Human dendritic cells respond to Porphyromonas gingivalis LPS by promoting a Th2 effector response in vitro
Abstract
Understanding how mucosal pathogens modulate the immune response may facilitate the development of vaccines for disparate human diseases. In the present study, human monocyte-derived DC (MDDC)were pulsed with LPS of the oral pathogen Porphyromonas gingivalis and Escherichia coli 25922 and analyzed for: (i) production of Th-biasing/inflammatory cytokines; (ii) maturation/costimulatory molecules; and (iii) induction of allogeneic CD4+ and naive CD45RA+ T cell proliferation and release of Th1 or Th2 cytokines. We show that E. coli LPS-pulsed MDDC released Th1-biasing cytokines — consisting of high levels of IL-12 p70, IFN-γ-inducible protein 10 (IP-10) – but also TNF-α, IL-10, IL-6 and IL-1β. In contrast, no IL-12 p70 or IP-10, and lower levels of TNF-α and IL-10 were induced by P. gingivalis LPS. These differences were sustained at LPS doses that yielded nearly equivalent maturation of MDDC; moreover the T cell response was consistent: E. coli LPS-pulsed MDDC induced higher T cell proliferation, and T cells released more IFN-γ and IL-2, but less IL-5 than T cells co-cultured with P. gingivalis LPS pulsed-MDDC. IL-13 was secreted by naive CD45RA+CD45RO–CD4+ T cells in response to P. gingivalisLPS-pulsed MDDC. These results suggest that human MDDC can be polarized by LPS from the mucosal pathogen P. gingivalis to induce a Th2 effector response in vitro.
Abbreviations:
-
- IP-10:
-
IFN-γ-inducible protein 10
-
- MDDC:
-
Monocyte-derived DC
1 Introduction
DC are innate immune cells within the skin and mucosa that respond rapidly to infection, carrying vital information about the infection to the lymph nodes, where an immune response can be initiated 1–3. This includes specific information about the identity of microbes and also about the inflammatory cytokines / growth factors encountered in the microenvironment of the skin and mucosa. DC can become cytokine-polarized by these microenvironmental signals, and can pass this information on to naive T cells in the lymph nodes, thereby promoting a Th1 or Th2 response (reviewed in 3). Although many factors have been implicated in cytokine polarization 3–9, our group is particularly interested in the ability of different pathogen-associated molecular patterns to modulate the immune response 9. Understanding this process may facilitate the improvement of vaccines to treat human diseases with an immunopathogenic etiology, such as autoimmune diseases, atopic dermatitis, asthma and periodontitis.
Our recent studies in mice 9 suggest that LPS from the oral mucosal pathogen Porphyromonas gingivalis, which does not require TLR4 10 and purportedly signals through TLR2 11, 12, can skew the murine response to ovalbumin towards a Th2-response. It is extremely important, however, to establish whether P. gingivalis LPS induces a Th2-effector response in the human system, in which the disease chronic periodontitis (CP) occurs. CP patients have elevated local levels of both Th2- and Th1-biasingcytokines (reviewed in 13, 14) and increased local numbers of DC subpopulations 15–19, CD4+ T cells 13–16 and B cells / plasma cells 14, 20. Many reports cite the induction of a strong humoral immune response towards P. gingivalis inadult periodontitis subjects (reviewed in 21), including evidence for induction of high IgG antibodies to P. gingivalis LPS 22, with elevated IgG4 23, 24. The lack of clear polarization in the cytokine response in CP 13 might be reflective of the complexity of the polymicrobial oral infection 25 and the different stages of the disease process during cytokine sampling 13. Also observed is the presence of P. gingivalis antigens, colocalized with immature DC in situ 18; moreover, our laboratory has now observed, using double-immunofluorescence staining and laser confocal microscopy, the presence of dermal DC and mature CD83+ DC in close association with CD4+ T cells in situ 19.
Accordingly, the present study was designed to establish whether human monocyte-derived DC (MDDC) can be polarized by highly purified P. gingivalis LPS to induce a Th2 effector response in vitro. The results indicate that, indeed, human MDDC pulsed with LPS of P. gingivalis undergo maturation but release a different profile of cytokines and induce a Th2-skewed response, relative to Escherichia coli LPS.
