed glass beads. To exclude false positives, the recovered library DNA was transformed into yeast cells that expressed the DNA binding domain of Gal4 alone. The proteins that interacted selectively with LAIR-1 were then identified by automated DNA sequencing of the library DNA.
For Csk interaction studies in yeast, full-length Csk, CskΔSH2 and the SH2 domain were cloned in frame to the activation domain of Gal4 in the pACT2 vector (BD Biosciences). Of these constructs or the Csk construct obtained in the screen (CskΔSH3), 1 μg was transformed in yeast cells expressing the DNA binding domain/hLAIR-1 or DNA binding domain/mLAIR-1 fusion protein. Three colonies were spotted on media and grown in the presence or absence of methionine for 48 h. Interactions were identified by the β-galactosidase assay as described above.
Immunoprecipitation and Western blot analysis
To assess the expression of SHP-1 in the DT40 cell lines, the cells were treated with 50 μM pervanadate and lysed in TNE lysis buffer (50 mM Tris-HCl, pH 8, 1% Nonidet P-40, 2 mM EDTA, 0.02% sodium azide) supplemented with protease inhibitors (complete mini EDTA-free protease inhibitor cocktail tablets, Roche, Mannheim, Germany) and 1 mM phenylmethylsulfonyl fluoride. Protein A/G PLUS-agarose beads (Santa Cruz Biotechnologies) were coated with anti-chicken SHP-1 antibody and immunoprecipitation was performed for 90 min in the presence of 0.5% BSA. Immune complexes were washed three times with lysis buffer supplemented with 1 mM phenylmethylsulfonyl fluoride and boiled in non-reducing Laemmli sample buffer. Western blotting was performed and phosphorylated SHP-1 was detected by anti-phosphotyrosine antibodies. To assess the expression of SHP-2, the cells were directly lysed in Laemmli sample buffer and equal amounts of cell lysate were analyzed by Western blotting.
To investigate the LAIR-1-mediated effects on BCR-induced tyrosine phosphorylation, DT40 cells were incubated with intact or F(ab’)2 rabbit anti-mouse IgM, followed by mouse IgM anti-chicken IgM as described for the calcium mobilization assay and left at 37°C for 1 min. The cells were immediately washed in ice-cold PBS containing 250 μM orthovanadate and lysed in non-reducing Laemmli sample buffer.
For the identification of LAIR-1/Csk interactions, RBL cells stably transfected with LAIR-1 or LAIR-1 mutants were washed in PBS and 25 × 106 cells were lysed in TNE lysis buffer. Immunoprecipitation was performed as described above using polyclonal anti-Csk antibodies.
To investigate binding of SHIP to LAIR-1 293T cells were transfected with FLAG-tagged LAIR-1 or FcγRIIB using FuGENE 6 (Roche) as transfection agent. To confirm expression of the FLAG-tagged receptors the cells were stained with M2 anti-FLAG antibody (Sigma) followed by phycoerythrin (PE)-conjugated goat anti-mouse IgG (Southern Biotechnology Associates) and analyzed by flow cytometry. After 48 h, the cells were treated with 50 μM pervanadate and lysed in Triton lysis buffer (containing 1% Triton X-100, 10 mM Tris-HCl, pH 7.5, 150 mM NaCl and 0.02% sodium azide) supplemented with protease inhibitors as described above. Immunoprecipitation was performed as described before 15 using precoated beads with anti-FLAG antibodies (Sigma). The samples were boiled in reducing Laemmli sample buffer.
Proteins were resolved by SDS-polyacrylamide gel electrophoresis and transferred to Immobilon-P membranes (Millipore, Bedford, MA). Western blot analyses were performed with anti-phosphotyrosine, anti-SHP-2, anti-PLCγ2, anti-SHIP, 8A8 anti-LAIR-1 or monoclonal anti-Csk antibodies, followed by HRP-linked secondary antibodies. Proteins were detected by enhanced chemiluminescence (Amersham, Little Chalfort, UK).
Acknowledgements
We thank Drs K. Fuller (GlaxoSmithKline, UK), T. Kurosaki, J. C. Cambier, D. E. Jackson, L. L. Lanier, T. van den Berg and J. van de Winkel for providing materials. We are also grateful to Drs P. Coffer, F. Miedema, H. Clevers and R. Lebbink for critically reading the manuscript and for their useful discussion. This work was supported by the Netherlands Organization for Scientific Research (NWO, Grants 901–07–230, 016–026–008 and 015–001–070).
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




