Quantitative, not qualitative, differences in CD8+ T cell responses to Theiler's murine encephalomyelitis virus between resistant C57BL/6 and susceptible SJL/J mice
Abstract
Theiler's murine encephalomyelitis virus (TMEV) infection of the CNS induces an immune-mediated demyelinating disease in susceptible mouse strains and serves as a relevant infection model for human multiple sclerosis. However, it is not yet clear what immunological parameters determine the susceptibility of SJL/J mice compared to resistant mice. We have here compared the TMEV-specific CD8+ T cell responses in highly susceptible SJL/J mice with those of highly resistant C57BL/6 mice. Our results clearly indicate that the levels of initial responses of infiltrating CD8+ T cells to viral capsid proteins are higher in resistant C57BL/6 mice compared to susceptible SJL/J mice. However, the level of virus-specific CD8+ T cells was much more rapidly reduced in resistant C57BL/6, resulting in a higher CD8+ T cell level in SJL/J mice later in viral infection. The activation states, cytokine production, as well as the cytolytic function of the CD8+ T cells were similar to each other in these mice. These results suggest that an initial induction of a vigorous CD8+ T cell response to TMEV is critically important for the resistance to virally induced demyelinating disease.
Abbreviations:
-
- CNS:
-
Central nervous system
-
- CNS-IL:
-
CNS-infiltrating lymphocytes
-
- TMEV:
-
Theiler's murine encephalomyelitis virus
-
- TMEV-IDD:
-
TMEV-induced demyelinating disease
1 Introduction
Intracerebral infection of the BeAn strain of Theiler's murine encephalomyelitis virus (TMEV) into susceptible strains of mice induces a chronic, progressive demyelinating disease that is similar clinically and histopathologically to human multiple sclerosis (MS) 1. In addition, the various immunological and genetic factors that affect disease outcome in TMEV-infected mice closely parallel those associated with the development of MS 2. Also, immune responses against myelin components have been demonstrated in both MS and TMEV-induced demyelinating disease (TMEV-IDD), suggesting that autoimmunity may play a role in the pathogenesis of both diseases 3. Combined with a suspected viral etiology for MS 4, these similarities make TMEV-IDD an attractive and relevant infection model for the study of this human demyelinating disease.
Development of TMEV-IDD in the highly susceptible SJL/J (H-2s) mouse is associated with viral persistence in the central nervous system (CNS) 5, whereas the prototypically resistant C57BL/6 (H-2b) mouse clears the virus within 2–4 weeks of infection 6. Thus, viral persistence appears to be a critical factor in disease development. Resistance to TMEV-IDD has been closely associated with the MHC class I genetic locus 7, suggesting that MHC class I-restricted CD8+ T cells may be important mediators of protection and/or pathogenesis. MHC class I-restricted CTL appear to confer protection from TMEV-IDD on resistant strains, as β2-microglobulin-deficient 8 and perforin-deficient 9, 10 mice on a resistant H-2b background fail to clear TMEV from the CNS and develop demyelinating lesions similar to susceptible strains. However, the lack of clinical signs (i.e. waddling gait and eventual paralysis) in these mice has led to the hypothesis that CTL may also mediate the CNS pathology responsible for clinical manifestation of disease 10, 11. On the other hand, susceptible SJL/J (H-2s) mice lacking β2-microglobulin exhibit higher levels of demyelination, increased inflammatory Th responses and earlier onset of clinical disease 12. These results indicate that MHC class I-restricted CD8+ T cells are not necessary for the development of demyelination or clinical symptoms and instead contribute to protection from disease. Thus, the role of CD8+ T cells in the pathogenesis of TMEV-IDD remains a controversial issue. However, the great majority of the previous studies on CD8+ T cells specific for TMEV have been performed using either prototypically resistant C57BL/6 or susceptible SJL/J mice. In order to determine the potential role of CD8+ T cells in the protection/pathogenesis, it is essential to define and characterize the MHC class I-restricted CD8+ T cell response in these resistant and susceptible mice. Previously, other studies have shown that resistant H-2b mice mount an H-2Db-restricted CTL response against one highly dominant (VP2121–130) viral capsid protein epitope, comprising as much as 60–75% of CNS-infiltrating CD8+ T cells 13. More recently, we reported that such mice also generate low but detectable H-2Db-restricted responses to the VP2165–173 and VP3110–120 determinants 14. In addition, we have shown that at least 60–75% of CNS-infiltrating CD8+ T cells in susceptible mice are TMEV capsid specific and recognize one of three (VP111–20, VP3159–166 or VP3173–181) H-2Ks-restricted epitopes 15. No other major CD8+ T cell epitopes are present, based on the epitope-screening with a 20-mer library overlapping ten amino acid residues for the leader/capsid 14, 15 and non-capsid proteins (unpublished data). Thus, similar levels of CNS-infiltrating CD8+ T cells appear to recognize TMEV capsid protein epitopes in highly resistant C57BL/6 and susceptible SJL/J mice. These findings offer an opportunity to directly compare the virus-specific CTL responses within the CNS of these resistant and susceptible mouse strains.