2 Results
2.1 Cytokine-polarization of human MDDC by P. gingivalis LPS leads to a differential T cell response
E. coli LPS induces high levels of IL-12 p70 from ex vivo-isolated murine CD8α+ DC (9). IL-12 p70 is instrumental in committing T cells to Th1-lineage differentiation 26. IFN-γ-inducible protein 10 (IP-10) is a CXC chemokine that, in addition to directing the migration of Th1 cells preferentially, also directly drives naive T cells into the Th1 lineage 27. To establish whether human MDDC can be cytokine-polarized by different LPS moieties, MDDC were pulsed with P. gingivalis or E. coli LPS at a range of concentrations, and supernatants were analyzed (Fig. 1). Our results indicate that E. coli LPS induced a dose-dependent increase in IL-12 p70 and of IP-10. P. gingivalis LPS did not induce IL-12 p70 or IP-10 at any dose. E. coli LPS also induced significantly higher levels than P. gingivalis LPS of TNF-α and IL-10, but not IL-6 or IL-1β (Table 1). Both LPS moieties induced saturating levels of IL-8 (>8,000 pg/ml; not shown). The essential difference in these cytokine profiles was retained when pulsed with either LPS moiety at doses (10 ng/ml for E. coli and 1000 ng/ml for P. gingivalis) that led to nearly equivalent maturation and costimulatory molecule expression (Fig. 1B and C, respectively).
MDDC were then co-cultured with CD4+ allogeneic T cells. MDDC pulsed with E. coli LPS were much more efficient at stimulating allogeneic T cell proliferation than P. gingivalis LPS was, particularly at 100 ng/ml of either LPS (Table 1). To determine the cytokine profile of CD4+ responder T cells, supernatants from MDDC / T cell co-cultures were analyzed. As shown in Table 1, E. coli LPS-pulsed MDDC induced T cells to secrete high levels of Th1 cytokines IFN-γ and IL-2. In contrast, P. gingivalis LPS-pulsed MDDC induced T cells to secrete lower levels of IFN-γ and IL-2 and higher levels of the Th2 cytokine IL-5. The classic Th2 cytokine IL-4 28 was not detected in any of the T cell supernatants (not shown), consistent with our previous murine studies of P. gingivalis LPS 9.
Th1 and Th2 subsets develop from the same T cell precursor, which is a naive CD4+ T lymphocyte 28. Functional receptors for IL-12 appear to be restricted to uncommitted cells and to Th1 cells 29. Accordingly, naive CD45RA+CD45RO–CD4+ T cells were isolated and used as responder cells for MDDC polarized by the two LPS moieties in the next set of experiments.
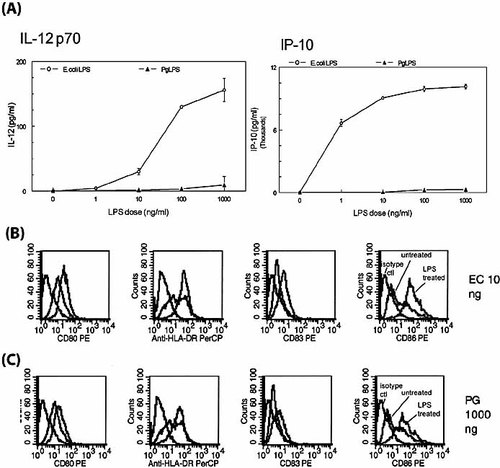
Maturation and differential secretion of IL-12 and IP-10 by MDDC treated with LPS from P. gingivalis or E. coli. (A) MDDC treated with E. coli LPS, but not with P. gingivalis (Pg) LPS, secreted IL-12 p70 and IP-10. Day 6 immature MDDC were grown as described in Sect. 4, and treated with indicated doses of LPS for 24 h. Culture supernatants were collected and assayed for IL-12 p70 and IP-10 by commercial ELISA (IL-10) and by CBA kit (IL-12 p70). Each data point represents mean ± S.D. of triplicate samples from a typical experiment. Unstimulated MDDC served as control and did not produce any detectable levels of IL-12 p70 or IP-10. Data are representative of three independent experiments. (B) MDDC treated with 10 ng/ml E. coli LPS (EC) upregulated expression of costimulatory molecules CD80, HLA-DR, CD83, and CD86 (red histograms), as compared with untreated controls (green histograms) and isotype controls (black histograms). FACS analysis was carried out as described in Sect. 4. (C) MDDC required 1,000 ng/ml P. gingivalis LPS (PG) to achieve near-comparable maturation/co-stimulatory molecule expression. Results are representative of three separate experiments.
