Expression of the NKG2D ligand UL16 binding protein-1 (ULBP-1) on dendritic cells
Abstract
Innate and adaptive immunity have not evolved separately. In this regard, the NKG2D molecule first identified on NK cells and classified as an activating NK cell receptor is also an important receptor for CD8+ T cells. Functional analyses of human NKG2D and its ligands, i.e. UL16 binding proteins (ULBP) and MHC class I chain-related (MIC), have so far focused on immune cell-target cell situations because of the expression of NKG2D ligands on infected, stressed or transformed cells. Here, however, we address a possible function of NKG2D/ULBP-1 during the initiation of T cell responses. ULBP-1 can be detected on mature dendritic cells both in situ in the T cell areas of lymph nodes as well as in vitro after artificial maturation. FCM analysis further demonstrated that although NKG2D is expressed to some degree on all analyzed T cell subsets from peripheral blood, in vitro stimulation of T cells results in up-regulation of NKG2D on proliferating T cells. Using the sentinel lymph nodes of primary melanoma as a model for induction of defined T cell responses in vivo, we were able to demonstrate the expression of NKG2D on melanoma-associated antigen-specific T cells. Thus, our results suggest a role for NGK2D-ULBP-1 in the induction or reactivation of T cell responses.
Abbreviations:
-
- IMP:
-
influenza matrix protein
-
- MIC:
-
MHC class I chain-related
-
- PCNA:
-
proliferating cell nuclear antigen
-
- ULBP:
-
UL16 binding protein
Introduction
The interface at prolonged intercellular contacts of immune effector cells, such as NK and T cells, with target cells or cells regulating the state of the effector cells is characterized by an immunological synapse. As the synapse matures, it evolves into a defined structure composed of an outer ring, the peripheral supramolecular activation cluster, and an inner ring, the central supramolecular activation cluster. For T cells, this central ring contains the TCR and costimulatory receptors (reviewed in 1 and 2). Recent work, however, demonstrated the rapid and profound loss of TCR from the synapse long before T cell commitment 3. Therefore, the engagement of other receptors is crucial, since the fate of the lymphocyte is dependent on the balance between the signaling of stimulatory and inhibitory receptors. Among these, the NK cell receptors, which directly recognize MHC class I molecules, have recently received increased attention. In humans, the NK cell receptors can be divided into two families, the killer cell immunoglobulin-like receptors and the lectin-like receptors 4. Although basically discovered on NK cells, the expression of NK cell receptors is not restricted to them; this also holds true for the C-type lectin NKG2D. The NKG2D protein forms a homodimeric structure and is encoded by a gene localized in the NK gene complex on chromosome 12. It assembles with the signal transducing adapter molecule DAP10 to form a stimulatory receptor complex 5. Initially identified as a cDNA expressed by human NK cells, the expression of NKG2D is also observed on γδ cells and CD8+ αβ T cells (reviewed in 6 and 7). For the murine system, it has been demonstrated that NKG2D expression is induced on CD8+ T cells by their activation 8. It should be noted, however, that although NKG2D is expressed on both NK cells and CD8+ T cells in the mouse, its biological role seems to be different for the two cell types; while NKG2D on activated T cells is solely a costimulatory receptor, NK cells are directly stimulated by NKG2D engagement 8.
Ligands for human NKG2D, i.e. MHC class I chain-related (MIC) and the UL16 binding proteins (ULBP), are all distantly related to MHC class I proteins 9. The MIC proteins are expressed at rather low levels on human intestinal epithelial cells but are up-regulated on transformed tumor or virus-infected cells, suggesting that they may mark these cells for immune rejection. In contrast, ULBP mRNA is expressed in a wide range of tissues, and ULBP proteins are expressed on a variety of cell lines 10. The ULBP family consists of several members and was identified based on the ability of the ULBP proteins to bind to the human cytomegalovirus glycoprotein UL16 9, 11. As the detailed expression pattern of ULBP proteins under physiological conditions remains elusive, their biological role has not been completely revealed. We therefore scrutinized the role of the NKG2D/ULBP-1 system in DC/T cell interactions. The expression of ULBP-1 on mature DC, together with the presence of NKG2D on antigen-specific T cells in the sentinel lymph nodes of primary melanoma, suggests an important function of NKG2D signaling for the induction of tumor-specific T cell responses.
