Primary induction of CD4 T cell responses in nasal associated lymphoid tissue during group A streptococcal infection
Abstract
CD4 T cells are important for development of long-term immunity to bacterial infections. Here we describe construction of a group A streptococcus (GAS) strain that expresses the model ovalbumin epitope (OVA) on its surface, and the use of this strain in adoptive transfer experiments to study CD4 T cell response to bacterial infection in nasal-associated lymphoid tissue (NALT), which was previously shown to be a specific target for GAS colonization. The OVA+ GAS, but not the wild-type strain was shown to activate CD4 T cells in an antigen-specific manner both in vitro and in vivo. After intranasal infection of mice with this strain, OVA-specific CD4 T cells were first activated in NALT, which is functionally equivalent to human tonsils, rather than in the cervical lymph nodes. During localized infection, OVA+ GAS induced rapid and prolonged activation of CD4 T cells at higher magnitudes in the NALT than in draining lymph nodes and spleen, where CD4 T cells underwent little or no activation. In contrast, systemic infection induced significantly higher activation of CD4 T cells in both lymph nodes and spleens, compared to when the infection was localized in NALT. Further investigation of cellular immune responses in NALT during GAS infection using adoptive T cell transfer, combined with the model antigen on the pathogen may ultimately shed light on mechanisms for failure of children to develop protective immune responses following streptococcal tonsillitis.
Abbreviations:
-
- GAS:
-
Group A streptococcus
-
- NALT:
-
Nasal-associated lymphoid tissue
-
- M cells:
-
Membranous cells
-
- CFSE:
-
Carboxyfluorescein diacetate succinimidyl ester
-
- SP:
-
Spleen
1 Introduction
Group A streptococcus (GAS) is an important human pathogen that causes pharyngitis, skin infections, necrotizing fasciitis, toxic shock, and septicemias, and has been implicated in the pathogenesis of psoriasis, glomerulonephritis, and rheumatic heart disease. Although more than five million people are annually diagnosed with streptococcal pharyngitis, and 1,200–1,500 die annually from complications of GAS infections in the United states, little is known about the protective immune response to this Gram-positive bacterium. One third of those treated with antibiotics continue to shed streptococci and a significant number of these carriers have recurrent disease by the same strain 1. Carriers are both a curiosity and an important public health problem; curious because an effective immune response fails to develop, problematic because they maintain the cycle of disease in a community 2–4.
Tonsils are known to harbor and shed streptococci, even after vigorous antibiotic therapy. Österlund et al. 4 found that tonsils excised from 13 of 14 children retained intracellular GAS, and others reported isolation of GAS from excised tonsils, confirming that this secondary lymphoid tissue is an important reservoir. Recent experiments in our laboratory addressed this issue using an intranasal infection model in mice. Instead of palatine tonsils, mice have two lobes of nasal-associated lymphoid tissue (NALT), suggested to be functionally equivalent to human tonsils, located at the base of their nasopharyngeal cavity 5. The ciliated epithelial lining separating underlying lymphoid tissue from the nasal cavity contains specialized membranous (M) cells that resemble those in the follicle-associated epithelium of Peyer's patches of the gut 5. Our recent studies showed that GAS uses M cells as a conduit to traverse the ciliated epithelium and specifically target NALT for colonization following intranasal inoculation 6. Similarities between this tropism and the ability of enteric pathogens to traverse Peyer's patches via M cells in the intestinal epithelium are striking 7.
The relationship between the anatomic distribution of a mucosal bacterial pathogen, such as GAS, and the primary induction of an adaptive immune response has not been investigated beyond serological responses. We presume that inhaled streptococci are deposited onto the oral-nasal mucosa where they adhere and ultimately multiply. Without penetration of the mucin-coated epithelium, it is not known how or where streptococci are detected and processed by the innate immune system. Without antigenic stimulation NALT is predominantly a naive immunological site, lacking germinal centers and consisting of unswitched IgM+ and IgD+ B cells 8, and naive Th0 T cells 9. However, immune responses following infection, notably secretory immunoglobulins (sIgA and sIgM) and locally produced IgG, are known to be induced in NALT, suggesting that it can serve as an important mucosal immune inductive site 8, 10, 11. In contrast, generation and regulation of specific cell-mediated immunity at this organ following infection are far less understood. As noted above GAS do not readily breach the ciliated columnar epithelial lining of the murine nasal canal, but instead rapidly invade and spread to the juxtaposed lymphoid tissue or NALT. Therefore, we postulate that the initial interaction between innate and adaptive immune response occurs in this tissue and that regional lymph nodes (LN) and spleen are secondary sites of naive T cell priming. We suggest that more distant sites only respond when bacteria manage to escape the innate defenses of local mucosal lymphoid tissues and disseminate via the blood or lymphatics.