We have here directly compared the TMEV-specific CTL responses between the prototypically susceptible SJL/J (H-2s) and resistant C57BL/6 (H-2b) strains. Our analyses reveal that virus-specific CTL in both susceptible and resistant strains have similar activation markers and effector function. However, the magnitude of the response is roughly threefold higher in resistant C57BL/6 mice at the peak of the CTL response. Interestingly, TMEV-specific CTL do not persist within the CNS of resistant C57BL/6 mice compared to SJL/J mice, reflecting a lack of viral persistence. These results suggest that the MHC haplotype determines the initial level of the CTL response, which is critically important in viral clearance in the CNS, resulting in resistance to chronic immune-mediated TMEV-IDD.
2 Results
2.1 TMEV-reactive CD8+ T cells in the acute response in the CNS and periphery of virus-infected resistant C57BL/6 and susceptible SJL/J mice
To assess the levels of virus-specific CD8+ T cells infiltrating the CNS of C57BL/6 and SJL/J mice, mononuclear cell preparations of brains and spinal cords of these mice infected with TMEV were analyzed at 8 days post infection (Fig. 1). As shown previously 14, the great majority (74%) of infiltrating CD8+ T cells from C57BL/6 mice recognize the predominant VP2 epitope (VP2121–130), a significant percentage (14%) is specific for VP3110–130, and a minimal level recognizes VP2165–173. Thus, as much as 86% of the infiltrating CD8+ cells appear to recognize TMEV capsid epitopes in resistant C57BL/6 mice at the peak of the CTL response. Similarly, the majority (∼65%) of CD8+ T cells infiltrating the CNS of susceptible SJL/J mice were reactive to virus capsid epitopes at this time (38% for VP3159–166, 18% for VP3173–181, and 9% for VP111–20). However, the level of virus-reactive CTL was somewhat lower in SJL/J mice than in resistant C57BL/6 mice, and the epitope dominance is less prominent in SJL/J mice. These results clearly indicate that the majority of CNS-infiltrating CD8+ T cells recognize capsid epitopes both in resistant C67BL/6 and in susceptible SJL/J mice during the peak responses. We believe that these TMEV-specific CD8+ T cells represent most of the CD8+ T cell responses in these mice, since no other major CD8+ T cell epitopes are present 14, 15.
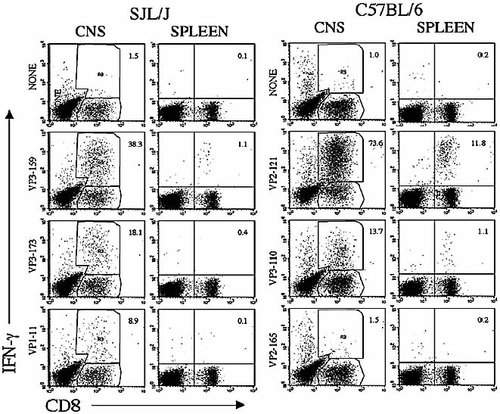
Comparison of IFN-γ production by CNS-infiltrating and splenic CD8+ T cells from TMEV-infected SJL/J and C57BL/6 mice in response to the respective capsid epitopes. CNS-infiltrating lymphocytes were isolated from four mice per strain at 8 days post TMEV infection. IFN-γ-producing cells were assessed by flow cytometry following intracellular staining of IFN-γ. The first residues of the minimal epitope sequences are indicated. Percentages of CD8+ T cells producing IFN-γ in the mononuclear cell population are shown in the figure. The total CD8+ T cells were 12.8% in the CNS and 13% in the spleens of SJL/J mice. The total CD8+ T cells were 21.4% in the CNS and 15% in the spleens of C57BL/6 mice. A representative result from three separate experiments is shown here.