Results
In situ expression of ULBP-1 on antigen-presenting cells
Numerous reports have demonstrated that triggering of NKG2D by transformed or virus-infected cells enhances NK or T cell immune responses, respectively. Therefore, we analyzed lymph nodes of melanoma patients for the presence of ULBP-1-expressing cells by immunohistochemistry. We indeed detected ULBP-1+ cells in 14 of the 16 analyzed lymph nodes from ten individual melanoma patients (Fig. 1A, Table 1). Double staining with anti-ULBP-1 and anti-NKG2D revealed the close vicinity of NKG2D+ and ULBP-1+ cells (Fig. 1B), hence we further characterized the latter cell population. As these cells did not possess characteristic tumor cell morphology, we performed double staining for ULBP-1 and CD83 or CD14. CD83 is a commonly used marker for mature DC, whereas CD14 is expressed by macrophages or immature DC. ULBP-1+ cells co-expressed CD83 but not CD14, indicating that these were - at least in part - mature DC (Fig. 1C, D). In fact, CD83 expression was necessary but not sufficient for ULBP-1 expression, as all ULBP-1+ cells were positive for CD83, but only 20–30% of the CD83+ cells expressed ULBP-1.
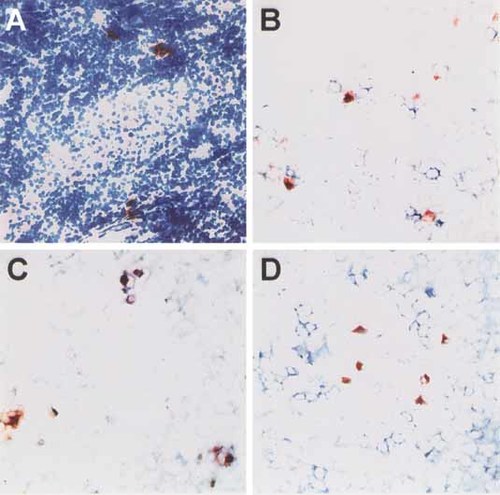
ULBP-1 expression in human lymph nodes. Cryosections of lymph nodes of melanoma patients were stained for ULBP-1 (red) alone (A) or together with anti-NKG2D (B), anti-CD83 (C) or anti-CD14 (D) antibodies (blue). In (A) the section is counterstained with hematoxylin. Double-positive cells appear purple.
Expression of ULBP-1 on in vitro-matured DC
The presence of ULBP-1 and CD83 double-positive cells in lymphatic tissue prompted us to test if this cell population was indeed composed of mature DC. Therefore, we analyzed ULBP-1 expression on in vitro-generated DC. For immunohistochemistry, T2 cells transfected with a plasmid coding for ULBP-1 served as a positive control (Fig. 2A). A similar staining pattern was observed for in vitro-matured DC (Fig. 2C) but not immature DC (Fig. 2B). The presence of ULBP-1-expressing DC among in vitro-generated DC was confirmed by FCM analyses (Fig. 2D–F). It should be noted, however, that the percentage of ULBP-1+ DC varied among the DC preparations. Thus, we initially determined relative ULBP-1 mRNA expression by real-time PCR in day 7 DC generated from leukapheresis from melanoma patients performed for vaccination purposes 12. All patients gave informed consent for the analysis of their DC. A total of 37 DC preparations from 13 different donors were analyzed. In 31 of the analyzed samples, mRNA coding for ULBP-1 was present. Only in 1 patient could no ULBP-1 be measured (in two separate DC preparations; data not shown). The relative expression of ULBP-1 mRNA normalized to CD74 showed strong intra- and inter-individual variations. Thus, we correlated the ULBP-1 expression with several markers representing the quality of the DC preparation and the maturation status, i.e. CD1a, CD25, CD14, CD80, CD83, CD86, CD95, MHC class I and MHC class II. For CD1a (pearson r=0.4285 with p=0.0414), CD25 (pearson r=0.4804 with p=0.0203) and CD95 (pearson r=0.4765 with p=0.0215), a moderate but significant correlation was present (Fig. 2G and data not shown). In contrast, the percentage of CD14-, CD80-, CD83-, CD86-, class I- or class II-positive cells did not correlate with ULBP-1 expression (data not shown). In order to test whether ULBP-1 expression is indeed regulated during the process of maturation, we quantified ULBP-1 mRNA expression in DC before and after a maturation cocktail containing IL-1β, IL-6, TNFα and PGE2 was added on day 6 in a kinetic study. ULBP-1 mRNA expression was normalized to GAPDH. The expression of ULBP-1 mRNA was tightly linked to the process of DC maturation. In fact, the kinetics of ULBP-1 expression were very comparable in all analyzed patients (n=3), with a peak of mRNA expression 4–6 h after addition of the cytokine cocktail (Fig. 2H). Notably, a similar kinetic was observed if ULBP-1 expression was normalized to CD74, demonstrating that CD74 mRNA is expressed early and at rather constant levels during DC maturation (data not shown). To test whether ULBP-1 expression is also tightly regulated on the protein level, we measured ULBP-1 expression during the maturation process by FCM on DC obtained from two different melanoma patients. These analyses confirmed our finding, as the peak of ULBP-1-expressing DC was observed 4–8 h after maturation. Interestingly, it didn't matter whether the DC were maturated with the maturation cocktail or with LPS (1 µg/ml) (Fig. 2I). As the extent of ULBP1 expression apparently varied between DC preparations, it should be noted that the preparation displaying the higher expression also contained more CD25+ cells 24 h after maturation induction (data not shown).