Adoptive transfer of antigen-specific transgenic T cells has proven to be a powerful tool for unraveling the complexities of T cell biology and to study development of T cell responses to only a few bacterial pathogens 12. Timing, tissue distribution and the magnitude of these responses are monitored by tracking antigen-specific T cells in naive animals 13 or dissected tissues 14.
Here, we describe the kinetics of antigen-specific CD4 T cell responses in NALT and in other peripheral lymphoid tissues following streptococcal infection. From this study we conclude that NALT is the primary inductive site for CD4 T cell activation in response to intranasal streptococcal infection. Moreover, these results encourage continued use of the T cell adoptive transfer system to evaluate the ability of CD4 T cell-mediated responses in the nasal lymphoid tissue to limit streptococcal oral-nasal colonization and dissemination into the deeper tissues.
2 Results
2.1 Construction of recombinant GAS strains that express the M1-OVA fusion protein on their surface
A streptococcal strain 90–226 was genetically engineered to express the OVA peptide (amino acids 323–339) on the bacterial surface as a fusion protein with streptococcal M protein. The hybrid emm1.0::ova gene was constructed in plasmid pGhost5 in E. coli and then introduced into the chromosomal emm1.0 gene locus by allelic replacement 15 as described in Materials and methods. The corresponding chimeric protein is, therefore, composed of the 17 amino acid OVA epitope inserted in frame after the first five amino acids of the mature M1 surface protein (Fig. 1A). The strain was designated 90–226 emm1.0::ova. Expression of the M1-OVA fusion protein on the surface of streptococci was confirmed by fluorescence microscopy of intact bacteria and by immunoblot analysis of cell surface protein extracts using rabbit anti-M1 and mouse anti-OVA serum (not shown). A protein of the expected size was found in extracts from the recombinant strain that reacted with both anti-M1 and anti-OVA antibodies, while a similar size protein in extracts from wild-type streptococci reacted only with anti-M1 antibodies. The amount M1-OVA fusion protein in extracts from the recombinant strain was found to be equal to that of M1 protein extracted from wild-type strain 90–226 (unpublished data), indicating that OVA insertion did not change the expression level of M1 protein on the bacterial surface. The strain is genetically stable without antibiotic selection due to the integration and replacement of the wild-type gene in the chromosome.
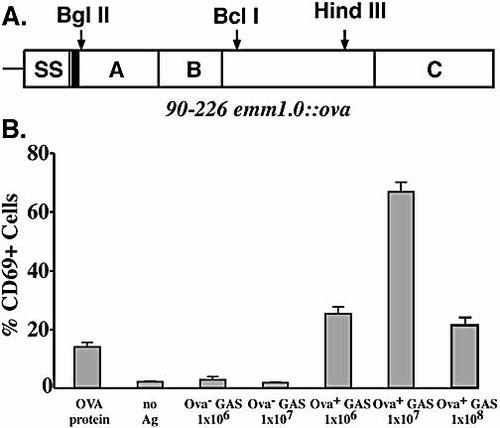
(A) Map of M1-OVA fusion protein. SS is signal sequence. Solid bar indicates position of the OVA epitope. A and B domains are required for fibronectin binding and invasion of human epithelial cells. C contains the cell wall anchor region. Restriction enzyme sites used for cloning are shown. (B) Expression of CD69 on DO11.10 T cells after in vitro incubation of splenocytes with OVA+ GAS. Spleen cells (1×106) were co-cultured with various numbers of heat-killed GAS for 24 h at 37°C, and the percentage of CD4+, KJ1–26+ T cells expressing CD69 was determined by three-color flow cytometry. The results (mean ± SD) represent two individual experiments.
To test whether or not insertion of the OVA epitope in M protein altered intrinsic properties of M protein, various functions of the M1 protein were assessed. Recombinant strain 90–226 emm1.0::ova was as resistant to phagocytosis as the parent culture in whole blood bactericidal assays, and promoted invasion of A549 human epithelial cells as efficiently as the parent 90–226 strain. The recombinant strain bound fibronectin equivalent to that of the parent culture (unpublished data). For all these experiments, the M– strain 90–226 emm1.0::km strain 16 was included as a negative control. In summary, the M1-OVA fusion protein retained all known functions of M1 protein pertinent to virulence.