To correlate the CNS-infiltrating CD8+ T cell response to viral epitopes with the CD8+ T cell response in the periphery, splenic CD8+ T cells specific for viral epitopes from these mice were similarly assessed by flow cytometric analysis for IFN-γ production and compared to those in the CNS (Fig. 1). Interestingly, as much as 12% of splenic CD8+ T cells from resistant C57BL/6 mice at day 8 after TMEV infection were specific for the predominant epitope (VP2121–130), while 1% or less splenic CD8+ T cells were reactive to any of the capsid epitopes in susceptible SJL/J mice. Similar differences in the splenic CD8+ T cells specific for viral capsid epitopes were observed in multiple experiments (data not shown). These results clearly indicate that the overall CD8+ T cell response in the periphery of resistant C57BL/6 mice is significantly higher than that of susceptible SJL/J mice at the peak of response. The lower level of peripheral virus-specific CD8+ T cells in susceptible SJL/J mice may consequently result in a lower level of CD8+ T cell infiltration into the CNS.
2.2 TMEV capsid-specific CD8+ T cells infiltrating the CNS during the course of viral infection
Development of TMEV-IDD has been shown to correlate with viral persistence 6, 16. In order to correlate the differential susceptibility to demyelination with the levels of virus-specific CTL in the CNS of these two strains during the course of viral infection, capsid-specific CD8+ T cells from TMEV-infected mice were enumerated by intracellular IFN-γ staining at different days post infection (Fig. 2). SJL/J mice up to 30 days post infection showed no clinical symptoms of TMEV-IDD, while disease symptoms (severe waddling gait) were clearly observable at 60 days, and full-blown disease, including severe hind limb paralysis, was seen at 90 and 120 days post infection (not shown). As expected, no virus-specific T cells were detectable in either SJL/J or C57BL/6 mice at 3 days post infection. However, by day 5, approximately 4% of the CNS-infiltrating CD8+ T cells in SJL/J mice were virus specific (for VP111–20, VP3159–166 and VP3173–181), while 14% of CD8+ T cells from C57BL/6 mice were reactive to the dominant VP2121–130 epitope (Fig. 2A). It should be noted here that responses to the minor capsid protein epitopes (VP3110–120 and VP2165–173) for C57BL/6 were negligible, as described previously 14. Nevertheless, the percentage of infiltrating CD8+ T cells that are TMEV specific is greater in C57BL/6 mice at this early time point, suggesting that the kinetics of the virus-specific CTL response differ between C57BL/6 and SJL/J mice. By 8 days post infection, high proportions (58% and 66%, respectively) of infiltrating CD8+ T cells were virus-specific in both SJL/J and C57BL/6 strains. TMEV-specific CD8+ T cells persist in the CNS of SJL/J mice at all late time points in relatively high numbers. In contrast, TMEV-specific CD8+ T cells were approximately threefold lower in C57BL/6 mice at the 30-day time point and were undetectable at later time points due to the low number of lymphocytes present in the CNS.
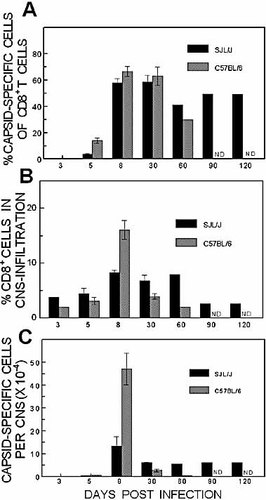
Assessment of the level of infiltration and capsid reactivity by CNS-infiltrating CD8+ T cells in SJL/J and C57BL/6 mice during the course of TMEV-infection. (A) The proportion of capsid-reactive T cells of the total CNS-infiltrating CD8+ T cells in TMEV-infected mice (four to six mice/group). The capsid reactivity was assessed based on IFN-γ production after stimulation with VP3159–166, VP3173–181 and VP111–20 for CNS cells from SJL/J and VP2121–130 for CNS cells from C57BL/6 mice. (B) Level of overall CD8+ T cell infiltration into the CNS during the course of TMEV infection. (C) The number of TMEV-specific CD8+ T cells per 104 mononuclear cells was calculated based on the levels of virus reactivity and CD8+ cell infiltration. ND: not determined. The values at days 5, 8 and 30 represent the means and standard errors of three to five separate experiments. The difference in the level of CD8+ T cell infiltration into the CNS between TMEV-infected C57BL/6 and SJL/J mice is significant (p=0.03).