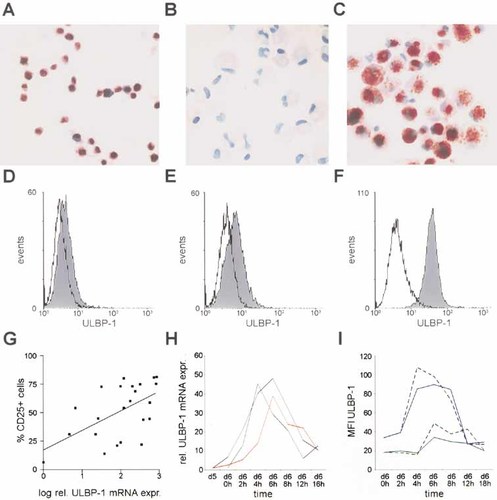
ULBP-1 expression on in vitro-generated DC. ULBP-1 protein expression was analyzed by immunohistochemistry on cytospins of T2 cells transfected with ULBP-1 plasmid (A), immature DC (B) or DC maturated with a cytokine cocktail (C). FCM analysis of ULBP-1 expression on three in vitro-matured DC preparations from different melanoma patients are depicted as histograms (D–F). Solid lines depict the isotype control, and the filled curves represent ULBP-1 staining. The relative expression of ULBP-1 mRNA (normalized to CD74) was correlated to the percentage of CD25+ cells for different in vitro-maturated DC preparations (G). ULBP-1 mRNA (H) and protein (I) expression in the course of DC maturation for DC preparations from individual patients (colored lines). On day 6 (d6 0 h) DC maturation was induced. The relative ULBP-1 mRNA expression (rel. ULBP-1 mRNA expr.) was normalized to GAPDH (H) and calculated by the ΔΔCT method; the starting point of the respective DC preparation served as a calibrator. For protein expression, the change in mean fluorescence intensity (MFI) of ULBP-1 is given for DC preparations matured on day 6 by the maturation cocktail (solid lines) or by LPS (broken lines).
NKG2D expression on CD8+ T cell subsets
NKG2D is known to be expressed on most CD8+ cells. Since this notion is based solely on mRNA analysis of total CD8+ populations 13, we analyzed NKG2D expression on CD8+ subpopulations in the blood by FCM. This analysis confirmed the earlier reports, demonstrating that in all analyzed T cell subpopulations, i.e. CD45RA-, CCR7-, CD45RO- or cutaneous lymphocyte-associated antigen-positive CD8+ cells, at least 20–40% of the cells expressed NKG2D (data not shown). Recently, Verneris et al. demonstrated the up-regulation of NKG2D on CD8+ T cells upon activation and expansion 14. We also confirmed this finding by analyzing CFSE-labeled CD8+ T cells stimulated by co-culture with HLA-mismatched DC or phytohemagglutinin. While NKG2D was expressed to some degree on basically all cells in the undivided cell population (day 2), proliferating T cells up-regulated NKG2D irrespective of the proliferation stimulus (Fig. 3).