2.2 Recombinant GAS activate transgenic DO11.10 T cells antigen specifically in vitro
An important question was whether the M1-OVA fusion protein is specifically recognized by DO11.10 T cells. To test this, splenocytes from transgenic mice were co-cultured with various numbers of heat-killed recombinant bacteria for 24 h in vitro and then examined for activation. Antigen-specific CD4 T cells were identified using KJ1–26 anti-clonotypic mAb, which uniquely binds to the DO11.10 TCR 17 along with anti-CD4 mAb. Activation status was measured by CD69 expression levels 18. Upon stimulation with heat-killed OVA+ GAS, DO11.10 CD4 T cells expressed high levels of CD69 on the surface, whereas the addition of wild-type GAS strain (OVA–) had no effect on induction of CD69 expression on DO11.10 T cells (Fig. 1B), indicating that CD4 T cell activation is OVA antigen specific. Most but not all GAS isolates are known to express superantigens, including streptococcal pyrogenic exotoxin SpeA, which binds to T cell subclasses Vβ8.1 and Vβ8.2. Since products of the Vβ8.2 genes are part of the DO11.10 transgenic TCR, SpeA should activate DO11.10 T cells. Despite carrying the gene, strain 90–226 does not express detectable level of SpeA (unpublished data), which could explain the failure to activate DO11.10 T cells. Interestingly, recombinant bacteria induced higher levels of CD69 on CD4 T cells than soluble OVA protein alone, consistent with the natural adjuvanticity of bacterial cells.
2.3 Characterization of the primary CD4 T cell response to intranasal GAS infection
The kinetics of the CD4 T cell response in NALT, cervical LN, and spleen following intranasal GAS infection was investigated. A preliminary experiment demonstrated that inoculation of mice with 2×107–5×107 CFU results in colonization of NALT without systemic translocation of bacteria to distant tissues. We infected naive BALB/c mice intranasally with 5×107 recombinant strain OVA+ GAS after adoptively transferring OVA-specific DO11.10 T cells. As expected, we found large numbers of streptococci in homogenates of NALT, but only occasionally in LN and in relatively few numbers (Table 1). The number of viable streptococci in NALT, determined by CFU on blood agar plates, decreased to below detection levels by 8 days after infection. No viable streptococci were found in the spleens of any infected mice. Thus, as expected the growth of the intranasally administered GAS was restricted largely to the NALT.
|
Inoculum size (CFU) |
Days after infection |
CFU/organa) |
||
|---|---|---|---|---|
|
NALT |
LN |
Spleen |
||
|
5×107 |
3 |
0.7×104 0.8×104 1.2×104 0.5×104 |
0 0 0 0 |
0 0 0 0 |
|
5 |
0.8×103 0.9×103 0.4×103 0.2×103 |
1.0×102 0 0 0 |
0 0 0 0 |
|
|
8 |
0 0 |
0 0 |
0 0 |
|
|
5×108 |
1 |
1.2×106 4.9×106 3.4×107 |
0 0 6.0×102 |
0 0 0 |
|
3 |
5.0×104 1.5×105 6.3×104 |
4.3×102 4.0×104 2.4×102 |
2.6×103 3.0×103 0 |
|
|
5 |
5.8×103 5.0×105 1.0×103 |
3.0×103 2.0×104 0 |
2.5×102 4.0×103 0 |
|
- a) NALT, LN, and spleens were removed from mice on various days after infection with two different doses of OVA+ GAS. Tissue homogenates were plated on blood agar to determine numbers of CFU. Similar results were obtained in two additional sets of experiments.
Lymphoid tissues from the above-infected mice were analyzed for antigen-specific T cell expansion. Percentage of DO11.10 T cells increased markedly in NALT by day 3 after infection with the OVA+ strain and decreased by day 5 to the level of uninfected mice (Fig. 2). This decreased percentage of DO11.10 T cells is explained by the great influx of endogenous T cells that recognize GAS antigen other than OVA, and by the migration of activated OVA-specific T cells out of the NALT into the periphery. Five days after inoculation small increases in percentage of OVA-specific CD4 T cells were observed in the LN of mice infected with OVA+ GAS, but no increase occurred in spleens. In contrast, CD4 T cells in mice infected with strain 90–226 (OVA–) failed to mount a response in any organs tested (Fig. 3), indicating that DO11.10 T cell expansion is antigen-specific and not due to polyclonal expansion, induced by streptococcal superantigen. Proliferation of DO11.10 CD4 T cells in mice was also monitored using a dye dilution method 19. Spleen and LN single-cell suspensions from transgenic mice were prelabeled with the fluorescent vital dye carboxyfluorescein diacetate succinimidyl ester (CFSE) before being transferred into naive BALB/c mice. Mice were then infected intranasally with GAS and killed for analyses at various time points after infection (Fig. 3). CFSE-labeled DO11.10 CD4 T cells from OVA– GAS-infected mice retained the high levels of CFSE, indicating that none had divided (Fig. 3A). However, DO11.10 T cells in NALT from OVA+ GAS-infected mice showed progressive dilution of CFSE, recognized on day 3 after infection (Fig. 3B), suggesting that these cells were undergoing considerable clonal expansion in this tissue. Moreover, 58% of the DO11.10 CD4 T cells had divided between one and eight times (Fig. 3B, C). In contrast, divided DO11.10 T cells did not appear in LN and spleen until day 5. The percentage of divided DO11.10 T cells in these organs remained low even 8 days after infection (Fig. 3); not surprisingly, since GAS rarely disseminate to LN and spleens following the small inoculum used in this experiment.