The level of overall CD8+ T cells infiltrating the CNS in these mice was assessed based on the percentage of CD8+ T cells among CNS mononuclear cells during the course of viral infection (Fig. 2B). The level of overall CD8+ T cell infiltration was significantly higher in SJL/J mice during the course of viral infection, except at the peak of the response, at day 8 post infection, when a higher level of CD8+ T cell infiltration was observed in C57BL/6 mice. However, despite higher CD8+ T cell infiltration into the CNS, SJL/J mice showed lower levels of virus-specific CD8+ T cells at the early time points (3 and 5 days post infection) compared to C57BL/6 mice. The levels of overall CD8+ T cells in the CNS were also somewhat reduced at the later time points (>60 days post infection). To further enumerate virus-specific CD8+ T cells infiltrating the CNS in these two strains, the percentage of epitope-specific CD8+ T cells was multiplied with the total number of CD8+ T cells infiltrating the CNS (total cells per mouse × %CD8+) for each strain (Fig. 2C). Virus-infected C57BL/6 mice contain close to twice as many TMEV-specific CD8+ T cells as SJL/J mice (∼6.0×103 vs. 3.7×103, respectively) at 5 days post infection. At 8 days post infection, infiltration of virus-specific CD8+ T cells increased greater than threefold (4.7×105 vs. 1.3×105 epitope-specific cells) in C57BL/6 mice compared to SJL/J mice. Thus, resistant C57BL/6 mice appear to mount a more vigorous early CTL response in the CNS, even though the proportion of capsid-reactive CD8+ T cells in the CNS is similar in these mouse strains.
2.3 Correlation of CD8+ T cell responses with viral persistence
To correlate the level of viral persistence in the above experiments with the level of virus-specific CD8+ T cells in the CNS, viral plaque-forming units (pfu) in the brain and spinal cord were determined (Fig. 3). At 7 days post infection (peak immune response), viral plaques from the brains of SJL/J mice were 2–3-fold higher than those from the brains of C57BL/6 mice, although no marked differences were found in the spinal cords. At 21 days (onset of disease) after viral infection, viral plaques were not detectable in the brain of either C57BL/6 or SJL/J mice. However, viral persistence in the spinal cords of susceptible SJL/J mice was >10-fold higher than in resistant C57BL/6 mice. These results are in agreement with earlier studies in these mouse strains 6. Together, these results suggest that SJL/J mice fail to efficiently clear virus, leading to viral replication in the spinal cords of susceptible mice at the late time point, while resistant C57BL/6 mice are able to eliminate virus. Thus, persistent infection may lead to the accumulation and retention of virus-specific CD8+ T cells within the CNS of susceptible SJL/J mice, whereas viral clearance leads to the resolution of the immune response in resistant C57BL/6 mice.
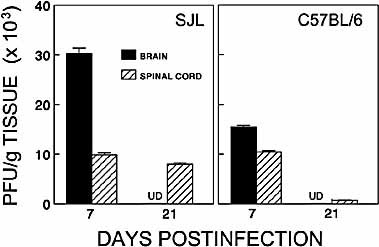
Comparison of viral persistence in the brains and spinal cords of SJL/J and C57BL/6 mice at 7 and 21 days post TMEV infection. The level of viral persistence in the brains and spinal cords (pooled from four mice) was assessed by plaque assay and expressed as the number of plaques per g tissue (mean of triplicates ± standard error of mean) at each time point. The differences in viral persistence between SJL/J brain and C57BL/6 brain, SJL/J brain and spinal cord, C57BL/6 brain and spinal cord at 7 days post infection are significant (p<0.001). However, the differences between SJL/J spinal cords at 7 days and 21 days, and SJL/J and C57BL/6 spinal cords at 7 days are not significant (p>0.05). UD: undetectable, which represents lower than 500 pfu/g tissue.
2.4 Similar cytokine profiles of CNS CD8+ T cells from SJL/J and C57BL/6 mice
CTL also produce TNF-α, which has powerful direct antiviral activity through non-cytotoxic mechanisms 17. On the other hand, TNF-α, a pro-inflammatory cytokine, could potentially contribute to the pathogenesis of immune-mediated diseases such as TMEV-IDD 18. Therefore, the production of TNF-α by TMEV-specific CTL from the CNS of SJL/J and C57BL/6 mice was further analyzed at 8 days post infection by intracellular staining after stimulation with epitope peptides. As shown in Fig. 4, very similar percentages of virus-specific CD8+ T cells from these strains produce both TNF-α and IFN-γ, regardless of the epitope specificity. Thus, no significant differences exist between virus-specific CTL from these two mouse strains with respect to the production of these antiviral and pro-inflammatory cytokines.