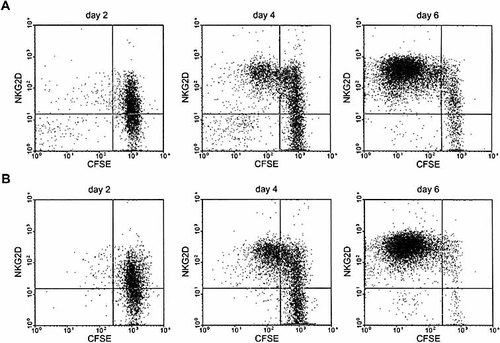
Up-regulation of NKG2D expression on proliferating CD8+ cells. CD8+ cells from healthy donors were co-cultured with either HLA-mismatched donor DC at a DC:CD8 ratio of 1:2 (A) or with PHA (B). Expression of NKG2D was measured each 2nd day by FCM. Loss of CFSE served as a marker for proliferating cells. The quadrant was set with the help of the isotype control (data not shown).
NKG2D expression on specific T cells in lymph nodes
The notion that NKG2D is up-regulated on activated T cells together with the fact that mature DC express ULBP-1 in vitro as well as in situ – notably in the place where T cell responses are initiated, i.e. the lymph node – prompted us to test if T cells reactive to antigens presented by DC may preferentially express NKG2D. Therefore, we analyzed five sentinel lymph nodes of melanoma patients by in situ detection of melanoma-associated antigen-specific T cells, i.e. cells recognizing the MART-126–35 or Mage-3271–279 epitopes presented by HLA-A2, and evaluated their NKG2D expression. These populations were chosen as it can be assumed that MART-1 and Mage-3 are presented by DC in the sentinel lymph nodes of melanoma patients. Detection of influenza matrix protein (IMP)58–66-specific T cells served as a control, since earlier studies demonstrated that this T cell population is present in the majority of patients displaying a memory phenotype 15, and in the absence of an acute infection, no IMP58–66-presenting DC should be present in the lymph node. The analysis revealed that the majority of HLA-A2-restricted MART-1- or Mage-3-specific T cells present in the sentinel lymph node expressed NKG2D (Fig. 4D, E). In contrast, basically none of the IMP-specific T cells expressed NKG2D (Fig. 4F). Importantly, these in situ observations were supported by FCM analyses of lymph node single-cell suspensions. In fact, NKG2D expression was not only detectable on CD8+ cells but also on MART-1-specific cells, whereas IMP-specific cells did not express NKG2D (Fig. 4G–I). To ensure that NKG2D expression is largely restricted to activated cells in the lymph node, we double stained lymph node cryosections with anti-NKG2D antibody and a marker for proliferating cells, i.e. anti-proliferating cell nuclear antigen (PCNA) or anti-Ki-67 antibodies. As depicted in Fig. 4B and C, all proliferating cells in the lymph node did express NKG2D.
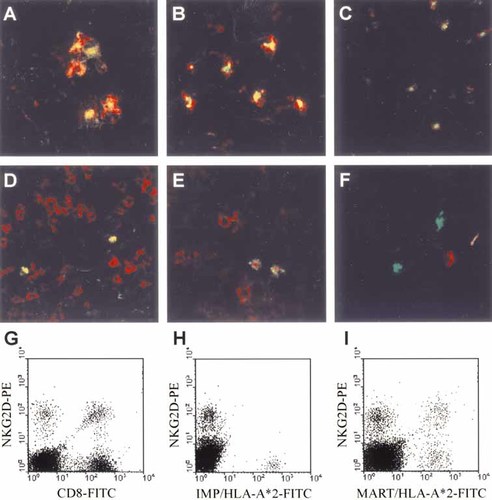
NKG2D and ULBP-1 expression in lymph nodes. Cryosections of lymph nodes from melanoma patients were subjected to double staining with antibodies specific for (A) ULBP-1 (green) and CD83 (red), (B) NKG2D (green) and PCNA (red) or (C) NKG2D (green) and Ki67 (red). (D–F) Staining of cryosections with Mart-126–35/HLA-A*0201 (green, D and E) or IMP58–66/HLA-A*0201 (green, F) multimers was combined with anti-CD8 (red, D) or anti-NKG2D (red, E and F) antibody staining. The digital overlays are given, and double-positive cells appear yellow. FCM analyses of single-cell suspensions from lymph nodes of melanoma patients stained with an anti-NKG2D antibody together with anti-CD8 antibody (G), IMP58–66/HLA-A*0201 (H) or Mart-126–35/HLA-A*0201 (I) multimeric complexes are shown.