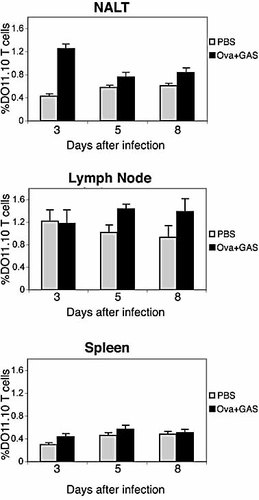
Expansion of DO11.10 T cells in secondary lymphoid organs after intranasal infection with GAS. BALB/c mice were either not infected (PBS), or infected intranasally with 2×107–5×107 CFU organisms 1 day after adoptive transfer of DO11.10 T cells. Mice were killed 3, 5, 8 days after infection. The percentages (mean ± SD) of CD4+/KJ1–26+ T cells in NALT, cervical LN, and spleen are shown.
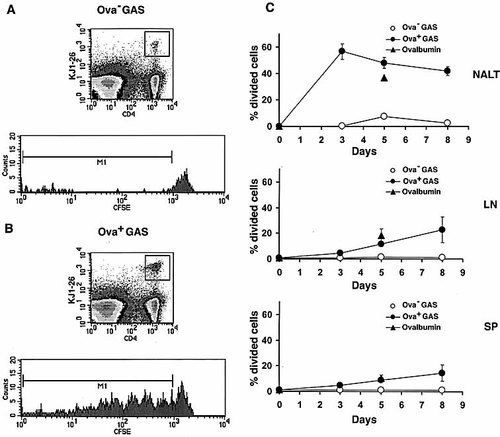
Clonal expansion of DO11.10 T cells in the NALT. One day following adoptive transfer of CSFE-labeled DO11.10 T cells, the recipient BALB/c mice were infected intranasally with 2×107–5×107 CFU or 30 μg OVA in 15 μl PBS. Groups of mice were killed at the indicated times. Dot plots (top) from two-color flow cytometric analysis of NALT: cells were stained with anti-CD4 and KJ1–26 mAb at 3 days after infection with either OVA– GAS (A) or OVA+ GAS (B). Histograms (bottom) of the CFSE content in DO11.10 T cells from NALT at 3 days after infection. Similar results were obtained in three separate experiments. (C) Percentage of divided DO11.10 T cells in NALT, cervical LN, and spleen (SP) at various time points after infection. The results represent the population of DO11.10 T cells containing lower levels of CFSE than the original amount of CFSE.
Since DO11.10 T cells appeared to divide first in NALT, then later in the other lymphoid organs we predicted that NALT is the initial inductive site for CD4 T cell activation. To test this prediction the level of CD69, an early activation marker, on OVA-specific T cells in NALT was assessed at an earlier time point. On day 1.5 after infection, a great number of DO11.10 T cells in NALT and LN expressed CD69 (Fig. 4A). Both levels of CD69+ expression on DO11.10 T cells and their frequency were higher in NALT than in LN. The expression of CD69 on DO11.10 T cells in NALT and LN had declined by 3.5 days after infection. In contrast, DO11.10 T cells in spleens showed no increase in expression of CD69, 1.5 or 3.5 days after OVA+ GAS infection.
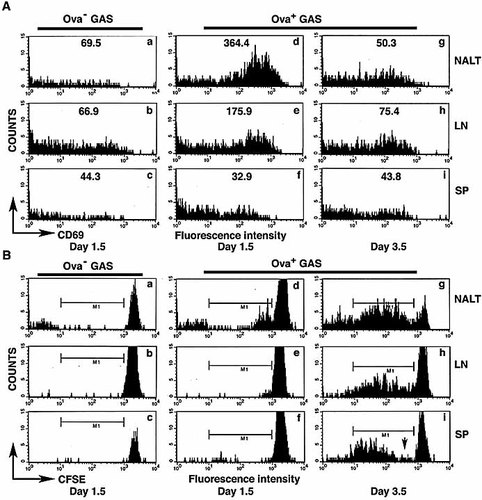
Induction of CD69 and division history of DO11.10 T cells in secondary lymphoid tissues. BALB/c mice received CFSE-labeled DO11.10 T cells were infected with 5×107 OVA– GAS or OVA+ GAS. At the indicated times, CD4+/KJ1–26+ cells in NALT, cervical LN, and spleen (SP) were analyzed for the induction of CD69 (A) and CFSE intensity (B) by flow cytometry. Numbers in (A) indicate mean fluorescences of CD69. Similar results were obtained from separate experiments. M1 represents the population of divided cells and was set based on staining of cells from control mice infected with OVA– GAS at each time point. Arrow indicates the lack of cells with lower division numbers.