|
|
MDDC cytokinesa) |
T cell proliferationb) |
T cell cytokinesc) |
|||||
|---|---|---|---|---|---|---|---|---|
|
|
TNF-α |
IL-10 |
IL-6 |
IL-1β |
cpm |
IFN-γ |
IL-2 |
IL-5 |
|
No LPS (control) |
42±16 |
5±2 |
24±8 |
n.d. |
3,000 |
300±20 |
60±7 |
16±3 |
|
P. gingivalis LPS (100 ng/ml) |
787±40 |
474±42 |
17,482±967 |
122±169 |
8,000 |
1,000±55 |
40±8 |
53±5 |
|
E. coli LPS (100 ng/ml) |
6,215±108 |
1,349±12 |
19,346±1113 |
41.4±47 |
25,000 |
2,500±75 |
115±10 |
36±6 |
|
No DC (control) |
– |
– |
– |
– |
1,000 |
60±30 |
10±4 |
4±2 |
- a) Mean pg/ml ± S.D. of triplicate determinations in supernatants from MDDC pulsed with LPS for 24 hrs. n.d., not detectable. —, not done.
- b) Proliferative response of allogeneic CD4+ T cells after 5 days determined by uptake of tritiated thymidine (1 μCi/well) for the last 16 h in counts per minute (cpm). Results are representative of three separate experiments.
- c) Mean pg/ml ± S.D. of triplicate determinations in supernatants from CD4+ T cells co-cultured with MDDC for 5 days.
2.2 MDDC that are cytokine-polarized by different LPS moieties differentially prime naive CD4+ T cells and promote the development of Th1 or Th2 cells
The results of co-culture experiments (Fig. 2A) indicate that indeed, MDDC that were polarized by E. coli LPS were much more efficient at stimulating proliferation of naive T cells than were MDDC polarized by P. gingivalis LPS. Moreover, the cytokines secreted by T cells in response to E. coli LPS-pulsed MDDC or P. gingivalis-pulsed MDDC (Fig. 2B, C) were consistent with Th1 and Th2 cells, respectively. Although IL-4 was not detected in any of the T cell supernatants, IL-13, which is crucial for Th2 generation in the absence of IL-4 30, as well as IL-5, was detected in naive T cells co-cultured with P. gingivalis-pulsed MDDC. Moreover, the ratio of Th1/Th2 cytokines revealed a pronounced Th1 bias of naive T cells co-cultured with E. coli LPS-pulsed MDDC.
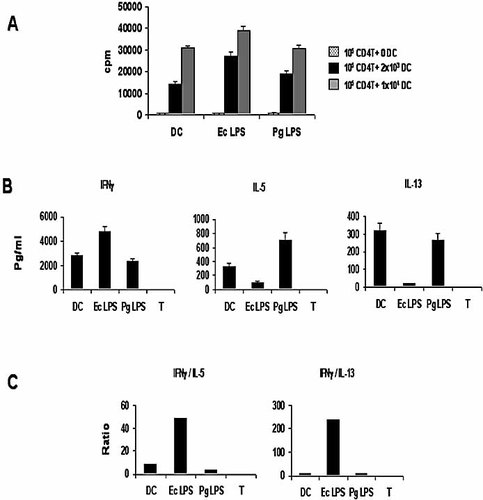
MDDC activated with E. coli (Ec) LPS and P. gingivalis (Pg) LPS induce distinct naive T-helper responses. Immature MDDC were cultured for 48 h, with the different stimuli, then washed and cultured at graded doses, with 105 naive, allogeneic CD4+ T cells. (A) After 5 days, T cell proliferation was assessed by overnight [3H]thymidine labeling (cpm, counts per minute). Speckled, black and gray histograms represent 0 DC, 2×103 DC and 1×104 DC, respectively. (B) The secretion of Th1 and Th2 cytokines in culture in triplicate was assessed by commercial ELISA. (C) The ratio of Th1/Th2 cytokines was calculated for each of the stimuli, as described previously 9.