Discussion
Since NKG2D was discovered in the early 1990s 16, much information about this receptor has been gathered. Initially identified on NK cells, NKG2D has meanwhile been detected on other immune cells such as γδ and αβ T cell subsets, connecting innate with adaptive immunity 13. Interestingly, although the identified ligands for NKG2D are quite heterogeneous, they all share the common property that they are inducible by cellular distress, namely malignant transformation, infection or cell stress 9, 17. Therefore, research on NKG2D has focused on its function during the interaction of immune and target cells. In this regard, NKG2D ligation was shown to mediate direct cytotoxicity in NK cells 18, whereas the receptor mediates costimulating signals in T cells 7, 8. This demarcation, however, is not that distinct. For example, a recent report demonstrated that NKG2D triggering accounts for the majority of MHC-unrestricted cytotoxicity of activated and expanded CD8+ T cells 14, confirming earlier reports that NKG2D may act as a direct activating molecule on T cell subsets 19. Additionally, a recent report demonstrated that dysregulated IL-15 expression can convert CTL into lymphokine-activated killers by a TCR-independent NKG2D signaling pathway 20.
In the present report we demonstrate that ULBP-1, one of the ligands for human NKG2D, is expressed on mature DC. For example, among the analyzed DC preparations from melanoma patients, ULBP-1 mRNA expression correlated with expression of markers for DC maturation. In the case of CD25, however, the kinetics of ULBP-1 and CD25 did not necessarily overlap for individual cells; double staining of DC preparations in time course experiments with anti-ULBP-1 and anti-CD25 antibodies did not reveal double-positive cells. Indeed, CD25 expression is only slowly induced, i.e. 8 h after addition of the maturation stimulus (data not shown), whereas the highest expression of ULBP-1 is observed within 4–8 h after maturation induction. Therefore, the percentage of CD25+ cells within the examined DC populations from the same time points, e.g. 24 h after the maturation cocktail was added on day 6, should be regarded as a marker for the quality of the DC preparation, reflecting the ability of the initial DC precursor population to subsequently mature. Notably, the kinetic of CD83 expression is more continuous, increasing constantly during maturation, and the majority of cells already express CD83 4–6 h after induction of maturation.
The time course experiments also revealed that ULBP-1 expression on DC after in vitro maturation is a tightly regulated process on both the RNA and protein levels. The tight regulation of ULBP-1 expression in vitro is reflected by the in vivo situation. CD83+ULBP-1+ cells were present in lymph nodes of melanoma patients, while peripheral blood mononuclear cells, which are virtually devoid of mature DC 21, did not express ULBP-1 protein 22. Moreover, these cells were in direct contact with melanoma-associated antigen-specific T cells.
Recent work demonstrated that NKG2D is expressed on most human CD8+ cells and is up-regulated upon activation and stimulation 13, 14. Our results are concordant with these findings, as NKG2D was expressed to various extents on all analyzed CD8+ T cell subsets, but expression was clearly increased after the CD8+ cells were stimulated. Our in situ observations further confirm this notion, as within a lymph node only those cells that were presumably taking part in an ongoing immune response and those that expressed proliferation markers preferentially expressed NKG2D.
The presence of NKG2D+ T cells probably involved in the current immune response in close vicinity to ULBP-1+ cells in the lymph node suggests that NKG2D/NKG2D ligand interactions play a role in the initiation and/or perpetuation of immune responses in addition to the role in mediating cytotoxicity in immune cell/target cell interactions. Indeed, recent findings support a possible interaction of T cells and DC through NKG2D/NKG2D ligand and a consequent impact on the immune response. For example, Somersalo et al. reported that MICA can substitute for ICAM in the formation of antigen-independent ring junctions. These structures are similar to peripheral supramolecular activation clusters of the immunological synapse 23. In addition, NKG2D/NKG2D ligand interactions can compensate for missing CD28 costimulation 19. Furthermore, vaccination with tumor cells expressing NKG2D ligand elicited a strong CD8+ T cell response 24. In the same line, this work demonstrated that tumor lines expressing NKG2D ligand evoke a strong CD8+ memory response towards the specific cell lines. Notably, the group of Reisfeld recently demonstrated the potential of NKG2D ligands to enhance specific T cell responses 25. NKG2D ligand expression and peptide presentation was targeted to antigen-presenting cells by DNA-based vaccination, as mice received plasmids containing the sequences for NKG2D ligand, H60 and tumor antigen peptides orally. In two distinct colon carcinoma models, this type of vaccination effectively inhibited tumor growth 25. The vaccination elicited an effective specific T cell response and enhanced NK cell activity. Thus, DC expressing NKG2D ligand can apparently influence immune cells. Similarly, proliferation of naive CD8+ T cells activated by TCR cross-linking is drastically increased when these cells are also stimulated by NKG2D triggering 26. Therefore, ULBP-1+ DC should stimulate NKG2D+ T cells in lymph nodes, suggesting a role for NKG2D/NKG2D ligand interactions in the initiation and/or perpetuation of cellular immune responses in addition to the well-characterized function of these interactions in mediating cytotoxicity.