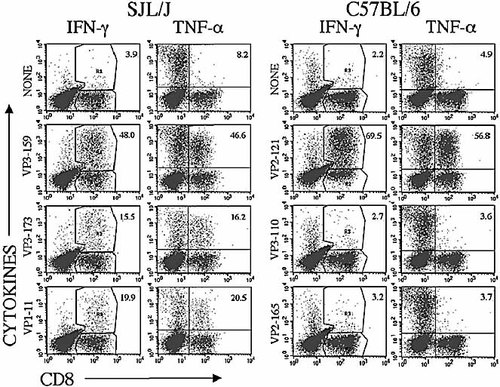
Similar levels of IFN-γ and TNF-α production by CNS-infiltrating virus-specific CD8+ T cells in SJL/J and C57BL/6 mice infected with TMEV. The levels of TNF-α- and IFN-γ-producing cells upon stimulation with epitope peptides were compared in SJL/J and C57BL/6 mice (four mice/group) at 8 days post infection. A representative result of three similar experiments is presented here.
2.5 Similar effector function and epitope avidity of CNS-infiltrating virus-specific CD8+ T cells from SJL/J and C57BL/6 mice
To determine the potential difference in the level of lytic effector function of CTL from susceptible SJL/J mice compared to that of C57BL/6 mice, the cytolytic activity of CNS-infiltrating T cells against EL-4 or EL-4Ks target cells loaded with the predominant C57BL/6 and SJL/J epitopes, respectively, was compared at 8 days post TMEV infection (Fig. 5A). When considering the overall levels of infiltrating viral epitope-specific CD8+ T cells, i.e. all capsid epitope-reactive CTL in SJL/J mice vs. the predominant epitope-reactive CTL in C57BL/6 mice, the level of target cell cytolysis by the infiltrating SJL/J CTL is not inferior to that by C57BL/6 CTL.
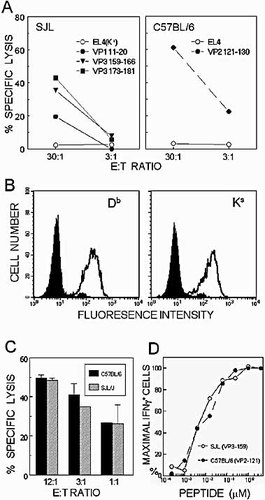
Similar cytolytic function and avidity to respective epitopes by CNS-infiltrating CD8+ T cells from virus-infected SJL/J and C57BL/6 mice. (A) Viral epitope-specific cytolytic function of CNS-infiltrating CD8+ T cells from SJL/J and C57BL/6 mice (six mice/group) at 8 days post TMEV infection. The cytolysis was assessed by standard 51Cr-release assay using peptide-loaded target cells. (B) The expression of Db and Ks molecules on the target cells was measured by flow cytometry using FITC-conjugated isotype controls (solid) and respective antibodies (open) specific for Db and Ks molecules. (C) The efficiencies of cytolysis by the same number of the predominant epitope-specific CD8+ T cells from the CNS of TMEV-infected mice (7 days post infection) were compared by 51Cr-release assay using VP3159–166-loaded EL-4Ks and VP2121–130-loaded EL-4 target cells. The proportions of the CD8+ T cells in the CNS mononuclear cell preparations and the epitope-specific cells in the infiltrating CD8+ T cells were considered to adjust the specific effector-to-target cell ratios. (D) Similar avidity of CNS-infiltrating CD8+ T cells toward the predominant epitopes of SJL/J and C57BL/6 mice infected with TMEV. The relative avidities were cytometrically assessed by determining the levels of IFN-γ-producing cells upon stimulation with varying concentrations of the respective epitope peptides. A representative result of two to three similar experiments is presented here.
To further dissect the cytolytic function of CNS-infiltrating CD8+ T cells, the expression levels of the relevant MHC class I molecules (i.e. H-2Db and H-2Ks) on the target cell surface were first assessed by flow cytometry (Fig. 5B). The results indicate that the levels of these molecules on the target cells are very similar to each other. Therefore, it is very unlikely that the above cytolytic assays (Fig. 5A) are affected by the potential differences in the MHC class I expression on the target cells. However, direct comparison of the cytolytic function of virus-specific CD8+ T cells from susceptible SJL/J and resistant C57BL/6 mice is rather difficult because the proportion of epitope-specific CD8+ T cells infiltrating the CNS is significantly different (Fig. 2). To directly compare the efficiencies of cytolysis by TMEV-specific CD8+ T cells from these mice, equal numbers of the predominant epitope-reactive CD8+ T cells were used to assess the cytolytic efficiencies for the target cells loaded with a low concentration (0.4 µM) of the respective peptides (Fig. 5C). The efficiencies of target cell cytolysis by the effector CD8+ T cells from SJL/J and C57BL/6 mice were very similar. Thus, these results strongly suggest that there is no significant difference in the cytolytic function of virus-specific CD8+ T cells in these susceptible and resistant mouse strains.