3 Discussion
In the present study we show that MDDC treated with LPS moieties from different bacterial species, purified identically, can induce a very different type of immune response in vitro. E. coli LPS and P. gingivalis LPS both induced a similar pattern of maturation/costimulatory molecule expression on MDDC, albeit P. gingivalis LPS was 100-fold less potent in this respect (Fig. 1B, C). Analysis of the cytokine profiles from MDDC pulsed with 100-fold more P. gingivalis LPS than E. coli LPS indicated a lack of IL-12 and IP-10 secreted (Fig. 1A). Despite concentrations of LPS that yield nearly comparable maturation status of MDDC, the cytokine profile elicited by P. gingivalis LPS (with the exception of IL-10 [Table 1]) was biased towards Th2, and towards a less-inflammatory response. This was confirmed by the weak activity of P. gingivalis LPS-pulsed MDDC for priming allogeneic CD4+ (Table 1) and naive (Fig. 2A) T cells and by the absence of Th1-cytokines secreted by these T cells (Fig. 2 and Table 1). Thus these data are consistent with the concept that MDDC can become polarized 3, 8, 28 when exposed to different bacterial pathogen-associated molecular patterns and that these MDDC can than induce a T helper cell subset response that is consistent with the cytokine profiles (and maturation status) of MDDC 28.
The lipid A of P. gingivalis is biologically weak and structurally unique, relative to the enterobacteriacea 10, 31. P. gingivalis lipid A has highly branched, long-chain fatty acids and lacks a phosphoryl group substitution in position 4′ of the non-reducing glucosamine, as well as other modifications 31. P. gingivalis LPS is active in C3H/HeJ mice that possess a point mutation in tlr4 9, 10, 31 and thus do not respond to enteric LPS moieties. TLR4 is the principal TLR species involved in LPS signaling, whereas TLR2 is the paradigmatic receptor for bacterial components other than LPS, including lipoteichoic acid, peptidoglycan and lipoproteins 8. Recent studies suggest that TLR2 might be the primary signal-transducing molecule for LPS from certain nonenterobacterial Gram-negative organisms, including P. gingivalis 9, 11, 12 and Leptospira interrogans 32. However, controversy still exists in the case of P. gingivalis LPS, as it appears to also have activity for TLR4 12, 33–35. P. gingivalis LPS has been shown to antagonize the activity of TLR4 agonists 33, and several studies in fibroblasts indicate that signaling in response to P. gingivalis LPS is mediated, in part, through TLR4 35, 36.
Several groups have analyzed the expression of TLR molecules by MDDC. Human MDDC express mRNA for TLR1, 2, 3, 4 and 5 37, whereas human immature CD11c+ DC isolated from peripheral blood express high levels of mRNA for TLR1, 2, and 3, low levels of TLR5, 6, 8, and 10, and undetectable levels of TLR4, 7, and 9 38. Another study analyzed TLR proteins on human peripheral blood DC and MDDC by flow cytometry – both DC sources expressed TLR2, but not TLR4 39. The MDDC used in the present study were 97% CD1a+, but expressed very low levels of TLR2, TLR4 and CD14 (<3%; not shown). The only difference we could discern between our methodology for culturing MDDC and that of Thoma-Uszynski 39 is that we use 10% FCS, whereas they use 10% human serum. Efforts are now underway in our laboratory to better characterize the expression of pattern-recognition receptors (PRR) on MDDC, as well as to establish how our MDDC are able to respond to LPS with such lowlevels of expression of PRR. One possibility being examined is that the TLR molecules are expressed internally, in vacuoles, thus requiring internalization for triggering a cytokine response 40.
Our study provides evidence that human MDDC can be polarized by LPS from the mucosal pathogen P. gingivalis and can convey this information to T cells. Through this rapid transfer of information the development of a disease- and pathogen-specific immune response can be fostered, but certain pathogens may modulate the response to their own benefit. These findings are particularlyapplicable to the pathogenesis of mucosal diseases and to the development of vaccine adjuvants for these diseases.