Materials and methods
Immunohistochemistry and antibodies
Frozen sections were fixed in cold acetone for 10 min, followed by removal of endogenous peroxidase with 0.03% H2O2 and blocking of non-specific binding to collagenous elements with 10% BSA. Sections were incubated for 30 min with biotinylated antibodies at predetermined dilutions (usually 20 µg/mL). Subsequently, the streptavidin-peroxidase complex (DAKO, Germany) was applied for 30 min, followed by 15 min incubation with the Chromogen AEC (DAKO). Finally, slides were counterstained with hematoxylin (DAKO) and mounted in aquatex (Merck Eurolab, Germany). Double staining was performed as previously described 27. For cytospins, 2 × 104 cells (in 10 µL PBS) were placed onto 3-amino-propyltriethoxy-silane (Roth, Germany)-coated slides, air dried overnight and then fixed in acetone for 10 min. Non-specific binding was blocked with 1% human immunoglobulin (Beriglobin, Centeon, Germany) in PBS. The ULBP-1 antibody (huULBP1-M291) was applied at a dilution of 1:100 in antibody diluent with background-reducing components (DAKO), and slides were incubated in a humid chamber for 30 min at room temperature. For detection of bound antibody by the Labeled-Streptavidin-Biotin-Methode® method, sections were incubated with the biotinylated secondary antibody and the HRP-conjugated streptavidin (both “ready to use”, DAKO) for 10 min. Slides were gently rinsed in TBS (Tris-buffered saline) between all incubation steps. As chromogene, the HistoGreen-Kit (Linaris, Germany) was used. Slides were counterstained with hematoxylin and embedded in Vitro-Clud® (Langenbrink, Germany).
The staining procedure for multimeric peptide/MHC complexes has been described recently 28. In brief, biotinylated peptide/HLA-A*0201 complexes were obtained from Proimmune (UK) and were multimerized using streptavidin-FITC-conjugated dextran molecules (kindly provided by L. Winther, DAKO, Denmark) to generate multivalent HLA-dextran complexes. Sections were dried overnight and subsequently fixed in cold acetone for 5 min. All incubation steps were performed in the dark at room temperature as follows: (i) primary antibody (diluted 1:100) for 45 min, (ii) Cy3-conjugated goat anti-mouse (diluted 1:500; code 115–165–100, Dianova, Germany) for 45 min and finally (iii) the multimers for 75 min. Between each step the slides were washed twice for 10 min in PBS/BSA 0.1%. They were then mounted in vectashield and observed under a Leica Confocal Microscope (TCS 4D, Leica, Germany).
The following commercially available antibodies were used: anti-CD83 (clone HB15e) and anti-CD8 (clone HIT8a; both BD Pharmingen, USA); anti-CD14 (clone TÜK4), anti-PCNA (clone PC10) and anti-Ki-67 (clone MIB-1; all from DAKO). The mAb against ULBP-1 (huULBP1-M291) and NKG2D (NKG2D-M291) were a kind gift from Amgen Corporation, Seattle. FCM analyses were performed with either the anti-ULBP-1 (clone 170818; R&D Systems, Germany) or the huULBP1-M291 antibody.