In the above experiment, division profiles of DO11.10 T cells confirmed that activation of DO11.10 T cells is first initiated in NALT following GAS infection (Fig. 4B). For example, as early as 1.5 days after infection, DO11.10 T cells that underwent one or two divisions were detected (Fig. 4B, panel d). In contrast all DO11.10 T cells in LN and spleen retained initial levels of CFSE, indicating that no division had occurred (Fig. 4B, panels e, f). By 3.5 days following infection DO11.10 T cells in NALT had further divided, some having undergone as many as seven divisions. As observed earlier, appearance of DO11.10 T cells containing diluted levels of CSFE in LN and spleens occurs at later time points. Interestingly, the division profile of DO11.10 T cells in spleen, observed by 3.5 days after infection, is discontinuous (Fig. 4B, arrow in panel i). This is consistent with the lack of CD69 up-regulation on DO11.10 T cells in the spleen, suggesting the possibility that these divided DO11.10 T cells had originated and divided elsewhere.
2.4 Activation of splenic CD4 T cells during disseminated GAS infection
The lack of DO11.10 T cell activation in spleen in situ following intranasal infection with 5×107 CFU of OVA+ GAS may reflect failure of streptococci to disseminate to this organ. An alternative explanation is that T cell activation is delayed in spleen relative to more local lymphoid tissue. Preliminary experiments showed that more than 80% of mice infected with high doses (2×108–5×108 CFU) of GAS developed bacteremia and culture positive spleens (data not shown). To investigate whether dissemination to spleen and LN induces vigorous T cell replication in those tissues, we infected mice with a higher inoculum (5×108 CFU), and analyzed cervical LN and spleens over 21 days. As expected with this high dose, GAS had frequently spread to the LN and spleen, with peak bacterial counts occurring between the 3rd and 5th day after inoculation (Table 1). Infection of mice with OVA+ GAS resulted in considerable expansion of the DO11.10 T cells both in LN and spleen (Fig. 5). The highest frequencies and total numbers of DO11.10 T cells measured in LN of OVA+ GAS-infected mice occurred at 5 days post infection (Fig. 5C). In contrast, DO11.10 T cells in the spleen increased more slowly, and peaked 7 days after infection with OVA+ streptococci (Fig. 5D). By this time the fraction of DO11.10 T cells in LN has already diminished (Fig. 5C). The magnitude of T cell expansion in spleen was eightfold, significantly greater than that in LN, which was fourfold. The total numbers of antigen-specific T cells in spleen were also significantly greater than that in LN, reflecting the larger size of this organ (Fig. 5B). The delay of the CD4 T cell response in spleens at day 5 may reflect the time of antigen appearance or GAS dissemination to this tissue (Table 1). In the spleen the T cell expansion peaks at day 7 (Fig 5B, D) possibly due to increased bacterial (antigen) load in the spleens later during the infection. Differences in kinetics of CD4 T cell responses between mice infected with OVA+ GAS and those that received soluble OVA protein were also observed. Consistent with previous studies 14, 20, the number of DO11.10 T cells both in spleens and LN spiked to maximal levels by day 3 post inoculation with OVA protein, and then rapidly returned to prechallenge levels by day 5 (Fig. 5A, B).
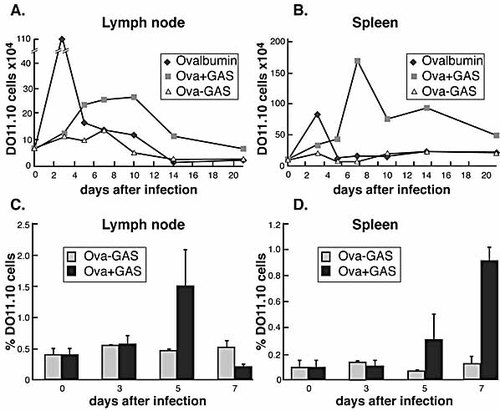
Clonal expansion of DO11.10 T cells at various times after infection with GAS. BALB/c mice that had received DO11.10 T cells were infected intranasally with 5×108 of OVA+ GAS, OVA– GAS, or OVA 1 day after adoptive transfer. Total number of DO11.10 T cells in cervical LN (A) and spleens (B) are shown. The percentages of OVA-specific CD4 T cells among total lymphocytes in each organ are shown in (C) and (D).