4 Materials and methods
4.1 DC cultures, and multiparameter flow cytometry analysis
Day 6 immature MDDC were generated as we have previously described 15, 18. Flow cytometry was performed to confirm the immature DC phenotype (CD14–CD83–CD1a+). Cell surface markers of DC were evaluated by four-color immunofluorescence staining with the following mAb: CD1a–FITC (Biosource), CD40–PE (Coulter/Immunotech), CD80–PE (Becton Dickinson), CD83–PE (Immunotech), CD86–PE (Pharmingen), HLA-DR–PerCP (Becton Dickinson), and CD14–APC (Caltag). After 30 min at 4°C and washing with staining buffer (PBS, pH 7.2, 2 mM EDTA, 2% FCS), cells were fixed in 1% paraformaldehyde. Analysis was performed with FACScaliburTM (Becton Dickinson). Marker expression was analyzed as the percentage of positive cells in the relevant population defined by forward scatter and side scatter characteristics. Expression levels were evaluated by assessing mean fluorescence intensity (MFI) indices calculated by relating MFI noted with the relevant mAb to that with the isotype control mAb for samples labeled in parallel and acquired using the same settings.
4.2 Purification of allogeneic CD4+ cells
CD4+ cells were purified from allogeneic PBMC, as described previously 15. Cells bearing the CD4 antigen were isolated from the mononuclear fraction through positive selection, using microbeads coated with anti-CD4 mAb and goat anti-mouse-IgG (Miltenyi Biotech Gmbh Gladbach, Germany). Isolation of CD4+ cells was achieved using Minimacs separation columns (Miltenyi Biotec Gmbh) as described by manufacturer. In all the experiments the isolated cells were 80–90% CD4+, as determined by staining with FITC-conjugated anti-CD4 mAb, followed byflow cytometry analysis (not shown). Naive, CD4+CD45RA+ T cells were isolated to 98% purity by FACS sorting.
4.3 LPS purification
The methodology for isolation and purification of LPS from P. gingivalis 381 and E. coli ATCC type strain 25922 was as previously described in our laboratory 9, 18, 41. LPS was analyzed for protein content by the bicinchoninic-acid protein assay (Pierce, Rockford, IL, USA). LPS samples were also separated by SDS-PAGE and stained for protein with Coomassie blue. Selected samples were also subjected to proteinase K digestion and nuclease treatment, and reanalyzed by SDS-PAGE to confirm the purity of the LPS moieties (data not shown). In certain experiments, the E. coli LPS and P. gingivalis LPS used were kind gifts of Tom Van Dyke (Boston University, Goldman School of Dental Medicine).
4.4 Cytokines from DC and T cells
To study cytokines produced by MDDC, cells were treated with LPS at a range of doses for 24 h, then culture supernatants were collected and analyzed by two methods: commercial ELISA (R&D systems, Minneapolis, MN, USA) and flow cytometry using a cytometric bead array (CBA Kit, BD Biosciences, San Diego, CA, USA). On the basis of a standard curve achieved for each cytokine, the CBA software calculates levels in pg/ml. For T cell cytokines, MDDC were washed, and then co-cultured with allogeneic CD4+ T cells for 5 days. The production of cytokines from co-cultures was determined using commercial ELISA kits (R&D Systems) and the CBA kit, as above.
4.5 T cell proliferation assay
The ability of LPS-pulsed MDDC to stimulate allogeneic CD4+ T cells to proliferate was performed as described previously 15. Day 6 MDDC were pulsed with LPS (1–1000 ng/ml) from P. gingivalis strain 381 and E. coli ATCC 25922, or no LPS, for 24 h at 37°C in complete RPMI. MDDC were washed extensively and cultured at graded doses (5000 DC / 200 μL, 1000 DC / 200 μL and 300 DC / 200 μL) in complete RPMI medium (plus 10% human AB serum) with allogeneic CD4+ T cells. (50,000 cells / 200 μl). Proliferation was determined after 5 days by uptake of tritiated thymidine (1 μCi/well for the last 16 h). The assay was repeated a minimum of three times on separate days.
Acknowledgements
We acknowledge the support of the NIH/NIDCR grants DE14328-03 (C. W. C.) and DE13154-01 (C. W. C.), NIH grants DK 57665-01 (B. P.) and AI48638-01 (B. P.) andsupport from the Centers for Disease Control (B. P.).
- WILEY-VCH
- WILEY-VCH