Cell preparation and culture
PBL were obtained by Ficoll separating solution (Biochrom, Germany) density gradient and cultured in RPMI 1640 with 10% FCS. All healthy donors and patients gave informed consent for the scientific workup of their blood. DC were generated either by (i) CD14+ cell isolation from peripheral blood of healthy donors and culture in the presence of IL-4 and GM-CSF for 7 days and TNFα and PGE2 for another 3 days or (ii) according to standard protocols for leukapheresis-derived DC for vaccination purposes in stage IV melanoma patients 12. In brief, adherent monocytes from leukapheresis were cultured for 6 days in RPMI 1640 medium (Invitrogen, Germany) supplemented with 1% autologous plasma, 2 mM L-glutamine, 1000 U/mL rhIL-4 (Strathmann, Germany) and 800 U/mL rhGM-CSF (Leukomax, Germany). For the maturation of DC, day 6 cells were cultured in a cytokine cocktail of IL-1β (2 ng/mL), IL-6 (1000 U/mL), TNFα (10 ng/mL) (Strathmann) and PGE2 (1 µg/mL) (Sigma, Germany) for 1 day. DC obtained prior to day 6 were regarded to be immature.
CD8+ cells were isolated from PBL of healthy donors by negative depletion. At least 2 × 107 freshly isolated PBL were incubated in the presence of 2 µg anti-CD4 (clone RPA-T4), 2 µg anti-CD16 (clone 3G8), 2 µg anti-CD19 (clone HIB19; BD Biosciences, Germany) and 50 µL supernatant from an anti-CD14-producing hybridoma (Dr. E. Kämpgen, Würzburg) in a total volume of 2 mL 0.1% BSA/PBS for 15 min at 4°C. After a single washing step and 45 min incubation at 4°C with goat-anti mouse IgG-coated magnetic microbeads at a concentration of 50 beads per cell (BioMag, Polysciences, USA), labeled cells were separated by magnetic attraction. Using this method, 99% of the resulting cell suspension was CD8+ as controlled by simple FACS analysis.
For CFSE labeling the protocol of Lyons and Parish was used 29. In brief, cells were incubated with 5 µmol/mL CFSE for 10 min at 37°C at a cell concentration of 5 × 107 cells/mL in RPMI 1640. Subsequently, cells were washed three times in cold FCS-containing medium. Cells were directly used for in vitro stimulation experiments. For in vitro stimulation experiments, cells were co-cultured with either HLA-mismatched donor DC at a ratio of 2:1 or with PHA (Invitrogen, Germany). The medium contained 10% human AB plasma and 100 U IL-2/mL (Aldesleukin, Chiron, Germany).
Flow cytometry
FCM analysis was performed by incubating cells with predetermined concentrations of the respective antibody or peptide/MHC multimer for 30 min at 4°C. Bound antibody was detected with R-PE- or FITC-conjugated goat anti-mouse IgG antibody in a 1:50 dilution (Dianova). The fluorescence was analyzed with a FACScan (BD Biosciences).
Quantitative PCR
The relative expression of ULBP-1 to CD74 (invariant polypeptide of the major histocompatibility complex, class II antigen-associated), which served as an endogenous control, was determined by real-time PCR using the comparative ΔΔCT method. The primers and probes for ULBP-1 and CD74 were designed with Primer Express software (Applied Biosystems, Germany): 5′-TGT GGT TCA GGT CTG GAC TTA GG-3′ (forward primer), 5′-TTC TGT GCC TCC CGC TTC T-3′ (reverse primer), 5′-FAM-AAG ACA GTG TGT GTC GAC CCA TCC TGC-TAMRA-3′ (probe) for ULBP-1; and 5′-TTG AAA TGA GCA GGC ACT CCT T-3′ (forward primer), 5′-AGG GAT GTG GCT GAC CTC TTC-3′ (reverse primer), 5′-FAM-CTC CAC CGA AAG TAC TGA CCA AGT GCC AG -TAMRA-3′ (probe) for CD74. The amplification efficiencies were comparable, i.e. virtually 100%. Amplifications were carried out in the GeneAmp SDS 5700 (Applied Biosystems). The amount of ULBP-1, normalized to CD74 or GAPDH and in relation to the calibrator, was calculated as 2–ΔΔCT with ΔΔCT = (CT ULBP-1, sample – CT CD74/GAPDH, sample) – (CT ULBP-1, calibrator DC – CT CD74/GAPDH, calibrator DC). CT is defined as the cycle when the threshold level of fluorescence is reached.
Acknowledgements
This work was supported by the Wilhelm-Sander-Stiftung grant 8260 and the Deutsche Forschungsgemeinschaft grant Be 1394/5–3. D. S. is presently supported by the Deutsche Krebshilfe #10–1845-Be I. We thank Eva Baumann and Claudia Siedel for excellent technical assistance.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