We also compared the CFSE dilution profiles in this experiment. DO11.10 CD4 T cells in uninfected mice retained high levels of CFSE as expected, indicating that none of these cells had divided (data not shown). In contrast, DO11.10 T cells in cervical LN from OVA+ GAS-infected mice showed progressive dilution of CFSE that was apparent 3 days after infection. In LN of infected mice nearly all DO11.10 T cells had proliferated, a response similar to mice injected with OVA protein (Fig. 6). However, by 3 days the fluorescence intensities of DO11.10 T cells in the spleen were more dilute and discontinuous (Fig. 6, arrow in panel k), showing that most of them had divided five to six times, yet there were very few cells in between, or that had undergone one to three divisions. This, together with the delay of splenic DO11.10 T cells responses, again suggests that activated and divided cells had migrated from elsewhere, presumably from draining LN or NALT. At 5 days after infection, almost all DO11.10 T cells in the spleen had under gone division, suggesting that division of DO11.10 T cells also occurs in the spleen, but at a later time. A discontinuous division history of DO11.10 T cells is also evident in spleens from mice that received OVA protein (Fig. 6e, f). These results demonstrate that the CD4 T cells activate efficiently in spleens when GAS disseminate into this tissue.
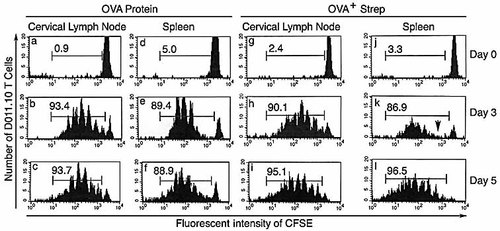
Histograms of CFSE-labeled DO11.10 T cells in response to GAS infection. BALB/c mice that had received CFSE-labeled DO11.10 T cells were infected with 5×108 OVA+ GAS or immunized with ovalbumin (200 μg) on 1 day after adoptive transfer. The bars indicate populations of divided cells. The percentages (mean ± SD) of divided cells obtained from three mice per group are shown in each histogram. Arrow indicates the lack of cells with lower division numbers.
3 Discussion
Anti-streptococcal antibody immune responses have been characterized in great detail 21, 22. However, neither the cellular immune response nor the response in tonsils, the predominant natural site of GAS infection, have been previously described. In this study we showed that intranasal infection by GAS initiated activation of CD4 T cells in NALT, an outcome that requires bacterial antigen acquisition by APC and presentation to T cells in this mouse, equivalent of human tonsils. NALT is known to contain macrophages and dendritic cells that lie under luminal epithelial surfaces. It is thought that APC acquire bacterial antigens from the basolateral surface of M cells and then present them to nearby T cells. We showed that GAS utilize M cells as a gateway for entry into the NALT 6. The fact that the CD4 T cellular response begins in NALT and not in the draining LN is consistent with descriptions of Salmonella infections 23. This organism induced CD4 T cell responses first in the Peyer's patches, rather than in nearby mesenteric LN. Together these results suggest that regional mucosal lymphoid tissues are the first to respond immunologically to bacterial pathogens that exploit M cells to disseminate within their host.
We also showed that the CD4 T cell response to surface-expressed OVA antigen in other lymphoid organs requires bacterial trafficking to those tissues. When GAS infection was localized to NALT, activation of CD4 T cells was more pronounced there than in LN or spleens, where the T cell response was marginal. However, when bacteria disseminated into these tissues, activation of CD4 T cells was also significant. Moreover, the kinetics of CD4 T cell responses in LN and spleens paralleled bacterial dissemination into these organs.
When infection was localized to NALT, or at early times after dissemination, we observed that spleens contained antigen-specific T cells that had extensively divided (CSFE-low cells), and large numbers of undivided naive T cells. Absence of up-regulated expression of CD69 on these cells suggests that these CSFE-low cells were initially activated and divided in NALT or LN, and then migrated to the spleen. This is consistent with the fact that T cells gain the potential to leave secondary lymphoid tissues and recirculate after activation 24. Activated T cells also acquire receptors that permit them to extravasate from the blood into inflamed peripheral tissue, such as the nasal mucosa. We postulate that, following intranasal inoculation, streptococci accumulate in NALT where they encounter macrophages and dendritic cells which present antigen (OVA) to specific T cells. When infection is restricted to the NALT, this tissue is postulated to be the primary source of effector and memory cells that can then migrate to regional LN and spleen via lymphatics or the blood stream. Even though other authors reported the potential of dendritic cells in tracheobronchial LN to present viral peptides after intranasal infection, such experiments lack the sensitivity to identify in vivo the location of primary activation of naive antigen-specific T cells. The requirements for activation of T cell clones are substantially different than for naive T cells, particularly when such activation is tested in vitro.