It is conceivable that a significant difference in the avidity of TCR towards epitopes between SJL/J and C57BL/6 mice may influence the efficiencies of the T cell activation and, consequently, the level of CTL. The differences in the level of dominance of the predominant epitopes between C57BL/6 (60–70%) and SJL/J (25–40%) mice may also reflect the differences in the avidity of T cells reactive to these epitopes. To address this possibility, the avidity of CNS-infiltrating CD8+ T cells reactive to the dominant epitopes (VP2121–130 for C57BL/6 and VP3159–166 for SJL/J) was assessed by enumerating IFN-γ-producing CD8+ T cells upon stimulation with varying concentrations of the epitope peptides (Fig. 5D). The proportions of IFN-γ-producing CD8+ T cells were virtually identical across the peptide concentrations, strongly suggesting that the avidity of these T cells toward the respective predominant epitopes is not different between resistant and susceptible mice. Therefore, avidity differences toward the epitopes are not likely to contribute to the level of CD8+ T cells infiltrating the CNS in these mouse strains.
2.6 Similarly activated but more CD25-expressing CD8+ T cells in the CNS of TMEV-infected C57BL/6 mice compared to SJL/J mice
To further characterize the CNS-infiltrating CD8+ T cells during early TMEV infection in both mouse strains, the activation state of these cells was determined by flow cytometry after staining with antibodies to several T cell activation markers. Fig. 6A shows a representative analysis using one activation marker (CD44), and Fig. 6B shows the percentage of CNS-infiltrating CD8+ T cells with “activated phenotype” as assessed with all these markers. The data suggest that most of the infiltrating CD8+ T cells in both strains are similarly activated (CD28hi CD44hi CD62Llo CD69hi). In addition, the levels of CD38+ T cells, which correlate well with the activation status and cytolytic function of virus-reactive CD8+ T cells 19, are similar in these mice. These data suggest that the activation status of CNS-infiltrating CD8+ T lymphocytes does not differ between these two strains.
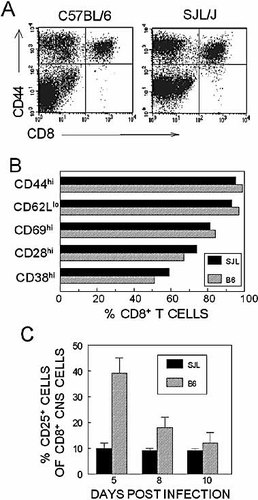
Similar activation state of CNS-infiltrating CD8+ T cells in SJL/J and C57BL/6 mice infected with TMEV. Several activation markers expressed on CNS-infiltrating CD8+ T cells at 8 days post viral infection were assessed by flow cytometry. (A) Comparison of CD44 expression on CD8+ T cells as an example. (B) Percentages of CNS-infiltrating CD8+ T cells bearing the activation markers in SJL/J and C57BL/6 mice. (C) The levels of CD25+ CD8+ T cells in the CNS of SJL/J and C57BL/6 mice were compared by flow cytometry. The percentage of CD25+ cells from total CNS-infiltrating CD8+ T cells is plotted on the y-axis. The values represent the means ± standard error of two to three separate experiments.
Since the quantity, but not the quality, of TMEV-specific CD8+ T cells differs in the CNS of virus-infected resistant and susceptible mice, it is conceivable that the initial expansion of virus-specific CD8+ T cells may be affected by potential differences in the IL-2 and/or IL-2 receptor (CD25) expression. Consequently, this could result in different levels of CD8+ T cell expansion in the periphery and CNS. In order to assess potential differences in the level of IL-2 production upon stimulation with viral epitopes, CNS-infiltrating cells from TMEV-infected C57BL/6 and SJL/J mice were stimulated with epitope peptides, and the IL-2 production was monitored by flow cytometric analysis. Greater than twofold more infiltrating CD8+ T cells (12% vs. 5%) produced IL-2 in resistant C57BL/6 mice than in SJL/J mice (not shown). Interestingly, a higher percentage of infiltrating CD8+ T cells from C57BL/6 mice (e.g. 39%, as compared to 10% in SJL/J at 5 days post infection) express the high-affinity IL-2 receptor, CD25, at early time points of viral infection (Fig. 6C). The relatively higher level of CD25 expression in CD8+ T cells from the CNS of C57BL/6 mice may lead to higher CD8+ T cell proliferation in the resistant mice.