Lymphocytes isolated from rat NALT preferentially homed backed to NALT, to cervical LN, and to mesenteric LN 25. Our experiments did not use T cells from NALT of DO11.10 mice, but we showed that the naive CD4 T cells from LN and spleens can circulate and park in NALT with a frequency of 0.4% of total endogenous CD4+ T cells. Such antigen-specific T cells can then be activated, divide in NALT and migrate out of NALT into the periphery.
Second only to otitis media, streptococcal pharyngitis is the most common childhood infection for which antibiotics are prescribed. Serious complications such as rheumatic fever, acute glomerulonephritis and toxic shock depend on immunological responses to GAS, which are poorly understood. Children frequently become non-immune carriers with recurrent infection. The gravity of the problem has recently prompted both the private sector and NIH to launch aggressive vaccine development projects. Yet the basis for protective immunity and the importance of cellular immunity has not been investigated in depth. This study is the first to apply the powerful tool of adoptive T cell transfer to investigate the cellular response to GAS infections, and the first to investigate that response in mucosal-associated lymphoid tissue in the upper respiratory tract. Advances in understanding immunity to Gram-positive bacterial infections will produce a foundation of information that will augment vaccine development and influence therapeutic strategies in the future.
4 Materials and methods
4.1 Mice
DO11.10 TCR transgenic mice were bred under pathogen-free conditions at the animal facility of the University of Minnesota according to National Institutes of Health guidelines and screened as described previously 17. Female BALB/c mice were purchased from the National Cancer Institute (Frederick, MD) and used at 7–10 weeks of age. Mice infected with GAS were housed in biosafety level 2 facilities.
4.2 Adoptive transfer of DO11.10 T cells
Superficial and posterior cervical, brachial, axillary, pelvic, mesenteric LN and spleen from DO11.10 donor mice were harvested by dissection, mashed on a nylon screen and washed twice in HBSS. No attempt was made to remove red blood cells. For some experiments pooled cells were resuspended at 5×107/ml and incubated at 37°C for 10 min in 7.5 μM CFSE/HBSS 19. To determine the frequency of DO11.10 T cells, an aliquot of pooled cells were counted and stained prior to CFSE labeling with CyChrome-labeled anti-CD4 and biotin-labeled KJ1–26 mAb followed by streptavidin-PE and analyzed using a FACScan flow cytometer (Becton Dickinson). CFSE-labeled LN and spleen cells containing 3×106–5×106 CD4+/KJ1–26+ T cells as determined by FACS were injected in the tail vein of recipient BALB/c mice. After 24 h, recipient mice were infected or immunized intranasally.
4.3 Harvesting NALT
NALT tissues were collected as previously described 6. Briefly, euthanized mice were decapitated. After the lower jaw, including tongue, was removed, palates were scored along the outer edge distal to the central incisors and lateral to the hard palate through the oral mucosa going distal up to the mesial surface of the first molar. If the hard palate was lifted gently, the NALT remained attached to it. Once exposed the entire hard palate and NALT were excised just distal to emergence of the palatine nerve. NALT from two mice were pooled and the resulting single-cell suspension was filtered over a nylon screen and washed before staining.
4.4 Flow cytometry and antibodies
Single-cell suspensions from NALT, cervical LN and spleens were incubated on ice with CyChrome-labeled anti-CD4 (PharMingen, San Diego, CA) and biotinylated anti-clonotypic KJ1–26 mAb, followed by streptavidin-PE (Caltag, South San Francisco, CA) as previously described 17. A Becton Dickinson FACScan flow cytometer was used to collect and analyze 2,000 events that had the light scatter properties of lymphocytes and were CD4+ and KJ1–26+. The total OVA-specific T cells were determined in flow cytometry by gating on CD4+, KJ1–26+ cells and by multiplying such percentages by the total number of cells of a specific compartment. Total proliferating DO11.10 T cells were calculated by multiplying the total number of CD4+, KJ1–26+ cells in each compartment by the percentage of cells that have diluted CFSE on the CFSE histogram.
4.5 Construction of recombinant GAS strains
The 17-amino acid epitope of OVA 323–339 was inserted after the 5th amino acid from the N-terminal end of the mature M1 surface protein. The epitope was inserted at this position to minimize the probability of interference with cleavage of the signal sequence peptide. In addition, the structure of the N terminus of the M protein is predicted to be random coil, while the remainder is almost completely in an α-helix conformation 26. By locating the insertion in the random coil region, the effect of the insertion on M1 protein function should be minimized. The emm1.0 of strain 90–226 is contiguous with mga at the 5′ end and with the sic at the 3′ end, and has its own promoter 27.