3 Discussion
CD8+ T cells are known to play an important role in clearing viruses and other intracellular pathogens from infected hosts. Thus, protection from disease caused by these pathogens may rely heavily on this important effector cell population. In contrast, the development and progression of demyelinating disease correlate well with the level of Th1 responses specific for viral epitopes 2. Thus, such protection by CD8+ T cells may reflect direct down-regulation of the pathogenic Th cell response by regulatory CD8+ T cells and/or CTL-mediated elimination of a source of stimulation (virus antigen) for pathogenic Th cells 20. These results are consistent with previous reports suggesting that MHC class I-restricted responses contribute to clearance of TMEV in both susceptible and resistant mouse strains 6. In the absence of a vigorous antiviral response, chronic infection may lead to the persistent activation and recruitment of pathogenic CD4+ T cells that are specific for virus or for CNS autoantigens 3.
Rodriguez et al. 21 have previously suggested that SJL/J mice and other H-2s strains cannot mount virus-specific CTL responses to TMEV in the CNS, in contrast to strong CTL responses in the resistant C57BL/6 or C57BL/10 (H-2b) strains by utilizing TMEV-infected fibroblast target cell lines. Another group has also reported that the level of CTL responses in SJL/J is lower than in C57BL/6 mice, using TMEV-infected fibroblast targets to assess the differences in the cytolytic functions of splenic T cells 22. However, recent studies utilizing defined epitope peptides indicated that strong virus-specific CD8+ T cell responses are present in the CNS of SJL/J mice infected with TMEV, based on epitope-specific intracellular IFN-γ production as well as cytolytic activity 15, 23. The utilization of peripheral T cells and TMEV-infected target cells for the cytolytic assays may not be able to exclude the involvement of NK cells, inefficient target cytolysis 15, as well as TMEV-induced nonspecific 21 or autoreactive CD8+ T cells 24. Therefore, the assessment of virus-specific CD8+ T cell responses that solely rely on the cytolytic function may not be able to accurately enumerate virus-specific CD8+ T cell levels as compared to intracellular cytokine production or TCR binding by H-2 MHC class I-peptide tetramers 25.
We have demonstrated here that the majority (>60%) of CNS-infiltrating CD8+ T cells in both mouse strains during the acute response are specific for viral capsid protein epitopes (Fig. 1, 2). However, the overall number of virus-specific CNS-infiltrating CD8+ T cells is significantly higher (>3-fold) in resistant C57BL/6 mice at early stages of viral infection compared to susceptible SJL/J mice. Therefore, the initial level of the antiviral CD8+ T cell response may be critically important in early containment of viral persistence, leading to resistance to TMEV-IDD. No significant level of CD8+ T cells reactive to non-structural proteins (encoded by P2 or P3 regions) were detectable either in the periphery or CNS of TMEV-infected mice by a similar screening of a 20-mer peptide library (data not shown). These results strongly suggest that the capsid-reactive CD8+ T cells 13–15 likely represent most of TMEV-specific CD8+ T cell responses in both strains. Thus, the direct comparison studies presented here provide a credible estimation of the actual magnitude and kinetics of the overall MHC class I-restricted T cell response to TMEV in these prototypical, resistant and susceptible mouse strains. The role of persisting virus-specific CD8+ T cells in the CNS of susceptible SJL/J mice is not yet clear. These CD8+ T cells may reflect an antiviral T cell response to continued viral persistence in the CNS. However, it is conceivable that such a late occurring CD8+ T cell population may play an important pathogenic role in the induction of demyelinating disease. It is possible that, in some circumstances, CD8+ CTL may be pathogenic due to destruction of virus-infected cells in an effort to clear the infectious agent 10, as shown with lymphochoriomeningitis virus (LCMV) infection in mice 26 and viral hepatitis in humans 27.