Plasmid pFW5 28 has two multiple cloning sites on either side of a spectinomycin resistance gene. The entire sic gene including its promoter region was inserted into the multiple cloning site downstream of the spectinomycin resistance gene in pFW5. This region was amplified from strain 90–226 using PCR. The forward primer, designated sicNcoIfor introduced an NcoI restriction site (underlined). This primer starts 8 bp after the end of the emm1.0 gene (5′-CCC CCC CCA TGG CTT TGT AAT ACT GAG TGA ACA-3′). The reverse primer, sicSpeIrev, includes the last five codons of the sic gene and adds a SpeI restriction site (5′-CCC CCC ACT AGT TTT ACG TTG CTG ATG GTG-3′). This 1.1-kb fragment was digested with NcoI and SpeI and inserted into the NcoI and SpeI restriction sites in pFW5 to construct pPE5.
A fragment containing the C-terminal half of mga through the entire emm1.0 gene was inserted into the multiple cloning sites on the other side of the spectinomycin resistance gene in pPE5. This fragment was created by a two-step method to insert the sequence of the OVA epitope peptide into the emm1.0 gene. In the first step, two DNA fragments, each coding for part of the ova epitope sequence were amplified by PCR. In the second step, these fragments were used as template DNA to amplify a 2.5-kb fragment, which was inserted into pPE5. In the first step, the two separate DNA fragments were amplified from the strain 90–226. The upstream fragment was generated by the forward primer, mgaBsiWIfor, which anneals 817 bp after the start of mga and has a BsiWI restriction site (5′-CCC CCC CGT ACG GAC CAA TTA GAA ATC GGT TAT-3′). The reverse primer, emmovarev, anneals to the first 5 amino acids of the mature M1 protein and the amino acid immediately before the cleavage site. In addition, 37 bp corresponding to the amino terminal end of the ova peptide were added (underlined) (5-′TGA TTT CTG CAT GTG CTG CAT GGA CAG CTT GAG ATA TAT TAC CAT CAC CGT TAG C-3′). The forward primer of the other fragment, emmovafor, anneals to codons 6–11 of the mature M1 protein, and also includes the sequence of the C-terminal 11 amino acids of the OVA peptide (underlined) (5′ GCA GCA CAT GCA GAA ATC AAT GAA GCA GGC AGA CCT AGG GAA GTT ATA GAA 3′). The reverse primer, emmXhoIrev, is located about 100 bp downstream of the emm1.0 sequence and adds an XhoI site (5′-CCC CCC CTC GAG TTA GTT TGT GAC CTC TCC TTA-3′). The first 18 bases of the ova epitope sequence included in emmovarev are complementary to the last 18 bases of the OVA epitope sequence in the emmovafor primer. In the second step, the two fragments generated above were added together and two PCR cycles were run with an annealing temperature of 48°C. After fragments were annealed, the primers mgaBsiWIfor and emmXhoIrev, and Bio-X-Act Polymerase (Intermountain Scientific Corp.) were added to the reaction and the annealing temperature was increased. Twenty-five additional PCR cycles resulted in a 2.5-kb fragment consisting of the C-terminal half of mga and the entire emm1.0 gene with the OVA epitope. This fragment was inserted into pPE5 using the BsiWI and XhoI restriction sites to construct pPE6. This plasmid was introduced into strain 90–226 emm1.0::km 16 for gene replacement, and transformants were selected on spectinomycin (100 μg/ml). The spectinomycin-resistant transformants were then screened for kanamycin sensitivity. The resulting strain was designated 90–226 emm1.0::ova. The DNA region including the OVA insertion was amplified and sequenced using the forward primer, all M forward 29 and the reverse primer, emm414, complementary to nucleotides 414–435 of the emm1.0 sequence (5′-GCG ATG CCA TGG ATA GAC AAA GAC TTG AAA AA-3′).
4.6 Intranasal inoculum and antigens
Adoptively transferred BALB/c mice were anesthetized with isoflurane/oxygen mixture for 1 min and inoculated intranasally with OVA+ GAS, OVA– GAS (2×107–5×107 or 2×108–5×108 CFU) or OVA (30 or 200 μg, Sigma, St. Louis, MO) in 15 μl PBS (7.5 μl/nostril). By placing fractionated droplets with a pipette tip near the entrance of the nostril the inoculum goes into nasal cavity by involuntary aspiration.
Acknowledgements
This work was supported by National Institute of Health Grant AI 34503.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