The CTL response in the CNS of TMEV-infected susceptible SJL/J mice seems to be qualitatively similar to that in resistant C57BL/6 mice with respect to the avidity to the epitopes, IFN-γ production, and cytolytic function (Fig. 4, 5). The activation status of the CD8+ T cells in the CNS of virus-infected mice appears to be also very similar in these mice (Fig. 6). Therefore, the major differences in the CD8+ T cell response between these mouse strains may lie in the quantity rather than the quality of virus-specific CD8+ T cells in the CNS. This notion is supported by the fact that greater than twofold increase of infiltrating CD8+ T cells expressing CD25 are found at early stages of viral infection in C57BL/6 mice compared to SJL/J mice (Fig. 6). The difference in CD25 expression is known to play an important role in rapid expansion of CD8+ T cells upon stimulation 28. Thus, the lower level of CD25+ CNS CD8+ T cells coupled with the lower level of IL-2 production (not shown) in susceptible SJL/J mice may result in lower numbers of virus-specific CD8+ T cells at early stages of viral infection compared to that in resistant C57BL/6 mice. However, the potential differences in the processing levels of respective epitopes and/or subtle differences in the cytolytic function by the overall CD8+ T cells between C57BL/6 and SJL/J mice may also critically affect the protection from TMEV infection, as demonstrated using recombinant vaccinia viruses 29.
4 Materials and methods
4.1 Animals
Female SJL/J and C57BL/6 mice were purchased from the Charles River Laboratories (Charles River, MA) through the National Cancer Institute (Frederick, MD). All mice were housed at the Center for Comparative Medicine Facility of Northwestern University.
4.2 Virus and cell lines
The BeAn strain of TMEV was propagated and titered in BHK cells grown in Dulbecco's modified Eagle medium supplemented with 7.5% donor calf serum. The EL-4 (H-2b) cell line was obtained from American Type Culture Collection (ATCC, Bethesda, MD). EL-4Ks cell lines were generated by transfection with RSV-Ks-SV40, as described 15. All cell lines were maintained in RPMI 1640 supplemented with 10% fetal calf serum, glutamine/pyruvate and antibiotics.
4.3 Synthetic peptides and antibodies
Synthetic peptides were generated with the RaMPS peptide synthesis system (DuPont Co., Wilmington, DE). All peptide stocks (2 mM) were dissolved in 8% dimethylsulfoxide in PBS. All antibodies used for flow cytometry were purchased from PharMingen (San Diego, CA).
4.4 Infection of mice with TMEV
For intracerebral (i.c.) infection, 30 µl (approximately 1×105 pfu) of TMEV BeAn was injected into the right cerebral hemisphere of 6–8-week-old mice anesthetized with methoxyflurane.
4.5 Isolation of CNS-infiltrating lymphocytes
Mice were perfused through the left ventricle with 30 ml HBSS. Excised brains and spinal cords were forced through wire mesh and incubated at 37°C for 45–60 min in 250 µg/ml collagenase type 4 (Worthington, Lakewood, NJ). CNS-infiltrating lymphocytes (CNS-IL) were then enriched on a continuous Percoll (Pharmacia, Piscataway, NJ) gradient after centrifugation for 30 min at 27,000×g.
4.6 CTL assays
All target cells were incubated for 4–6 h with peptides, labeled with 51Cr (50 µCi per target) for 2 h. 51Cr-labeled target cells were added to a 96-well round-bottom plate at 100 µl/well. CNS effector cells were added to the target cells immediately after isolation. Supernatants were harvested after 6 h of incubation at 37°C, and mean radioactivity values were calculated from duplicate wells. Percentage of specific lysis was calculated according to the standard formula. Spontaneous release for all experiments was <15% of the maximum release.
4.7 Intracellular cytokine staining
CNS-IL were cultured in 96-well plates in the presence of relevant or control peptide and Golgi-PlugTM for 6 h at 37°C. Cells were then incubated in 50 µl 2.4G2 hybridoma (ATCC) supernatant for 20 min at 4°C to block FcR. FITC-conjugated anti-CD8 antibody (clone 53–6.7) diluted in 50 µl 2.4G2 supernatant was added, and cells were incubated for an additional 30 min at 4°C. After two washes, intracellular IFN-γ staining was performed according to the manufacturer's instructions (PharMingen) using a PE-labeled anti-IFN-γ and an isotype control antibody. Cells were analyzed on a Becton Dickinson FACSCalibur. Live cells were gated based on light scatter properties.
4.8 Plaque assay
After cardiac perfusion with cold HBSS, brain and spinal cords were homogenized in PBS as a 10% (w/v) solution, using a tissue homogenizer, and clarified by low-speed centrifugation (600×g). A standard plaque assay was performed on BHK-21 cell monolayers 6. Plaques in the BHK monolayer were visualized by staining with 0.1% crystal violet solution.
Acknowledgements
This work was supported by grants NS23349, NS28752 and NS33008 from USPHS.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




